Challenges in Liver Transplantation for Hepatocellular Carcinoma: A Review of Current Controversies
Abstract
Simple Summary
Abstract
1. Introduction
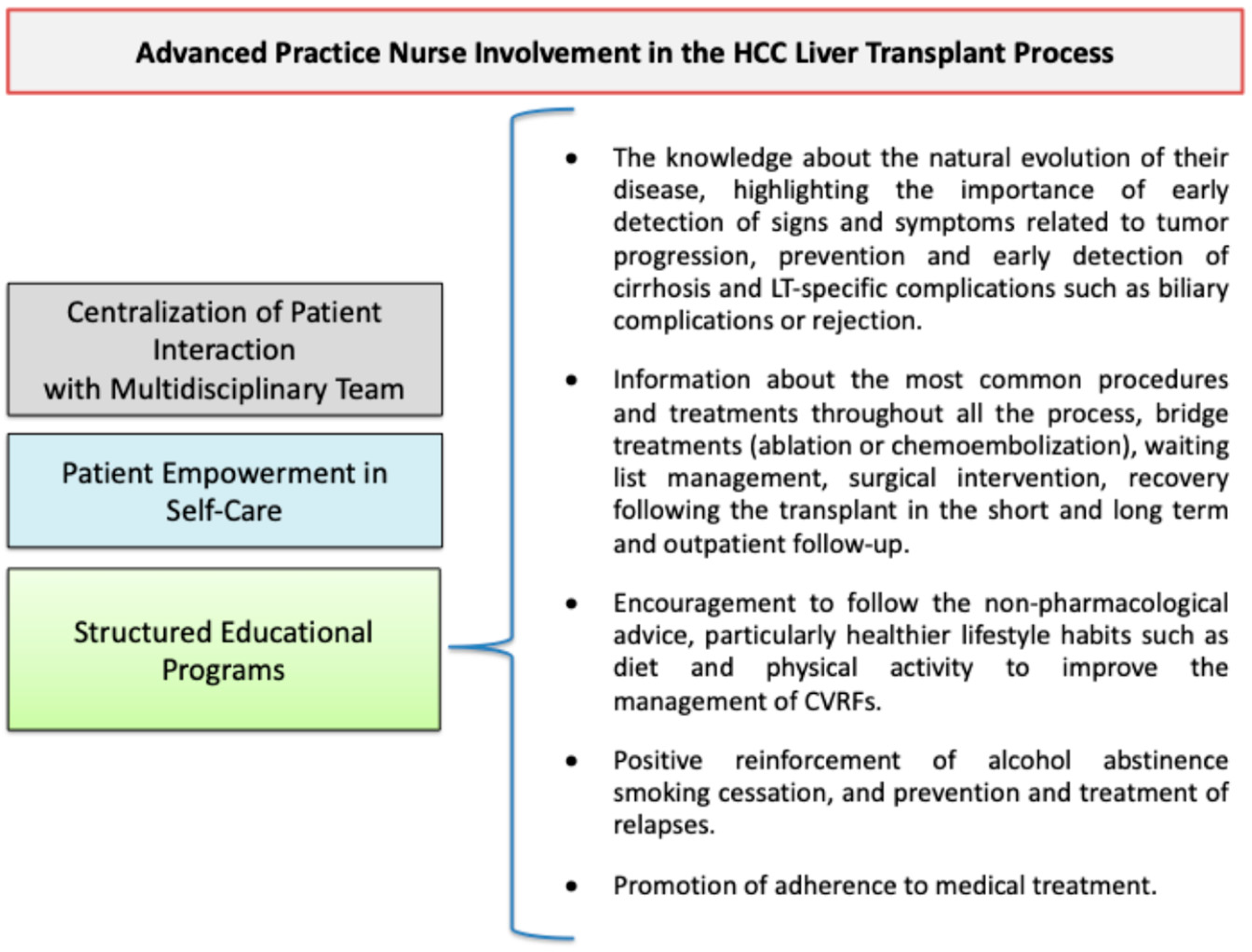
2. The Role of Advanced Practice Nurses in Patient Education and Managing Comorbidities
3. Prevalence and Impact of Frailty on Waitlists and Post-LT Outcomes in Patients with HCC
4. Selection Criteria for LT in HCC: Should Downstaging Be Universal or Tailored to the Tumor Burden and AFP Limits?
Is the “All Comers” Approach Worth It?
5. The Pivotal Role of Radiology in Patient Selection for LT
6. Immunotherapy Pre- and Post-LT: Expanding Horizons and Challenges
6.1. Pre-LT: Downstaging and Bridging Therapy
6.2. Implications of Immunotherapy Use in the Post-LT Setting
7. Immunosuppression Management of LT in HCC
8. Post-LT Surveillance for HCC Recurrence
8.1. Selection of Candidates for Surveillance
8.2. Duration, Intervals, and Imaging Techniques for Surveillance of HCC Recurrence after LT
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Burroughs, A.; Graziadei, I.; Pirenne, J.; Valdecasas, J.C.; Muiesan, P.; Samuel, D.; Forns, X. EASL Clinical Practice Guidelines: Liver Transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef]
- Navasa, M.; Bruix, J. Multifaceted Perspective of the Waiting List for Liver Transplantation: The Value of Pharmacokinetic Models. Hepatology 2010, 51, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.; Trota, N.; Londoño, M.C.; Mauro, E.; Baliellas, C.; Castells, L.; Castellote, J.; Tort, J.; Forns, X.; Navasa, M. The Efficacy of Direct Anti-HCV Drugs Improves Early Post-Liver Transplant Survival and Induces Significant Changes in Waiting List Composition. J. Hepatol. 2018, 69, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A Randomized Trial of Normothermic Preservation in Liver Transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Markmann, J.F.; Abouljoud, M.S.; Ghobrial, R.M.; Bhati, C.S.; Pelletier, S.J.; Lu, A.D.; Ottmann, S.; Klair, T.; Eymard, C.; Roll, G.R.; et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022, 157, 189–198. [Google Scholar] [CrossRef]
- O’Mahony, C.A.; Goss, J.A. The Future of Liver Transplantation. Tex. Heart Inst. J. 2012, 39, 874–875. [Google Scholar] [CrossRef]
- Li, M.; Bhoori, S.; Mehta, N.; Mazzaferro, V. Immunotherapy for Hepatocellular Carcinoma: The next Evolution in Expanding Access to Liver Transplantation. J. Hepatol. 2024. [CrossRef]
- Mikulic, D.; Mrzljak, A. Liver Transplantation and Aging. World J. Transplant. 2020, 10, 256–266. [Google Scholar] [CrossRef]
- Chen, H.P.; Tsai, Y.F.; Lin, R.; Liu, F.C.; Yu, H.P. Recipient Age and Mortality Risk after Liver Transplantation: A Population-Based Cohort Study. PLoS ONE 2016, 11, e0152324. [Google Scholar] [CrossRef]
- Crabb, D.W.; Im, G.Y.; Szabo, G.; Mellinger, J.L.; Lucey, M.R. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 2020, 71, 306–333. [Google Scholar] [CrossRef]
- Winder, G.S.; Shenoy, A.; Dew, M.A.; DiMartini, A.F. Alcohol and Other Substance Use after Liver Transplant. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101685. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, J.; Tabrizian, P.; Bhangui, P.; Pinato, D.J.; Rodríguez-Perálvarez, M.L.; Sapisochin, G.; Bhoori, S.; Pascual, S.; Senzolo, M.; Al-Adra, D.; et al. De Novo Malignancy After Liver Transplantation: Risk Assessment, Prevention, and Management-Guidelines From the ILTS-SETH Consensus Conference. Transplantation 2022, 106, e30–e45. [Google Scholar] [CrossRef]
- López-Lazcano, A.I.; Gual, A.; Colmenero, J.; Caballería, E.; Lligoña, A.; Navasa, M.; Crespo, G.; López, E.; López-Pelayo, H. Active Smoking Before Liver Transplantation in Patients with Alcohol Use Disorder: Risk Factors and Outcomes. J. Clin. Med. 2020, 9, 2710. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.G.; Benages, E.L. Enfermería Clínica Avanzada. In Enfermera de Práctica Avanzada y Trasplante de Órganos El Trasplante Hepático en Adultos; Elsevier: Amsterdam, The Netherlands, 2023; pp. 55–72. [Google Scholar]
- Lieber, S.R.; VanWagner, L.B. Monitoring Cardiovascular Risk Factors after Liver Transplantation May Improve Outcomes. Liver Transplant. 2022, 28, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, F.; Golfieri, L.; Nascimbeni, F.; Andreone, P.; Gitto, S. Metabolic Disorders in Liver Transplant Recipients: The State of the Art. J. Clin. Med. 2024, 13, 1014. [Google Scholar] [CrossRef]
- Neuberger, J. Long-Term Care of the Adult Liver Transplant Recipient. J. Clin. Exp. Hepatol. 2022, 12, 1547–1556. [Google Scholar] [CrossRef]
- Wong, L.L.; Lee, L.Y.; Karasaki, K.; Ogihara, M.; Tran, C. Management of Hepatocellular Carcinoma in Patients Who Are 70 Years or Older. Surg. Open Sci. 2022, 10, 53–58. [Google Scholar] [CrossRef]
- Kelly, D.; Fernández-Ortega, P.; Arjona, E.T.; Daniele, B. The Role of Nursing in the Management of Patients with Renal and Hepatic Cancers: A Systematic Literature Review. Eur. J. Oncol. Nurs. 2021, 55, 102043. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Sastre, L.; García, R.; Viñals, C.; Amor, A.J.; Yago, G.; Hervás, A.; Sánchez, L.; Trabal, J.; Molero, J.; Escudé, L.; et al. Results of a Multidisciplinary Strategy to Improve the Management of Cardiovascular Risk Factors after Liver Transplantation. Liver Transplant. 2022, 28, 1332–1344. [Google Scholar] [CrossRef]
- Cheung, R.; O’Donnell, S.; Madi, N.; Goldner, E.M. Factors Associated with Delayed Diagnosis of Mood and/or Anxiety Disorders. Health Promot. Chronic Dis. Prev. Can. Res. Policy Pract. 2017, 37, 137–148. [Google Scholar] [CrossRef]
- Tsai, W.C.; Kung, P.T.; Wang, Y.H.; Kuo, W.Y.; Li, Y.H. Influence of the Time Interval from Diagnosis to Treatment on Survival for Early-Stage Liver Cancer. PLoS ONE 2018, 13, e0199532. [Google Scholar] [CrossRef] [PubMed]
- Fabrellas, N.; Künzler-Heule, P.; Olofson, A.; Jack, K.; Carol, M. Nursing Care for Patients with Cirrhosis. J. Hepatol. 2023, 79, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Fabrellas, N.; Carol, M.; Palacio, E.; Aban, M.; Lanzillotti, T.; Nicolao, G.; Chiappa, M.T.; Esnault, V.; Graf-Dirmeier, S.; Helder, J.; et al. Nursing Care of Patients With Cirrhosis: The LiverHope Nursing Project. Hepatology 2020, 71, 1106–1116. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K. Conceptual Models of Frailty: Accumulation of Deficits. Can. J. Cardiol. 2016, 32, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rockwood, K. Frailty in Older Adults. N. Engl. J. Med. 2024, 391, 538–548. [Google Scholar] [CrossRef]
- Lai, J.C.; Covinsky, K.E.; Dodge, J.L.; Boscardin, W.J.; Segev, D.L.; Roberts, J.P.; Feng, S. Development of a Novel Frailty Index to Predict Mortality in Patients with End-Stage Liver Disease. Hepatology 2017, 66, 564–574. [Google Scholar] [CrossRef]
- Wang, C.W.; Lebsack, A.; Chau, S.; Lai, J.C. The Range and Reproducibility of the Liver Frailty Index. Liver Transplant. 2019, 25, 841–847. [Google Scholar] [CrossRef]
- Thuluvath, A.J.; Duarte-Rojo, A.; Lai, J.C.; Peipert, J.; Dietch, Z.C.; Siddiqui, O.; Morrissey, S.; Belfanti, K.; Zhao, L.; Guo, K.; et al. Brief PROMIS Assessment Screens for Frailty and Predicts Hospitalizations in Liver Transplant Candidates. Transplantation 2024, 108, 491–497. [Google Scholar] [CrossRef]
- Tandon, P.; Tangri, N.; Thomas, L.; Zenith, L.; Shaikh, T.; Carbonneau, M.; Ma, M.; Bailey, R.J.; Jayakumar, S.; Burak, K.W.; et al. A Rapid Bedside Screen to Predict Unplanned Hospitalization and Death in Outpatients With Cirrhosis: A Prospective Evaluation of the Clinical Frailty Scale. Off. J. Am. Coll. Gastroenterol.|ACG 2016, 111, 1759–1767. [Google Scholar] [CrossRef]
- Haugen, C.E.; McAdams-Demarco, M.; Holscher, C.M.; Ying, H.; Gurakar, A.O.; Garonzik-Wang, J.; Cameron, A.M.; Segev, D.L.; Lai, J.C. Multicenter Study of Age, Frailty, and Waitlist Mortality Among Liver Transplant Candidates. Ann. Surg. 2020, 271, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Volk, M.L.; Strasburg, D.; Alexander, N. Performance-Based Measures Associate with Frailty in Patients with End-Stage Liver Disease. Transplantation 2016, 100, 2656–2660. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Covinsky, K.E.; McCulloch, C.E.; Feng, S. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients With Cirrhosis. Off. J. Am. Coll. Gastroenterol.|ACG 2018, 113, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Whitlock, R.; Xu, C.; Taneja, S.; Singh, S.; Abraldes, J.G.; Burak, K.W.; Bailey, R.J.; Lai, J.C.; Tandon, P. Frailty Is Associated with Increased Risk of Cirrhosis Disease Progression and Death. Hepatology 2022, 75, 600–609. [Google Scholar] [CrossRef]
- Lai, J.C.; Dodge, J.L.; Kappus, M.R.; Dunn, M.A.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; Rahimi, R.S.; McCulloch, C.E.; Haugen, C.E.; et al. Changes in Frailty Are Associated with Waitlist Mortality in Patients with Cirrhosis. J. Hepatol. 2020, 73, 575–581. [Google Scholar] [CrossRef]
- Tandon, P.; Reddy, K.R.; O’Leary, J.G.; Garcia-Tsao, G.; Abraldes, J.G.; Wong, F.; Biggins, S.W.; Maliakkal, B.; Fallon, M.B.; Subramanian, R.M.; et al. A Karnofsky Performance Status-Based Score Predicts Death after Hospital Discharge in Patients with Cirrhosis. Hepatology 2017, 65, 217–224. [Google Scholar] [CrossRef]
- Lai, J.C.; Shui, A.M.; Duarte-Rojo, A.; Ganger, D.R.; Rahimi, R.S.; Huang, C.Y.; Yao, F.; Kappus, M.; Boyarsky, B.; McAdams-Demarco, M.; et al. Frailty, Mortality, and Health Care Utilization after Liver Transplantation: From the Multicenter Functional Assessment in Liver Transplantation (FrAILT) Study. Hepatology 2022, 75, 1471–1479. [Google Scholar] [CrossRef]
- Wang, M.; Shui, A.M.; Ruck, J.; King, E.; Rahimi, R.; Kappus, M.; Volk, M.L.; Ganger, D.R.; Ladner, D.P.; Duarte-Rojo, A.; et al. The Liver Frailty Index Is a Predictor of Healthcare Utilization after Liver Transplantation in Older Adults. Clin. Transplant. 2024, 38, e15219. [Google Scholar] [CrossRef]
- DeMaria, S.; Khromava, M.; Schiano, T.D.; Lin, H.M.; Kim, S. Standardized Measures of Frailty Predict Hospital Length of Stay Following Orthotopic Liver Transplantation for Hepatocellular Carcinoma. Clin. Transplant. 2019, 33, e13746. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Shui, A.M.; Duarte-Rojo, A.; Rahimi, R.S.; Ganger, D.R.; Verna, E.C.; Volk, M.L.; Kappus, M.; Ladner, D.P.; Boyarsky, B.; et al. Association of Frailty With Health-Related Quality of Life in Liver Transplant Recipients. JAMA Surg. 2023, 158, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Nikirk, S.; Parikh, N.D.; Zhao, L. Falls Are Common, Morbid, and Predictable in Patients with Cirrhosis. J. Hepatol. 2021, 75, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Ramai, D.; Dang-Ho, K.P.; Kewalramani, A.; Bandaru, P.; Sacco, R.; Giacomelli, L.; Shah, A.; Papa, S.; Cappellini, F.; Perversi, F.; et al. Hospital Frailty Risk Score Is Independently Associated with Mortality and Encephalopathy in Hospitalized Patients with Hepatocellular Carcinoma. Biomedicines 2021, 9, 1693. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Tanaka, S.; Shinkawa, H.; Ohira, G.; Kinoshita, M.; Amano, R.; Kimura, K.; Nishio, K.; Tauchi, J.; Uchida-Kobayashi, S.; et al. Impact of Frailty on Long-Term Outcomes after Liver Resection for Hepatocellular Carcinoma in Elderly Patients: A Prospective Study. Asian J. Surg. 2024, 47, 147–153. [Google Scholar] [CrossRef]
- Yamada, S.; Shimada, M.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Saito, Y.; Yoshikawa, M.; Miyazaki, K. Significance of Frailty in Prognosis After Hepatectomy for Elderly Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2021, 28, 439–446. [Google Scholar] [CrossRef]
- Hirota, K.; Kawaguchi, T.; Koya, S.; Nagamatsu, A.; Tomita, M.; Hashida, R.; Nakano, D.; Niizeki, T.; Matsuse, H.; Shiba, N.; et al. Clinical Utility of the Liver Frailty Index for Predicting Muscle Atrophy in Chronic Liver Disease Patients with Hepatocellular Carcinoma. Hepatol. Res. 2020, 50, 330–341. [Google Scholar] [CrossRef]
- Tsuchihashi, J.; Koya, S.; Hirota, K.; Koga, N.; Narao, H.; Tomita, M.; Kawaguchi, T.; Hashida, R.; Nakano, D.; Tsutsumi, T.; et al. Effects of In-Hospital Exercise on Frailty in Patients with Hepatocellular Carcinoma. Cancers 2021, 13, 194. [Google Scholar] [CrossRef]
- Orange, S.T.; Hallsworth, K.; Brown, M.C.; Reeves, H.L. The Feasibility and Acceptability of a Home-Based, Virtual Exercise Intervention for Older Patients with Hepatocellular Carcinoma: Protocol for a Non-Randomised Feasibility Study (TELEX-Liver Cancer). Pilot Feasibility Stud. 2022, 8, 113. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Kusakabe, J.; Akabane, M.; Maspero, M.; Zervos, B.; Modaresi Esfeh, J.; Whitsett Linganna, M.; Imaoka, Y.; Khalil, M.; Pita, A.; et al. Expanding Selection Criteria in Deceased Donor Liver Transplantation for Hepatocellular Carcinoma: Long-Term Follow-up of a National Registry and 2 Transplant Centers. Transplantation 2024, 10-1097. [Google Scholar] [CrossRef]
- Piñero, F.; Mauro, E.; Casciato, P.; Forner, A. From Evidence to Clinical Practice: Bridging the Gap of New Liver Cancer Therapies in Latin America. Ann. Hepatol. 2024, 29, 101185. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Dodge, J.L.; Grab, J.D.; Yao, F.Y. National Experience on Down-Staging of Hepatocellular Carcinoma Before Liver Transplant: Influence of Tumor Burden, Alpha-Fetoprotein, and Wait Time. Hepatology 2020, 71, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Duvoux, C.; Roudot–Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Citterio, D.; Bhoori, S.; Bongini, M.; Miceli, R.; De Carlis, L.; Colledan, M.; Salizzoni, M.; Romagnoli, R.; Antonelli, B.; et al. Liver Transplantation in Hepatocellular Carcinoma after Tumour Downstaging (XXL): A Randomised, Controlled, Phase 2b/3 Trial. Lancet Oncol. 2020, 21, 947–956. [Google Scholar] [CrossRef]
- Yao, F.Y.; Fidelman, N. Reassessing the Boundaries of Liver Transplantation for Hepatocellular Carcinoma: Where Do We Stand with Tumor Down-staging? Hepatology 2016, 63, 1014–1025. [Google Scholar] [CrossRef]
- Localuser OPTN Policies Effective as of May 29, 2024 [Organ Offer Acceptance Limit]. Available online: https://optn.transplant.hrsa.gov/media/jqmmcqii/policy-notice_opo_acceptance-limits_dec-2023.pdf (accessed on 29 August 2024).
- Sinha, J.; Mehta, N.; Dodge, J.L.; Poltavskiy, E.; Roberts, J.; Yao, F. Are There Upper Limits in Tumor Burden for Down-Staging of Hepatocellular Carcinoma to Liver Transplant? Analysis of the All-Comers Protocol. Hepatology 2019, 70, 1185–1196. [Google Scholar] [CrossRef]
- Degroote, H.; Piñero, F.; Costentin, C.; Notarpaolo, A.; Boin, I.F.; Boudjema, K.; Baccaro, C.; Chagas, A.L.; Bachellier, P.; Ettorre, G.M.; et al. International Study on the Outcome of Locoregional Therapy for Liver Transplant in Hepatocellular Carcinoma beyond Milan Criteria. JHEP Rep. 2021, 3, 100331. [Google Scholar] [CrossRef]
- Tran, N.H.; Muñoz, S.; Thompson, S.; Hallemeier, C.L.; Bruix, J. Hepatocellular Carcinoma Downstaging for Liver Transplantation in the Era of Systemic Combined Therapy with Anti-VEGF/TKI and Immunotherapy. Hepatology 2022, 76, 1203–1218. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.; Sirlin, C.B.; Abecassis, M.; Roberts, L.R.; Zhu, A.; Murad, M.H.; Marrero, J. Aasld Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2017, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi Burgio, M.; Garzelli, L.; Cannella, R.; Ronot, M.; Vilgrain, V. Hepatocellular Carcinoma: Optimal Radiological Evaluation before Liver Transplantation. Life 2023, 13, 2267. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.M.; Baek, J.H.; Shin, C.I.; Han, J.K.; Choi, B.I. Diagnostic Performance of Gadoxetic Acid-Enhanced Liver MR Imaging in the Detection of HCCs and Allocation of Transplant Recipients on the Basis of the Milan Criteria and UNOS Guidelines: Correlation with Histopathologic Findings. Radiology 2015, 274, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Yoo, J.; Park, J.Y. Comparison of Guidelines for Diagnosis of Hepatocellular Carcinoma Using Gadoxetic Acid-Enhanced MRI in Transplantation Candidates. Eur. Radiol. 2020, 30, 4762–4771. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Oh, N.E.; Choi, S.H.; Kim, S.; Lee, H.; Jang, H.J.; Byun, J.H.; Won, H.J.; Shin, Y.M. Suboptimal Performance of LI-RADS V2018 on Gadoxetic Acid-Enhanced MRI for Detecting Hepatocellular Carcinoma in Liver Transplant Candidates. Eur. Radiol. 2024, 34, 465–474. [Google Scholar] [CrossRef]
- Lyshchik, A.; Wessner, C.E.; Bradigan, K.; Eisenbrey, J.R.; Forsberg, F.; Yi, M.; Keith, S.W.; Kono, Y.; Wilson, S.R.; Medellin, A.; et al. Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System: Clinical Validation in a Prospective Multinational Study in North America and Europe. Hepatology 2024, 79, 380–391. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.Y.; Shin, J.; Son, W.J.; Roh, Y.H.; Choi, J.Y.; Sirlin, C.B.; Chernyak, V. Percentages of Hepatocellular Carcinoma in LI-RADS Categories with CT and MRI: A Systematic Review and Meta-Analysis. Radiology 2023, 307, e220646. [Google Scholar] [CrossRef]
- Ferrer-Fàbrega, J.; Sampson-Dávila, J.; Forner, A.; Sapena, V.; Díaz, A.; Vilana, R.; Navasa, M.; Fondevila, C.; Miquel, R.; Ayuso, C.; et al. Limited Tumour Progression beyond Milan Criteria While on the Waiting List Does Not Result in Unacceptable Impairment of Survival. J. Hepatol. 2021, 75, 1154–1163. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus Rivoceranib versus Sorafenib as First-Line Therapy for Unresectable Hepatocellular Carcinoma (CARES-310): A Randomised, Open-Label, International Phase 3 Study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef]
- Galle, P.R.; Decaens, T.; Kudo, M.; Qin, S.; Fonseca, L.; Sangro, B.; Karachiwala, H.; Park, J.-W.; Gane, E.; Pinter, M.; et al. Nivolumab (NIVO) plus Ipilimumab (IPI) vs Lenvatinib (LEN) or Sorafenib (SOR) as First-Line Treatment for Unresectable Hepatocellular Carcinoma (UHCC): First Results from CheckMate 9DW. J. Clin. Oncol. 2024, 42, LBA4008. [Google Scholar] [CrossRef]
- Ronca, V.; Wootton, G.; Milani, C.; Cain, O. The Immunological Basis of Liver Allograft Rejection. Front. Immunol. 2020, 11, 2155. [Google Scholar] [CrossRef]
- Tabrizian, P.; Florman, S.S.; Schwartz, M.E. PD-1 Inhibitor as Bridge Therapy to Liver Transplantation? Am. J. Transplant. 2021, 21, 1979–1980. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Ling, Q.; Xu, L.; Wang, T.; Zhu, J.; Lin, Y.; Lu, X.; Qu, W.; Zhang, F.; et al. Pretransplant Use of Immune Checkpoint Inhibitors for Hepatocellular Carcinoma: A Multicenter, Retrospective Cohort Study. Am. J. Transplant. 2024. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I Study of Single-Agent Anti-Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Rezaee-Zavareh, M.S.; Yeo, Y.H.; Wang, T.; Guo, Z.; Tabrizian, P.; Ward, S.C.; Barakat, F.; Hassanein, T.I.; Shravan, D.; Veeral, A.; et al. Impact of Pre-Transplant Immune Checkpoint Inhibitor Use on Post-Transplant Outcomes in HCC: A Systematic Review and Individual Patient Data Meta-Analysis. J. Hepatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Salerno, D.M.; Shertel, T.; Brown, R.S. Pre-Transplant Immune Checkpoint Inhibitor Use: The Intersection Between Medicine, Surgery and Pharmacy. J. Hepatol. 2024. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Colmenero, J.; González, A.; Gastaca, M.; Curell, A.; Caballero-Marcos, A.; Sánchez-Martínez, A.; Di Maira, T.; Herrero, J.I.; Almohalla, C.; et al. Cumulative Exposure to Tacrolimus and Incidence of Cancer after Liver Transplantation. Am. J. Transplant. 2022, 22, 1671–1682. [Google Scholar] [CrossRef]
- Lencioni, R.; Kudo, M.; Erinjeri, J.; Qin, S.; Ren, Z.; Chan, S.; Arai, Y.; Heo, J.; Mai, A.; Escobar, J.; et al. EMERALD-1: A Phase 3, Randomized, Placebo-Controlled Study of Transarterial Chemoembolization Combined with Durvalumab with or without Bevacizumab in Participants with Unresectable Hepatocellular Carcinoma Eligible for Embolization. J. Clin. Oncol. 2024, 42, LBA432. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-Year Overall Survival Update from the Phase III HIMALAYA Study of Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kayali, S.; Pasta, A.; Plaz Torres, M.C.; Jaffe, A.; Strazzabosco, M.; Marenco, S.; Giannini, E.G. Immune Checkpoint Inhibitors in Malignancies after Liver Transplantation: A Systematic Review and Pooled Analysis. Liver Int. 2023, 43, 8–17. [Google Scholar] [CrossRef]
- Charlton, M.; Levitsky, J.; Aqel, B.; O’Grady, J.; Hemibach, J.; Rinella, M.; Fung, J.; Ghabril, M.; Thomason, R.; Burra, P.; et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef]
- McAlister, V.C.; Haddad, E.; Renouf, E.; Malthaner, R.A.; Kjaer, M.S.; Gluud, L.L. Cyclosporin versus Tacrolimus as Primary Immunosuppressant after Liver Transplantation: A Meta-Analysis. Am. J. Transplant. 2006, 6, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; Shipkova, M.; Van Gelder, T.; Wieland, E.; Sommerer, C.; Budde, K.; Haufroid, V.; Christians, U.; López-Hoyos, M.; Barten, M.J.; et al. Barcelona Consensus on Biomarker-Based Immunosuppressive Drugs Management in Solid Organ Transplantation. Ther. Drug Monit. 2016, 38 (Suppl. 1), S1–S20. [Google Scholar] [CrossRef]
- Carenco, C.; Assenat, E.; Faure, S.; Duny, Y.; Danan, G.; Bismuth, M.; Herrero, A.; Jung, B.; Ursic-Bedoya, J.; Jaber, S.; et al. Tacrolimus and the Risk of Solid Cancers after Liver Transplant: A Dose Effect Relationship. Am. J. Transplant. 2015, 15, 678–686. [Google Scholar] [CrossRef]
- Abrahamsson, J.; Sternby Eilard, M.; Rizell, M.; Bennett, W.; Åberg, F. Reduced Calcineurin Inhibitor Exposure with Antibody Induction and Recurrent Hepatocellular Carcinoma after Liver Transplantation. Scand. J. Gastroenterol. 2022, 57, 325–332. [Google Scholar] [CrossRef]
- Vivarelli, M.; Cucchetti, A.; Barba, G. La; Ravaioli, M.; Del Gaudio, M.; Lauro, A.; Grazi, G.L.; Pinna, A.D. Liver Transplantation for Hepatocellular Carcinoma under Calcineurin Inhibitors: Reassessment of Risk Factors for Tumor Recurrence. Ann. Surg. 2008, 248, 857–862. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; García-Caparrós, C.; O’Beirne, J.; Poyato-González, A.; Ferrín-Sánchez, G.; Montero-Álvarez, J.L.; Patch, D.; et al. Reduced Exposure to Calcineurin Inhibitors Early after Liver Transplantation Prevents Recurrence of Hepatocellular Carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef]
- Neuberger, J.M.; Mamelok, R.D.; Neuhaus, P.; Pirenne, J.; Samuel, D.; Isoniemi, H.; Rostaing, L.; Rimola, A.; Marshall, S.; Mayer, A.D. Delayed Introduction of Reduced-Dose Tacrolimus, and Renal Function in Liver Transplantation: The “ReSpECT” Study. Am. J. Transplant. 2009, 9, 327–336. [Google Scholar] [CrossRef]
- Saliba, F.; De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Jonas, S.; Sudan, D.; Fischer, L.; Duvoux, C.; et al. Renal Function at Two Years in Liver Transplant Patients Receiving Everolimus: Results of a Randomized, Multicenter Study. Am. J. Transplant. 2013, 13, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Pópulo, H.; Lopes, J.M.; Soares, P. The MTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bravo, M.; Prieto Castillo, M.; Navasa, M.; Sánchez-Antolín, G.; Lladó, L.; Otero, A.; Serrano, T.; Jiménez Romero, C.; García González, M.; Valdivieso, A.; et al. Effects of Everolimus plus Minimized Tacrolimus on Kidney Function in Liver Transplantation: REDUCE, a Prospective, Randomized Controlled Study. Rev. Española de Enfermedades Dig. 2022, 114, 335–342. [Google Scholar] [CrossRef]
- De Simone, P.; Fagiuoli, S.; Cescon, M.; De Carlis, L.; Tisone, G.; Volpes, R.; Cillo, U. Use of Everolimus in Liver Transplantation: Recommendations From a Working Group. Transplantation 2017, 101, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Grigg, S.E.; Sarri, G.L.; Gow, P.J.; Yeomans, N.D. Systematic Review with Meta-Analysis: Sirolimus- or Everolimus-Based Immunosuppression Following Liver Transplantation for Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2019, 49, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.; Guerrero, M.; Barrera, L.; Ferrín, G.; Álamo, J.M.; Ayllón, M.D.; Artacho, G.S.; Montero, J.L.; Briceño, J.; Bernal, C.; et al. Impact of Early Initiated Everolimus on the Recurrence of Hepatocellular Carcinoma After Liver Transplantation. Transplantation 2018, 102, 2056–2064. [Google Scholar] [CrossRef]
- Geissler, E.K.; Schnitzbauer, A.A.; Zölke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; Burra, P.; Jauch, K.W.; Rentsch, M.; Ganten, T.M.; et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Liu, F.C.; Chen, C.Y.; Lin, J.R.; Yu, H.P. Effect of Mycophenolate Mofetil Therapy on Recurrence of Hepatocellular Carcinoma after Liver Transplantation: A Population-Based Cohort Study. J. Clin. Med. 2021, 10, 1558. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Rodríguez-Perálvarez, M.L.; Pageaux, G.P.; Sanchez-Fueyo, A.; Feng, S. Liver Transplantation Immunology: Immunosuppression, Rejection, and Immunomodulation. J. Hepatol. 2023, 78, 1199–1215. [Google Scholar] [CrossRef]
- Angelico, R.; Parente, A.; Manzia, T.M. Using a Weaning Immunosuppression Protocol in Liver Transplantation Recipients with Hepatocellular Carcinoma: A Compromise between the Risk of Recurrence and the Risk of Rejection? Transl. Gastroenterol. Hepatol. 2017, 2, 74. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.; Guillemin, A.; Duvoux, C.; Neuzillet, C.; Tlemsani, C.; Compagnon, P.; Azoulay, D.; Salloum, C.; Laurent, A.; de la Taille, A.; et al. Optimal Oncologic Management and MTOR Inhibitor Introduction Are Safe and Improve Survival in Kidney and Liver Allograft Recipients with de Novo Carcinoma. Int. J. Cancer 2019, 144, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Ivanics, T.; Claasen, M.P.; Muaddi, H.; Sapisochin, G. The Management of Post-Transplantation Recurrence of Hepatocellular Carcinoma. Clin. Mol. Hepatol. 2022, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sanduzzi-Zamparelli, M.; Díaz-Gonzalez, Á.; Reig, M. New Systemic Treatments in Advanced Hepatocellular Carcinoma. Liver Transplant. 2019, 25, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Welch, K.; Hussain, H.; Pelletier, S.J.; Fontana, R.J.; Marrero, J.; Merion, R.M. Incidence and Risk Factors of Hepatocellular Carcinoma Recurrence after Liver Transplantation in the MELD Era. Dig. Dis. Sci. 2012, 57, 806–812. [Google Scholar] [CrossRef]
- Berenguer, M.; Burra, P.; Ghobrial, M.; Hibi, T.; Metselaar, H.; Sapisochin, G.; Bhoori, S.; Kwan Man, N.; Mas, V.; Ohira, M.; et al. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1143–1149. [Google Scholar] [CrossRef]
- Lee, D.D.; Sapisochin, G.; Mehta, N.; Gorgen, A.; Musto, K.R.; Hajda, H.; Yao, F.Y.; Hodge, D.O.; Carter, R.E.; Harnois, D.M. Surveillance for HCC After Liver Transplantation: Increased Monitoring May Yield Aggressive Treatment Options and Improved Postrecurrence Survival. Transplantation 2020, 104, 2105–2112. [Google Scholar] [CrossRef]
- Bodzin, A.S.; Lunsford, K.E.; Markovic, D.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann. Surg. 2017, 266, 118–125. [Google Scholar] [CrossRef]
- Kornberg, A.; Küpper, B.; Tannapfel, A.; Katenkamp, K.; Thrum, K.; Habrecht, O.; Wilberg, J. Long-Term Survival after Recurrent Hepatocellular Carcinoma in Liver Transplant Patients: Clinical Patterns and Outcome Variables. Eur. J. Surg. Oncol. 2010, 36, 275–280. [Google Scholar] [CrossRef]
- Banerjee, S.; Wang, D.S.; Kim, H.J.; Sirlin, C.B.; Chan, M.G.; Korn, R.L.; Rutman, A.M.; Siripongsakun, S.; Lu, D.; Imanbayev, G.; et al. A Computed Tomography Radiogenomic Biomarker Predicts Microvascular Invasion and Clinical Outcomes in Hepatocellular Carcinoma. Hepatology 2015, 62, 792–800. [Google Scholar] [CrossRef]
- Partitt, J.R.; Marotta, P.; AlGhamdi, M.; Wall, W.; Khakhar, A.; Suskin, N.G.; Quan, D.; McAllister, V.; Ghent, C.; Levstik, M.; et al. Recurrent Hepatocellular Carcinoma after Transplantation: Use of a Pathological Score on Explanted Livers to Predict Recurrence. Liver Transpl. 2007, 13, 543–551. [Google Scholar] [CrossRef]
- Mehta, N.; Heimbach, J.; Harnois, D.M.; Sapisochin, G.; Dodge, J.L.; Lee, D.; Burns, J.M.; Sanchez, W.; Greig, P.D.; Grant, D.R.; et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017, 3, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Degroote, H.; Geerts, A.; Verhelst, X.; Vlierberghe, H. Van Different Models to Predict the Risk of Recurrent Hepatocellular Carcinoma in the Setting of Liver Transplantation. Cancers 2022, 14, 2973. [Google Scholar] [CrossRef]

| Combination | Objective Response (RECIST v1.1), %, (95% CI) | Objective Response (mRECIST), %, (95% CI) | Duration of Response Months, Median (IQR) |
|---|---|---|---|
| Atezolizumab–Bevacizumab (IMbrave150) | 30 (25–35) | 33.2 (28.1–38.6) | 18.1 (4.6–NE) |
| Tremelimumab–Durvalumab (HIMALAYA) | 20 (NA) | NA | 22.3 (8.5–NE) |
| Camrelizumab–Rivoceranib (CARES-310) | 26 (20–35) | NA | 14.8 (8.4–NE) |
| Durvalumab (HIMALAYA) | 17 | NA | 16.8 (7.4–NE) |
| Tislelizumab (RATIONALE-301) | 14.3 (10.8–18.5) | NA | 36.1 (16.8–NE) |
| Ipilimumab–Nivolumab (Checkmate 9DW) | 36 (31–42) | NA | 30.4 (21.2–NE) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, E.; Sanduzzi-Zamparelli, M.; Jutras, G.; Garcia, R.; Soler Perromat, A.; Llarch, N.; Holguin Arce, V.; Ruiz, P.; Rimola, J.; Lopez, E.; et al. Challenges in Liver Transplantation for Hepatocellular Carcinoma: A Review of Current Controversies. Cancers 2024, 16, 3059. https://doi.org/10.3390/cancers16173059
Mauro E, Sanduzzi-Zamparelli M, Jutras G, Garcia R, Soler Perromat A, Llarch N, Holguin Arce V, Ruiz P, Rimola J, Lopez E, et al. Challenges in Liver Transplantation for Hepatocellular Carcinoma: A Review of Current Controversies. Cancers. 2024; 16(17):3059. https://doi.org/10.3390/cancers16173059
Chicago/Turabian StyleMauro, Ezequiel, Marco Sanduzzi-Zamparelli, Gabrielle Jutras, Raquel Garcia, Alexandre Soler Perromat, Neus Llarch, Victor Holguin Arce, Pablo Ruiz, Jordi Rimola, Eva Lopez, and et al. 2024. "Challenges in Liver Transplantation for Hepatocellular Carcinoma: A Review of Current Controversies" Cancers 16, no. 17: 3059. https://doi.org/10.3390/cancers16173059
APA StyleMauro, E., Sanduzzi-Zamparelli, M., Jutras, G., Garcia, R., Soler Perromat, A., Llarch, N., Holguin Arce, V., Ruiz, P., Rimola, J., Lopez, E., Ferrer-Fàbrega, J., García-Criado, Á., Colmenero, J., Lai, J. C., & Forner, A. (2024). Challenges in Liver Transplantation for Hepatocellular Carcinoma: A Review of Current Controversies. Cancers, 16(17), 3059. https://doi.org/10.3390/cancers16173059






