Immune-Related Adverse Events of Genitourinary Cancer Patients, a Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Acquisition
2.2. Definition of irAE
2.3. Definition of Adverse Event Grade
2.4. Censoring of Time Data
2.5. Statistics
3. Results
3.1. Patient Characteristics by Tumor Type
3.2. Immune-Related Adverse Events by Tumor Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Harthy, M.; Redman, J.; Madan, R.A. Novel immunotherapy combinations for genitourinary cancers. Expert Opin. Biol. Ther. 2020, 20, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.; Jones, R.J. Immune checkpoint inhibitors in renal cell carcinoma. Clin. Sci. 2017, 131, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Hammers, H.; Lipson, E.J. Nivolumab: Targeting PD-1 to bolster antitumor immunity. Future Oncol. 2015, 11, 1307–1326. [Google Scholar] [CrossRef]
- Ward Grados, D.F.; Ahmadi, H.; Griffith, T.S.; Warlick, C.A. Immunotherapy for Bladder Cancer: Latest Advances and Ongoing Clinical Trials. Immunol. Investig. 2022, 51, 2226–2251. [Google Scholar] [CrossRef]
- Claps, F.; Pavan, N.; Ongaro, L.; Tierno, D.; Grassi, G.; Trombetta, C.; Tulone, G.; Simonato, A.; Bartoletti, R.; Mertens, L.; et al. BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer: Current Treatment Landscape and Novel Emerging Molecular Targets. Int. J. Mol. Sci. 2023, 24, 12596. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.; Climent, M.; Petrylak, D.; Choueiri, T.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Sharma, P.; Callahan, M.K.; Bono, P.; Kim, J.; Spiliopoulou, P.; Calvo, E.; Pillai, R.; Ott, P.; de Braud, F.; Morse, M.; et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016, 17, 1590–1598. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullen, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.I.; Rosenberg, S.A.; Fyfe, G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J. Sci. Am. 2000, 6 (Suppl. S1), S55–S57. [Google Scholar]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.; Venugopal, B.; Kollmannsberg, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthelemy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juarez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguie, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sawar, N.; et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef]
- Necchi, A.; Faltas, B.M.; Slovin, S.F.; Meeks, J.J.; Pal, S.K.; Schwartz, L.H.; Huang, R.S.P.; Li, R.; Manley, B.; Chahoud, J.; et al. Immunotherapy in the Treatment of Localized Genitourinary Cancers. JAMA Oncol. 2023, 9, 1447–1454. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Tarhini, A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: The underlying mechanisms and clinical management. Scientifica 2013, 2013, 857519. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, V.; Gallo, F.; Alberti, A.; Paloschi, D.; Ferrari Bravo, W.; Esposito, A.; Cosentini, D.; Grisanti, S.; Pedersini, R.; Petrelli, F.; et al. Immune-related adverse events as potential surrogates of immune checkpoint inhibitors’ efficacy: A systematic review and meta-analysis of randomized studies. ESMO Open 2023, 8, 100787. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Nishio, M.; Mok, T.S.K.; Reck, M.; Finley, G.G.; Kaul, M.D.; Yu, W.; Paranthaman, N.; et al. Association of Immune-Related Adverse Events with Efficacy of Atezolizumab in Patients With Non-Small Cell Lung Cancer: Pooled Analyses of the Phase 3 IMpower130, IMpower132, and IMpower150 Randomized Clinical Trials. JAMA Oncol. 2023, 9, 527–535. [Google Scholar] [CrossRef]
- Gogas, H.; Ioannovich, J.; Dafni, U.; Stavropoulou-Giokas, C.; Frangia, K.; Tsoutsos, D.; Pangiotou, P.; Polyzos, A.; Papadopoulos, O.; Stratigos, A.; et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N. Engl. J. Med. 2006, 354, 709–718. [Google Scholar] [CrossRef]
- Sun, X.; Roudi, R.; Dai, T.; Chen, S.; Fan, B.; Li, H.; Zhou, Y.; Zhou, M.; Zhu, B.; Yin, C.; et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: A PRISMA systematic review and meta-analysis. BMC Cancer 2019, 19, 558. [Google Scholar] [CrossRef]
- Berner, F.; Bomze, D.; Diem, S.; Ali, O.H.; Fassler, M.; Ring, S.; Niederer, R.; Ackermann, C.J.; Baumgaertner, P.; Pikor, N.; et al. Association of Checkpoint Inhibitor-Induced Toxic Effects with Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 1043–1047. [Google Scholar] [CrossRef]
- Kawai, T.; Sato, Y.; Makino, K.; Yamada, Y.; Nomiya, A.; Nakamura, M.; Yamada, D.; Suzuki, M.; Igawa, Y.; Kume, H. Immune-related adverse events predict the therapeutic efficacy of pembrolizumab in urothelial cancer patients. Eur. J. Cancer 2019, 116, 114–115. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Q.; Qu, L.; Li, M.; Wang, L.; Mir, M.C.; Carbonara, U.; Pandolfo, S.D.; Black, P.C.; Paul, A.K.; et al. Adverse Events of Immune Checkpoint Inhibitors Therapy for Urologic Cancer Patients in Clinical Trials: A Collaborative Systematic Review and Meta-analysis. Eur. Urol. 2022, 81, 414–425. [Google Scholar] [CrossRef]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chretien, B.; Da-Silva, A.; Plane, A.F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; NIH: Bethesda, MD, USA, 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 31 March 2024).
- Shankar, B.; Zhang, J.; Naqash, A.R.; Forde, P.M.; Feliciano, J.L.; Marrone, K.A.; Ettinger, D.S.; Hann, C.L.; Brahmer, J.R.; Ricciuti, B.; et al. Multisystem Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors for Treatment of Non–Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 1952. [Google Scholar] [CrossRef] [PubMed]
- Schafer, E.J.; Jemal, A.; Wiese, D.; Sung, H.; Kratzer, T.B.; Islami, F.; Dahut, W.L.; Knudsen, K.E. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur. Urol. 2023, 84, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Daneshmand, S.; Fisch, M.; Lotan, Y.; Noon, A.P.; Resnick, M.J.; Shariat, S.F.; Zolotta, A.R.; Boorjian, S.A. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur. Urol. 2016, 69, 300–310. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Hunting, J.C.; Faucheux, A.T.; Price, S.N.; Elko, C.A.; Quattlebaum, A.; Bloomer, C.; Olson, E.; Petty, W.J.; Lycan, T.W., Jr. Patterns of neurological adverse events among a retrospective cohort of patients receiving immune checkpoint inhibitors. Immunotherapy 2024, 16, 381–390. [Google Scholar] [CrossRef] [PubMed]
| Renal Cell (n = 196) | Urothelial (n = 170) | Other (n = 2735) | Total (N = 3101) | p Value | |
|---|---|---|---|---|---|
| Age | 62.51 (11.01) | 68.48 (11.99) | 63.16 (11.77) | 63.41 (11.80) | <0.001 1 |
| Female | 47 (23.98%) | 44 (25.88%) | 1145 (41.86%) | 1236 (39.86%) | <0.001 2 |
| Race | 0.036 2 | ||||
| Black/African American | 11 (5.61%) | 16 (9.41%) | 333 (12.18%) | 360 (11.61%) | |
| Other | 9 (4.59%) | 4 (2.35%) | 78 (2.85%) | 91 (2.93%) | |
| White/Caucasian | 176 (89.80%) | 150 (88.24%) | 2324 (84.97%) | 2650 (85.46%) | |
| Smoking History | <0.001 2 | ||||
| Current | 25 (12.76%) | 30 (17.65%) | 621 (22.71%) | 676 (21.80%) | |
| Former | 90 (45.92%) | 109 (64.12%) | 1425 (52.10%) | 1624 (52.37%) | |
| Never | 81 (41.33%) | 31 (18.24%) | 689 (25.19%) | 801 (25.83%) | |
| Tumor Primary | |||||
| Head/neck | na | na | 253 (9.25%) | na | |
| Melanoma | na | na | 444 (16.23%) | na | |
| NSCLC | na | na | 1114 (40.73%) | na | |
| Other | na | na | 924 (33.78%) | na | |
| Disease Stage | <0.001 2 | ||||
| 1 | 20 (12.12%) | 18 (14.40%) | 217 (9.02%) | 255 (9.45%) | |
| 2 | 22 (13.33%) | 38 (30.40%) | 284 (11.80%) | 344 (12.75%) | |
| 3 | 37 (22.42%) | 38 (30.40%) | 678 (28.17%) | 753 (27.92%) | |
| 4 | 86 (52.12%) | 31 (24.80%) | 1228 (51.02%) | 1345 (49.87%) | |
| Missing | 31 (.%) | 45 (.%) | 328 (.%) | 404 | |
| Immunotherapy line of treatment | <0.001 2 | ||||
| 1 | 102 (52.04%) | 49 (28.82%) | 1281 (46.84%) | 1432 (46.18%) | |
| 2 | 61 (31.12%) | 82 (48.24%) | 976 (35.69%) | 1119 (36.09%) | |
| 3 | 17 (8.67%) | 31 (18.24%) | 280 (10.24%) | 328 (10.58%) | |
| 4 | 16 (8.16%) | 8 (4.71%) | 198 (7.24%) | 222 (7.16%) |
| Comorbidity | Renal Cell (n = 196) | Urothelial (n = 170) | Other (n = 2735) | Total (N = 3101) | p Value |
|---|---|---|---|---|---|
| Gastrointestinal | 104 (53.06%) | 94 (55.29%) | 1381 (50.49%) | 1579 (50.92%) | 0.397 1 |
| Psychological | 80 (40.82%) | 63 (37.06%) | 1236 (45.19%) | 1379 (44.47%) | 0.067 1 |
| COPD | 48 (24.49%) | 68 (40.00%) | 1166 (42.63%) | 1282 (41.34%) | <0.001 1 |
| Disk degeneration | 112 (57.14%) | 119 (70.00%) | 1036 (37.88%) | 1267 (40.86%) | <0.001 1 |
| Rheumatological | 74 (37.76%) | 82 (48.24%) | 1043 (38.14%) | 1199 (38.66%) | 0.032 1 |
| Cardiovascular disease | 85 (43.37%) | 70 (41.18%) | 981 (35.87%) | 1136 (36.63%) | 0.049 1 |
| Visual impairment | 59 (30.10%) | 58 (34.12%) | 716 (26.18%) | 833 (26.86%) | 0.045 1 |
| Diabetes mellitus | 66 (33.67%) | 53 (31.18%) | 695 (25.41%) | 814 (26.25%) | 0.013 1 |
| Prior malignancy | 31 (15.82%) | 48 (28.24%) | 673 (24.61%) | 752 (24.25%) | 0.008 1 |
| Thyroid dysfunction | 48 (24.49%) | 25 (14.71%) | 462 (16.89%) | 535 (17.25%) | 0.020 1 |
| Hearing impairment | 24 (12.24%) | 32 (18.82%) | 304 (11.12%) | 360 (11.61%) | 0.013 1 |
| Asthma | 23 (11.73%) | 19 (11.18%) | 308 (11.26%) | 350 (11.29%) | 0.973 1 |
| Neurological | 19 (9.69%) | 14 (8.24%) | 289 (10.57%) | 322 (10.38%) | 0.658 1 |
| Osteoporosis | 8 (4.08%) | 9 (5.29%) | 167 (6.11%) | 184 (5.93%) | 0.540 1 |
| HIV | 0 (0.00%) | 2 (1.18%) | 16 (0.59%) | 18 (0.58%) | 0.267 1 |
| Irritable bowel disease | 2 (1.02%) | 1 (0.59%) | 19 (0.69%) | 22 (0.71%) | 0.770 1 |
| Renal Cell (N = 196) | Urothelial (N = 170) | Other (N = 2735) | p-Values | ||
|---|---|---|---|---|---|
| RCC vs. Other | Urothelial vs. Other | ||||
| Dermatitis | 32 (16.33%) | 17 (10.00%) | 218 (7.97%) | <0.001 1 | 0.346 1 |
| Thyroiditis | 22 (11.22%) | 16 (9.41%) | 169 (6.18%) | 0.006 1 | 0.104 2 |
| Colitis | 18 (9.18%) | 10 (5.88%) | 158 (5.78%) | 0.052 1 | 0.954 1 |
| Pneumonitis | 11 (5.61%) | 8 (4.71%) | 170 (6.22%) | 0.735 1 | 0.512 2 |
| Hepatitis | 9 (4.59%) | 2 (1.18%) | 53 (1.94%) | 0.013 1 | 0.769 2 |
| Acute kidney injury | 7 (3.57%) | 4 (2.35%) | 34 (1.24%) | 0.007 1 | 0.279 2 |
| Hypophysitis | 6 (3.06%) | 1 (0.59%) | 20 (0.73%) | 0.001 1 | 1.000 2 |
| Pancreatitis/DM | 5 (2.55%) | 0 (0.00%) | 7 (0.26%) | <0.001 1 | 0.999 2 |
| Arthritis | 4 (2.04%) | 4 (2.35%) | 61 (2.23%) | 0.999 2 | 0.790 2 |
| Myositis | 4 (2.04%) | 1 (0.59%) | 16 (0.59%) | 0.040 2 | 0.999 2 |
| Central nervous system | 3 (1.53%) | 0 (0.00%) | 4 (0.15%) | 0.008 2 | 0.999 2 |
| Peripheral nervous system | 3 (1.53%) | 0 (0.00%) | 13 (0.48%) | 0.087 2 | 0.999 2 |
| Cardiovascular | 3 (1.53%) | 2 (1.18%) | 8 (0.29%) | 0.500 2 | 0.112 2 |
| Hematological | 1 (0.51%) | 0 (0.00%) | 9 (0.33%) | 0.033 2 | 0.999 2 |
| Infusion reaction | 4 (2.04%) | 3 (1.76%) | 20 (0.73%) | 0.072 2 | 0.148 2 |
| Renal Cell (n = 196) | Urothelial (n = 170) | Other (n = 2735) | |
|---|---|---|---|
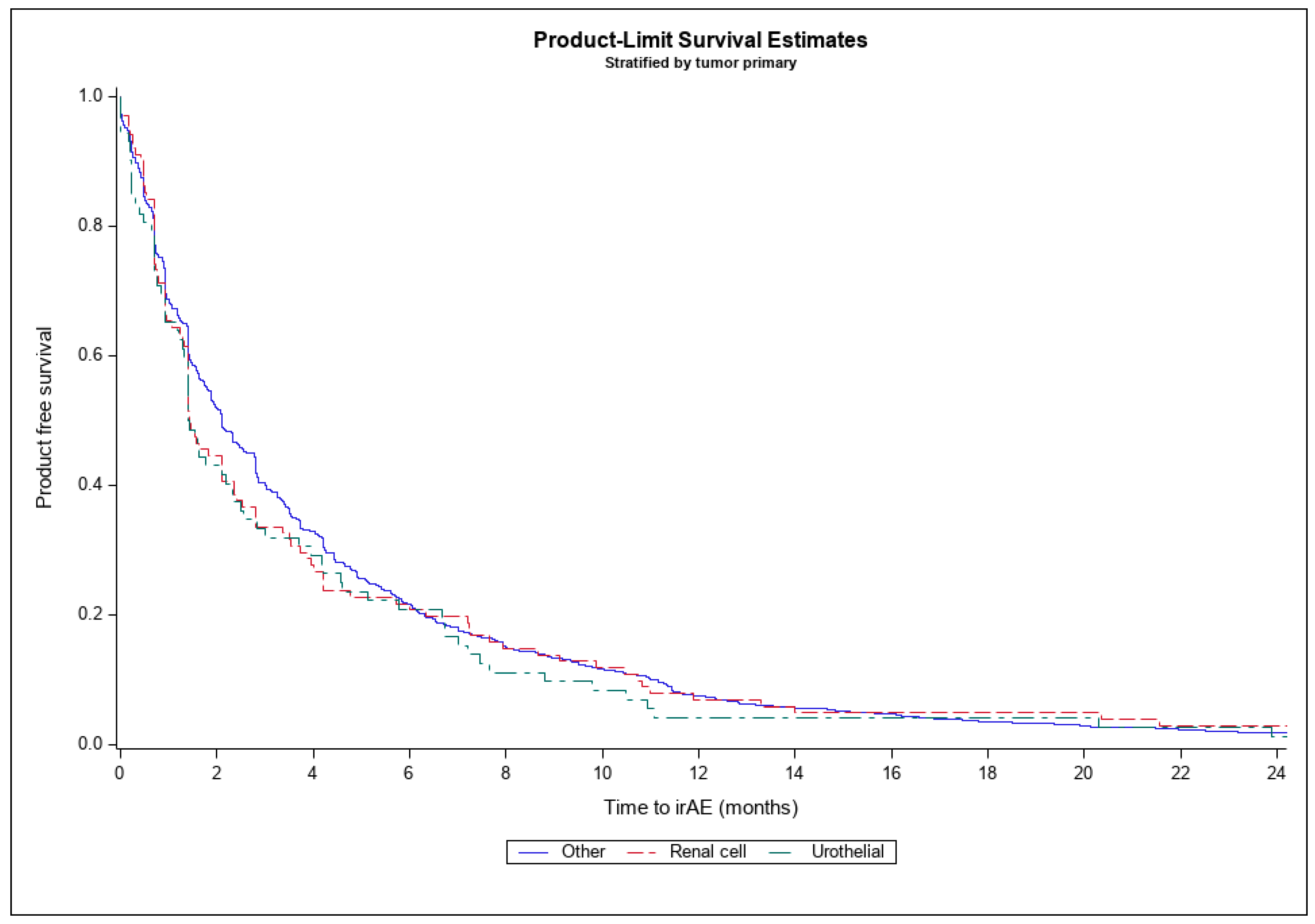
| Time to irAE | 4.5 (8.0) | 3.6 (5.1) | 4.5 (7.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunting, J.C.; Deyo, L.; Olson, E.; Faucheux, A.T.; Price, S.N.; Lycan, T.W., Jr. Immune-Related Adverse Events of Genitourinary Cancer Patients, a Retrospective Cohort Study. Cancers 2024, 16, 3045. https://doi.org/10.3390/cancers16173045
Hunting JC, Deyo L, Olson E, Faucheux AT, Price SN, Lycan TW Jr. Immune-Related Adverse Events of Genitourinary Cancer Patients, a Retrospective Cohort Study. Cancers. 2024; 16(17):3045. https://doi.org/10.3390/cancers16173045
Chicago/Turabian StyleHunting, John C., Logan Deyo, Eric Olson, Andrew T. Faucheux, Sarah N. Price, and Thomas W. Lycan, Jr. 2024. "Immune-Related Adverse Events of Genitourinary Cancer Patients, a Retrospective Cohort Study" Cancers 16, no. 17: 3045. https://doi.org/10.3390/cancers16173045
APA StyleHunting, J. C., Deyo, L., Olson, E., Faucheux, A. T., Price, S. N., & Lycan, T. W., Jr. (2024). Immune-Related Adverse Events of Genitourinary Cancer Patients, a Retrospective Cohort Study. Cancers, 16(17), 3045. https://doi.org/10.3390/cancers16173045





