Albumin Leakage Level during Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Is Associated with Major Complications
Abstract
Simple Summary
Abstract
1. Introduction
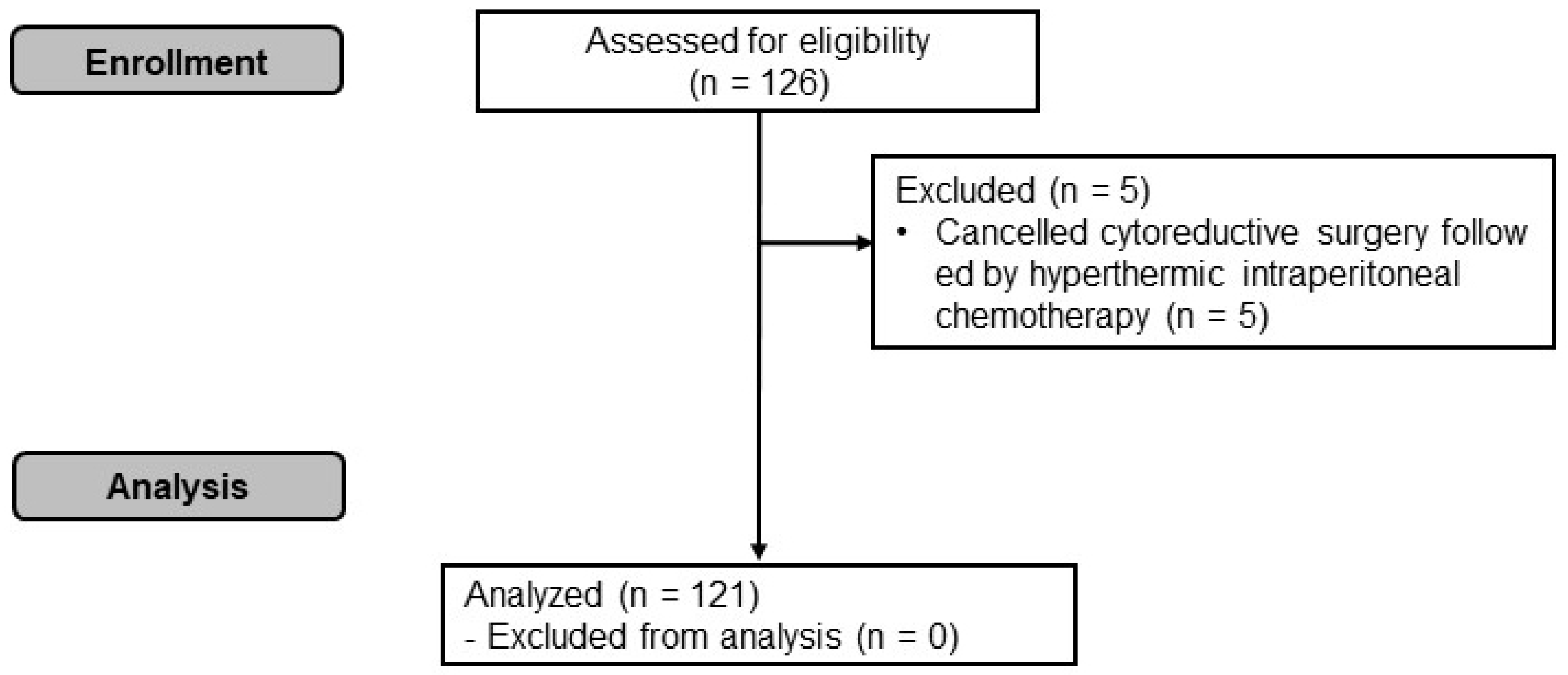
2. Materials and Methods
2.1. Albumin-Related Variables
2.2. Data Collection
2.3. Statistical Analyses
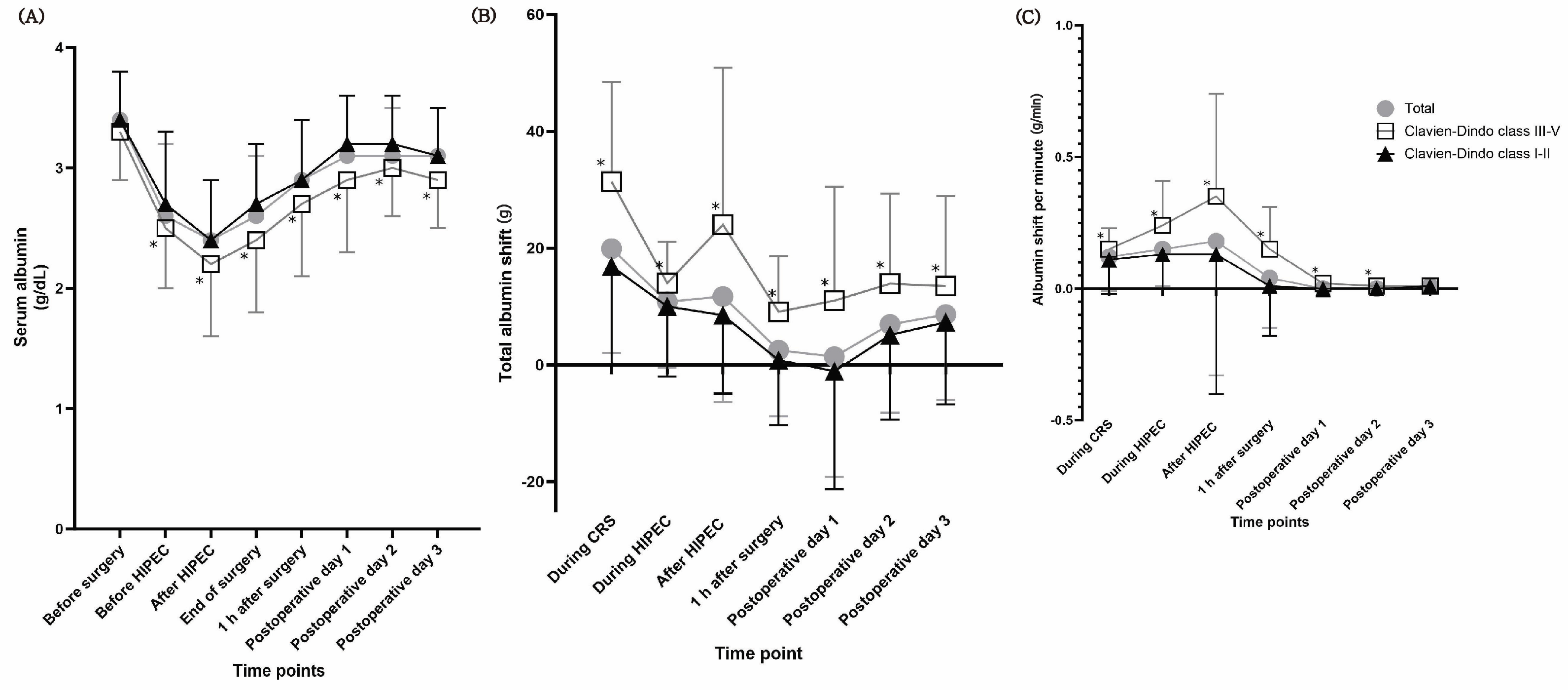
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Jahanban-Esfahlan, R.; Roufegarinejad, L.; Tabibiazar, M.; Amarowicz, R. Recent developments in the detection of bovine serum albumin. Int. J. Biol. Macromol. 2019, 138, 602–617. [Google Scholar] [CrossRef]
- Amouzandeh, M.; Nowak, G.; Januszkiewicz, A.; Wernerman, J.; Rooyackers, O.; Norberg, A. Albumin mass balance and kinetics in liver transplantation. Crit. Care 2018, 22, 152. [Google Scholar] [CrossRef]
- Norberg, A.; Rooyackers, O.; Segersvard, R.; Wernerman, J. Leakage of albumin in major abdominal surgery. Crit. Care 2016, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Norberg, A.; Rooyackers, O.; Segersvard, R.; Wernerman, J. Albumin kinetics in patients undergoing major abdominal surgery. PLoS ONE 2015, 10, e0136371. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, J.; Lai, Y.; Wang, Y.; Wang, X.; Su, J.; Che, G. Perioperative changes of serum albumin are a predictor of postoperative pulmonary complications in lung cancer patients: A retrospective cohort study. J. Thorac. Dis. 2018, 10, 5755–5763. [Google Scholar] [CrossRef] [PubMed]
- Joliat, G.R.; Schoor, A.; Schafer, M.; Demartines, N.; Hubner, M.; Labgaa, I. Postoperative decrease of albumin (deltaalb) as early predictor of complications after gastrointestinal surgery: A systematic review. Perioper. Med. 2022, 11, 7. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lunati, M.P.; Grabel, Z.J.; Staley, C.A.; Schwartz, A.M.; Schenker, M.L. Hypoalbuminemia is an independent risk factor for 30-day mortality, postoperative complications, readmission, and reoperation in the operative lower extremity orthopaedic trauma patient. J. Orthop. Trauma 2019, 33, 284–291. [Google Scholar] [CrossRef]
- Park, D.G. Is cytoreductive surgery and hyperthermic intraperitoneal chemotherapy a safe and effective procedure for treating patients with a peritoneal surface malignancy? Ann. Coloproctol. 2017, 33, 3–4. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, C.H. Treatment for peritoneal metastasis of patients with colorectal cancer. Ann. Coloproctol. 2021, 37, 425–433. [Google Scholar] [CrossRef]
- Roh, S.J.; Park, S.C.; Choi, J.; Lee, J.S.; Lee, D.W.; Hong, C.W.; Han, K.S.; Park, H.C.; Sohn, D.K.; Oh, J.H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy with mitomycin c used for colorectal peritoneal carcinomatosis. Ann. Coloproctol. 2020, 36, 22–29. [Google Scholar] [CrossRef]
- Dai, D.; Balega, J.; Sundar, S.; Kehoe, S.; Elattar, A.; Phillips, A.; Singh, K. Serum albumin as a predictor of survival after interval debulking surgery for advanced ovarian cancer (aoc): A retrospective study. J. Investig. Surg. 2022, 35, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Seretis, C.; Gill, J.; Malik, A.; Elhassan, A.M.; Shariff, U.; Youssef, H. Low preoperative serum albumin levels are associated with impaired outcome after cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal surface malignancies. J. Clin. Med. Res. 2020, 12, 773–779. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, D.H.; Kwon, T.D.; Han, D.W.; Baik, S.H.; Jung, H.H.; Kim, J.Y. Effect of intraoperative dexmedetomidine on renal function after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A randomized, placebo-controlled trial. Int. J. Hyperth. 2019, 36, 1–8. [Google Scholar] [CrossRef]
- Kim, H.C.; Park, J.; Oh, J.; Kim, M.; Park, E.J.; Baik, S.H.; Song, Y. Analgesic effects of combined transversus abdominis plane block and intramuscular electrical stimulation in patients undergoing cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy: A randomized controlled trial. Int. J. Surg. 2023, 109, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Nadler, S.B.; Hidalgo, J.H.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar] [PubMed]
- Svensen, C.; Hahn, R.G. Volume kinetics of ringer solution, dextran 70, and hypertonic saline in male volunteers. Anesthesiology 1997, 87, 204–212. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dumitra, S.; O’Leary, M.; Raoof, M.; Wakabayashi, M.; Dellinger, T.H.; Han, E.S.; Lee, S.J.; Lee, B. The comprehensive complication index: A new measure of the burden of complications after hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2018, 25, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Grange, R.; Rousset, P.; Williet, N.; Guesnon, M.; Milot, L.; Passot, G.; Phelip, J.M.; Le Roy, B.; Glehen, O.; Kepenekian, V. Metastatic colorectal cancer treated with combined liver resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy (hipec): Predictive factors for early recurrence. Ann. Surg. Oncol. 2024, 31, 2378–2390. [Google Scholar] [CrossRef]
- Mertens, L.S.; Behrendt, M.A.; Mehta, A.M.; Stokkel, L.; de Jong, J.; Boot, H.; Horenblas, S.; van der Heijden, M.S.; Moonen, L.M.; Aalbers, A.G.J.; et al. Long-term survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (hipec) for patients with peritoneal metastases of urachal cancer. Eur. J. Surg. Oncol. 2019, 45, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the eastern cooperative oncology group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Choudry, M.H.A.; Shuai, Y.; Jones, H.L.; Pai, R.K.; Pingpank, J.F.; Ahrendt, S.S.; Holtzman, M.P.; Zeh, H.J.; Bartlett, D.L. Postoperative complications independently predict cancer-related survival in peritoneal malignancies. Ann. Surg. Oncol. 2018, 25, 3950–3959. [Google Scholar] [CrossRef]
- Schneider, M.A.; Eshmuminov, D.; Lehmann, K. Major postoperative complications are a risk factor for impaired survival after crs/hipec. Ann. Surg. Oncol. 2017, 24, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Deballon, P.; Radais, F.; Facy, O.; d’Athis, P.; Masson, D.; Charles, P.E.; Cheynel, N.; Favre, J.P.; Rat, P. C-reactive protein is an early predictor of septic complications after elective colorectal surgery. World J. Surg. 2010, 34, 808–814. [Google Scholar] [CrossRef]
- Raspe, C.; Flother, L.; Schneider, R.; Bucher, M.; Piso, P. Best practice for perioperative management of patients with cytoreductive surgery and hipec. Eur. J. Surg. Oncol. 2017, 43, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Eshmuminov, D.; Laminger, F.; Koppitsch, C.; Schneider, M.; Graf, T.R.; Gupta, A.; Kober, F.; Roka, S.; Gertsch, P.; et al. Systemic inflammatory response after hyperthermic intraperitoneal chemotherapy (hipec): The perfusion protocol matters! Eur. J. Surg. Oncol. 2019, 45, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Guo, F.; Sun, Y.; Ren, D.; Zhao, J.; Hu, J.; Zhang, Y.; Jin, X.; Wu, H. Albumin difference as a new predictor of postoperative complications following pancreatectomy. Dig. Surg. 2021, 38, 166–174. [Google Scholar] [CrossRef]
- Liu, Z.J.; Ge, X.L.; Ai, S.C.; Wang, H.K.; Sun, F.; Chen, L.; Guan, W.X. Postoperative decrease of serum albumin predicts short-term complications in patients undergoing gastric cancer resection. World J. Gastroenterol. 2017, 23, 4978–4985. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Jiang, J.; Cao, X.; Liu, Q. Early decrease in postoperative serum albumin predicts severe complications in patients with colorectal cancer after curative laparoscopic surgery. World J. Surg. Oncol. 2018, 16, 192. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402. [Google Scholar] [CrossRef]
- Bentzer, P.; Fisher, J.; Kong, H.J.; Morgelin, M.; Boyd, J.H.; Walley, K.R.; Russell, J.A.; Linder, A. Heparin-binding protein is important for vascular leak in sepsis. Intensive Care Med. Exp. 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Schaller, S.J.; Fuest, K.; Ulm, B.; Schmid, S.; Bubb, C.; von Eisenhart-Rothe, R.; Friess, H.; Kirchhoff, C.; Stadlbauer, T.; Luppa, P.; et al. Substitution of perioperative albumin deficiency disorders (superadd) in adults undergoing vascular, abdominal, trauma, or orthopedic surgery: Protocol for a randomized controlled trial. Trials 2020, 21, 726. [Google Scholar] [CrossRef]
- Shimizu, A.; Kawai, M.; Hirono, S.; Okada, K.I.; Miyazawa, M.; Kitahata, Y.; Ueno, M.; Hayami, S.; Miyamoto, A.; Kimoto, Y.; et al. Postoperative visceral tissue edema assessed by computed tomography is a predictor for severe complications after pancreaticoduodenectomy. J. Gastrointest. Surg. 2018, 22, 77–87. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Phases of fluid management and the roles of human albumin solution in perioperative and critically ill patients. Curr. Med. Res. Opin. 2020, 36, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ortega, A.J.; Pinar-Gutierrez, A.; Serrano-Aguayo, P.; Gonzalez-Navarro, I.; Remon-Ruiz, P.J.; Pereira-Cunill, J.L.; Garcia-Luna, P.P. Perioperative nutritional support: A review of current literature. Nutrients 2022, 14, 1601. [Google Scholar] [CrossRef]
- Loftus, T.J.; Brown, M.P.; Slish, J.H.; Rosenthal, M.D. Serum levels of prealbumin and albumin for preoperative risk stratification. Nutr. Clin. Pract. 2019, 34, 340–348. [Google Scholar] [CrossRef]
- Franssen, B.; Tabrizian, P.; Weinberg, A.; Romanoff, A.; Tuvin, D.; Labow, D.; Sarpel, U. Outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on patients with diaphragmatic involvement. Ann. Surg. Oncol. 2015, 22, 1639–1644. [Google Scholar] [CrossRef]
- Chouliaras, K.; Levine, E.A.; Fino, N.; Shen, P.; Votanopoulos, K.I. Prognostic factors and significance of gastrointestinal leak after cytoreductive surgery (crs) with heated intraperitoneal chemotherapy (hipec). Ann. Surg. Oncol. 2017, 24, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Prien, T.; Backhaus, N.; Pelster, F.; Pircher, W.; Bunte, H.; Lawin, P. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. J. Clin. Anesth. 1990, 2, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhou, C.; Hua, Q.; Yang, L.; Zhao, W.; Xu, P. Impact of operation duration on short-term and long-term prognosis in patients undergoing radical colorectal surgery. J. Cancer 2022, 13, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Valles, J.; Castillo, J.; Sabate, S.; Mazo, V.; Briones, Z.; Sanchis, J.; et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef]

| Clavien–Dindo Class III–V | p-Value | ||
|---|---|---|---|
| Yes (n = 25) | No (n = 96) | ||
| Preoperative data | |||
| Age, years | 55 (14) | 56 (18) | 0.758 |
| Female (%) | 13 (52%) | 49 (51%) | 0.932 |
| Male (%) | 12 (48%) | 47 (49%) | |
| Height, cm | 164 ± 8 | 163 ± 8 | 0.308 |
| Weight, kg | 59.4 (13.0) | 59.9 (12.6) | 0.347 |
| Albumin, g/dL | 3.5 (0.6) | 3.4 (0.3) | 0.757 |
| Comorbidity (%) | |||
| Hypertension, yes | 9 (36%) | 19 (20%) | 0.087 |
| Diabetes, yes | 4 (16%) | 13 (14%) | 0.753 |
| Cerebrovascular disease, yes | 1 (4%) | 3 (3%) | 0.827 |
| Kidney disease, yes | 0 (0%) | 1 (1%) | 1.000 |
| ECOG score (0/1/2/3/4/5, %) | 20 (80%)/3 (12%)/2 (8%)/0 (0%)/0 (0%)/0 (0%) | 78 (81%)/10 (10%)/5 (5%)/3 (3%)/0 (0%)/0 (0%) | 0.777 |
| PSS (0/1/2/3, %) | 11 (44%)/10 (40%)/3 (12%)/1 (4%) | 33 (34%)/32 (33%)/18 (19%)/13 (14%) | 0.417 |
| Primary cancer (%) | 0.312 | ||
| Colorectal cancer, yes | 20 (80%) | 89 (93%) | |
| Gastric cancer, yes | 2 (8%) | 9 (9%) | |
| Pseudomyxoma peritonei, yes | 1 (4%) | 5 (5%) | |
| Mesothelioma, yes | 1 (4%) | 3(3%) | |
| Intraoperative data | |||
| PCI score | 21 (23) | 13 (21) | 0.051 |
| PCI score > 19, yes | 15 (60%) | 28 (29%) | 0.009 |
| Mesothelioma or pseudomyxoma peritonei with a PCI score greater than 19, yes | 2 (8%) | 5 (5%) | 0.633 |
| Lymph node dissection, yes | 8 (32%) | 30 (31%) | 1.000 |
| Vascular resection, yes | 1 (4%) | 4 (4%) | 1.000 |
| Number of anastomosis | 1.9 ± 1.0 | 1.3 ± 1.0 | 0.009 |
| Number of resected organs | 5.6 ± 2.6 | 4.7 ± 2.6 | 0.102 |
| Resected organs | |||
| Colon, yes | 17 (68%) | 67 (70%) | 0.863 |
| Intestine, yes | 16 (64%) | 41 (43%) | 0.057 |
| Stomach, yes | 3 (12%) | 11 (12%) | 1.000 |
| Pancreas, yes | 0 (0%) | 2 (2%) | 1.000 |
| Gallbladder, yes | 4 (16%) | 30 (31%) | 0.210 |
| Liver, yes | 7 (28%) | 18 (19%) | 0.309 |
| Diaphragm, yes | 15 (60%) | 28 (29%) | 0.004 |
| Uterus, yes | 6 (24%) | 17 (18%) | 0.475 |
| Ovary, yes | 6 (24%) | 25 (26%) | 0.835 |
| Omentum, yes | 12 (48%) | 58 (60%) | 0.263 |
| Peritoneum, yes | 20 (80%) | 69 (72%) | 0.412 |
| Spleen, yes | 7 (28%) | 11 (12%) | 0.056 |
| Bladder, yes | 8 (32%) | 18 (19%) | 0.175 |
| Diaphragmatic injury, yes | 15 (60%) | 28 (29%) | 0.004 |
| Completeness of cytoreduction (0/1/2/3, %) | 12 (48%)/6 (24%)/3 (12%)/4 (16%) | 68 (71%)/5 (5%)/10 (10%)/13 (14%) | 0.024 |
| Crystalloid input, mL | 7050 (3675) | 5400 (2538) | 0.022 |
| Colloid input, mL | 760 ± 542 | 467 ± 462 | 0.018 |
| pRBC input, unit | 2.0 ± 4.6 | 1.2 ± 2.2 | 0.395 |
| FFP input, unit | 1.0 ± 3.1 | 0.3 ± 1.2 | 0.305 |
| Albumin input, mL | 60 (60) | 23 (50) | <0.001 |
| Urine output, mL | 1650 (1008) | 1000 (889) | 0.007 |
| Blood loss, mL | 1891 ± 2187 | 1047 ± 951 | 0.070 |
| Norepinephrine, mcg | 1558 ± 3202 | 1017 ± 1753 | 0.466 |
| Need of vasopressin, yes | 2 (8%) | 15 (16%) | 0.520 |
| Operation time, min | 710 (410) | 443 (304) | 0.011 |
| Anesthesia time, min | 795 (403) | 548 (308) | 0.013 |
| Postoperative data | |||
| ICU duration, day | 1.8 ± 2.1 | 1.1 ± 1.1 | 0.146 |
| Complications | |||
| Pulmonary disease, yes | 4 (16%) | 2 (2%) | 0.004 |
| Acute kidney injury, yes | 0 (0%) | 6 (6%) | 0.343 |
| Gastrointestinal anastomosis leakage, yes | 9 (36%) | 0 (0%) | <0.001 |
| Ileus, yes | 3 (12%) | 6 (6%) | 0.329 |
| Infection, yes | 6 (24%) | 4 (4%) | 0.005 |
| Mortality, yes | 7 (28%) | 15 (16%) | 0.153 |
| Need of vasopressor, yes | 6 (24%) | 18 (19%) | 0.558 |
| Length of hospital stay, day | 18 (23) | 15 (6) | 0.098 |
| Main IV | Covariates | |||||||
|---|---|---|---|---|---|---|---|---|
| Main IV | Operation Time | Crystalloid Input | Colloid Input | Blood Loss | Number of Anastomoses | Diaphragmatic Injury (Yes) | PCI Score > 19 | |
| ∆Alb during surgery | ||||||||
| Odds ratio (95% CI) | 1.023 (0.994–1.053) | 1.002 (0.998–1.006) | 1.000 (1.000–1.000) | 1.000 (0.999–1.001) | 1.000 (1.000–1.001) | 1.456 (0.833–2.542) | 1.498 (0.449–5.004) | 2.511 (0.898–7.023) |
| p-value | 0.128 | 0.346 | 0.239 | 0.890 | 0.279 | 0.187 | 0.511 | 0.079 |
| ∆Alb during surgery and POD 3 | ||||||||
| Odds ratio (95% CI) | 1.052 (0.998–1.108) | 1.001 (0.997–1.005) | 1.000 (0.999–1.000) | 1.000 (0.999–1.001) | 1.000 (1.000–1.001) | 1.622 (0.909–2.896) | 1.546 (0.462–5.169) | 2.955 (1.02–8.532) |
| p-value | 0.060 | 0.681 | 0.327 | 0.854 | 0.197 | 0.102 | 0.480 | 0.045 |
| Albshift during surgery | ||||||||
| Odds ratio (95% CI) | 1.037 (1.016–1.058) | 1.002 (0.998–1.006) | 1.000 (0.999–1.000) | 1.000 (0.999–1.001) | 1.000 (1.000–1.001) | 1.374 (0.748–2.525) | 0.948 (0.244–3.686) | 2.523 (0.803–7.930) |
| p-value | 0.001 | 0.365 | 0.208 | 0.957 | 0.235 | 0.306 | 0.939 | 0.113 |
| Albshift before HIPEC | ||||||||
| Odds ratio (95% CI) | 1.040 (1.006–1.076) | 1.040 (1.006–1.076) | 1.040 (1.006–1.076) | 1.040 (1.006–1.076) | 1.040 (1.006–1.076) | 1.040 (1.006–1.076) | 1.040 (1.006–1.076) | 1.040 (1.006–1.076) |
| p-value | 0.023 | 0.492 | 0.320 | 0.700 | 0.363 | 0.177 | 0.811 | 0.102 |
| Albshift after HIPEC | ||||||||
| Odds ratio (95% CI) | 1.045 (1.013–1.078) | 1.045 (1.013–1.078) | 1.000 (0.999–1.000) | 1.000 (0.999–1.001) | 1.000 (0.999–1.001) | 1.334 (0.750–2.373) | 1.305 (0.357–4.770) | 2.845 (0.948–8.538) |
| p-value | 0.005 | 0.340 | 0.198 | 0.945 | 0.217 | 0.327 | 0.687 | 0.062 |
| Albshift during 3 days after surgery | ||||||||
| Odds ratio (95% CI) | 1.058 (1.029–1.088) | 1.000 (0.996–1.005) | 1.000 (0.999–1.000) | 1.001 (0.999–1.002) | 1.000 (1.000–1.001) | 1.420 (0.726–2.778) | 4.186 (0.977–17.939) | 0.886 (0.232–3.387) |
| p-value | <0.001 | 0.942 | 0.291 | 0.499 | 0.292 | 0.306 | 0.054 | 0.859 |
| Albshift during surgery and POD 3 | ||||||||
| Odds ratio (95% CI) | 1.046 (1.025–1.067) | 1.001 (0.996–1.006) | 1.000 (0.999–1.000) | 1.000 (0.999–1.002) | 1.000 (1.000–1.001) | 1.444 (0.663–3.143) | 1.912 (0.415–8.822) | 0.917 (0.214–3.924) |
| p-value | <0.001 | 0.703 | 0.169 | 0.748 | 0.213 | 0.355 | 0.406 | 0.907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-C.; Han, D.W.; Park, E.J.; Hong, Y.H.; Song, Y. Albumin Leakage Level during Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Is Associated with Major Complications. Cancers 2024, 16, 2874. https://doi.org/10.3390/cancers16162874
Kim H-C, Han DW, Park EJ, Hong YH, Song Y. Albumin Leakage Level during Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Is Associated with Major Complications. Cancers. 2024; 16(16):2874. https://doi.org/10.3390/cancers16162874
Chicago/Turabian StyleKim, Hyun-Chang, Dong Woo Han, Eun Jung Park, Yeon Hwa Hong, and Young Song. 2024. "Albumin Leakage Level during Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Is Associated with Major Complications" Cancers 16, no. 16: 2874. https://doi.org/10.3390/cancers16162874
APA StyleKim, H.-C., Han, D. W., Park, E. J., Hong, Y. H., & Song, Y. (2024). Albumin Leakage Level during Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Is Associated with Major Complications. Cancers, 16(16), 2874. https://doi.org/10.3390/cancers16162874







