Mitochondrial Dynamics in Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
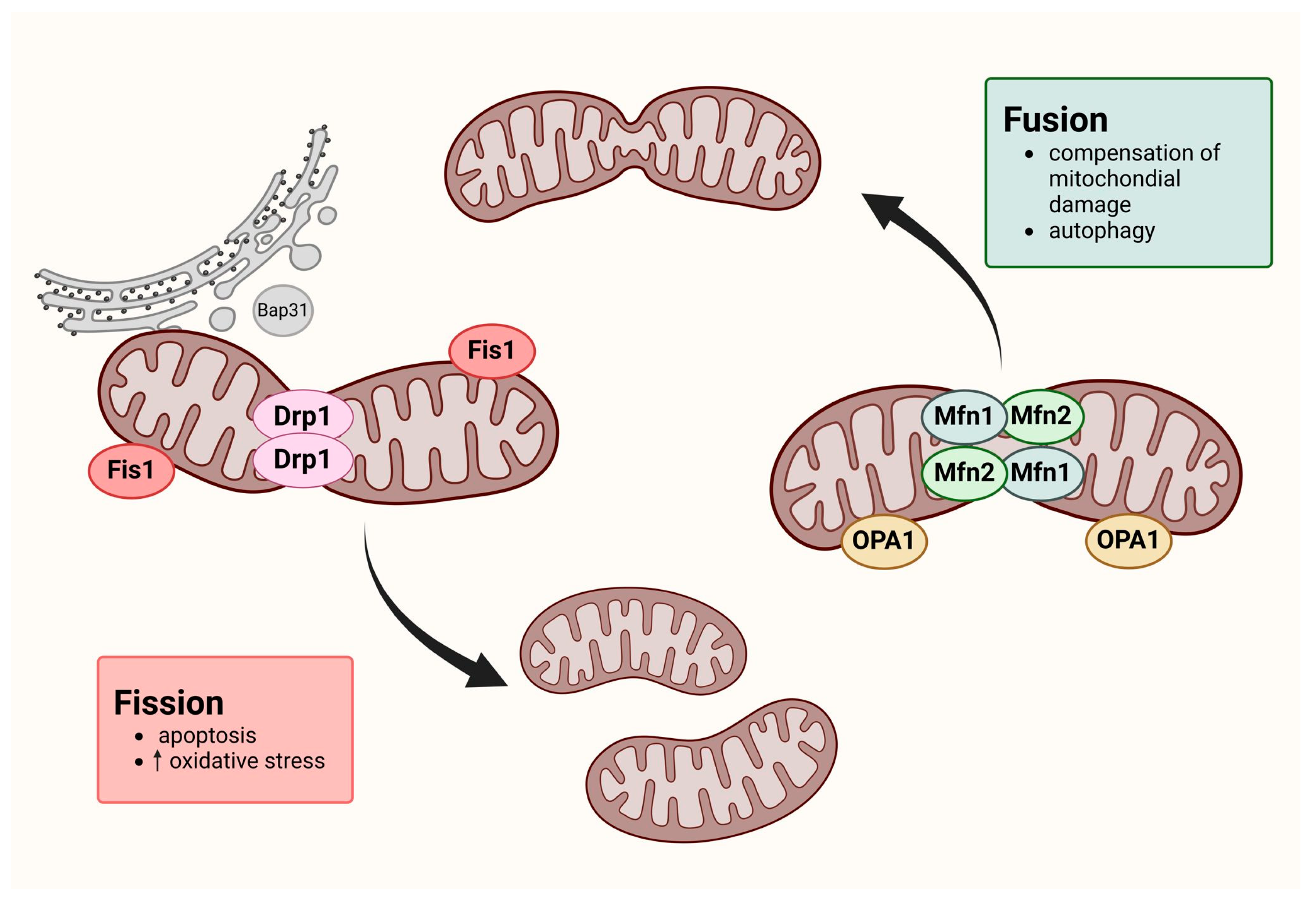
1. Introduction
2. Materials and Methods
2.1. Laboratory Procedures
- The ELx800—single-channel reader-assay system, designed to automatically perform endpoint analysis for ELISA-based applications.
- Gen5TM Microplate Software for Windows.
2.2. Statistical Analysis
3. Results
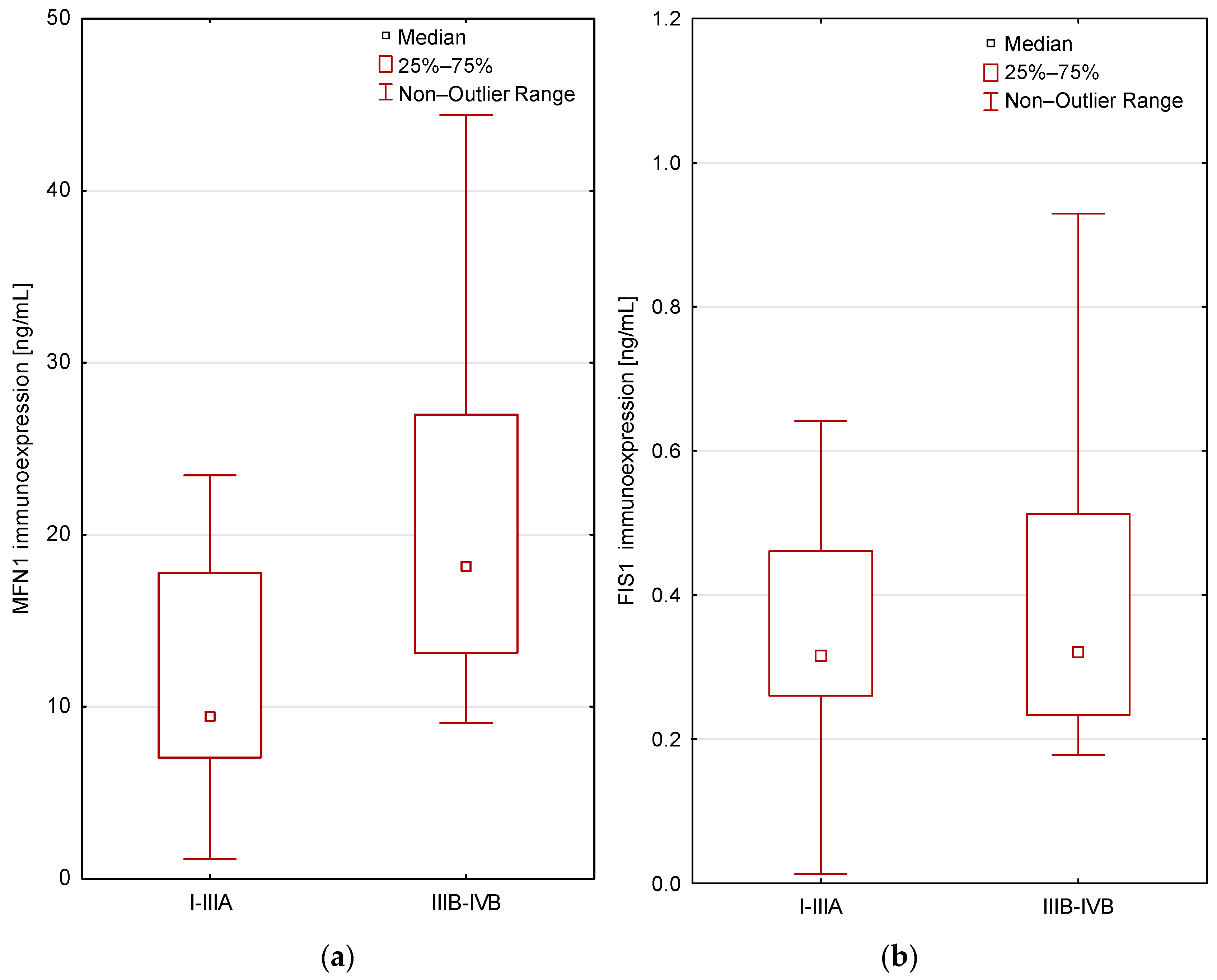
Protein Expression Analysis in Relation to Patient Age, Gender, Smoking History, and Body Mass Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Tuo, J.; Xiao, Y.; Tang, D.; Zhou, X.; Jiang, Y.; Ji, X.; Tan, Y.; Yuan, H.; Xiang, Y. Observed and Relative Survival Trends of Lung Cancer: A Systematic Review of Population-based Cancer Registration Data. Thorac. Cancer 2024, 15, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-M.; Kim, H.C.; Jung, C.Y.; Cho, D.G.; Jeon, J.H.; Lee, J.E.; Ahn, J.S.; Kim, S.J.; Kim, Y.; Choi, Y.-D.; et al. Report of the Korean Association of Lung Cancer Registry (KALC-R), 2014. Cancer Res. Treat. 2019, 51, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from Non-Small-Cell Lung Cancer to Small-Cell Lung Cancer: Molecular Drivers and Cells of Origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef]
- Hou, L.K.; Zhang, L.P.; Zhang, W.; Huang, Y.; Wu, W.; Dong, Z.W.; Wu, C.Y. Clinicopathologic features and genetic profile of the redefined large cell lung carcinoma. Chin. J. Pathol. 2017, 46, 298–302. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021. Available online: https://www.healthdata.org/research-analysis/library/global-burden-disease-2021-findings-gbd-2021-study (accessed on 18 June 2024).
- Lahoti, S.; Dixit, P. Declining Trend of Smoking and Smokeless Tobacco in India: A Decomposition Analysis. PLoS ONE 2021, 16, e0247226. [Google Scholar] [CrossRef] [PubMed]
- Fosgaard, T.R.; Pizzo, A.; Sadoff, S. Sustained Decline in Tobacco Purchasing in Denmark during the COVID-19 Pandemic. Commun. Med. 2022, 2, 96. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tobacco Use Declines Despite Tobacco Industry Efforts to Jeopardize Progress; World Health Organization: Geneva, Switzerland, 2024.
- Riudavets, M.; Garcia De Herreros, M.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.M. Warburg Effect(s)—A Biographical Sketch of Otto Warburg and His Impacts on Tumor Metabolism. Cancer Metab. 2016, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Cargill, K.R.; Hasken, W.L.; Gay, C.M.; Byers, L.A. Alternative Energy: Breaking Down the Diverse Metabolic Features of Lung Cancers. Front. Oncol. 2021, 11, 757323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Q.; Zhu, Q.; Zhan, Y.; Li, Y.; Huang, X. Role and Therapeutic Targeting of Glutamine Metabolism in Non-small Cell Lung Cancer (Review). Oncol. Lett. 2023, 25, 159. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Dai, D.; Vudem, A.; Varner, J.D.; Stroock, A.D. Multi-Scale Computational Study of the Warburg Effect, Reverse Warburg Effect and Glutamine Addiction in Solid Tumors. PLoS Comput. Biol. 2018, 14, e1006584. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Schafer, X.L.; Ambeskovic, A.; Spencer, C.M.; Land, H.; Munger, J. Addiction to Coupling of the Warburg Effect with Glutamine Catabolism in Cancer Cells. Cell Rep. 2016, 17, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial Fusion and Fission: The Fine-tune Balance for Cellular Homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef]
- Hales, K.G. Mitochondrial Fusion and Division. Nat. Educ. 2010, 3, 12. Available online: https://www.nature.com/scitable/topicpage/mitochondrial-fusion-and-division-14264007/#:~:text=Mitochondrial%20fusion%20is%20the%20physical,give%20rise%20to%20mitochondrial%20networks (accessed on 18 June 2024).
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial Fission and Fusion: A Dynamic Role in Aging and Potential Target for Age-Related Disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Colpman, P.; Dasgupta, A.; Archer, S.L. The Role of Mitochondrial Dynamics and Mitotic Fission in Regulating the Cell Cycle in Cancer and Pulmonary Arterial Hypertension: Implications for Dynamin-Related Protein 1 and Mitofusin2 in Hyperproliferative Diseases. Cells 2023, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Nguyen, M.; Chang, N.C.; Shore, G.C. Fis1, Bap31 and the Kiss of Death between Mitochondria and Endoplasmic Reticulum: Fis1, Bap31 and the Kiss of Death between Mitochondria and ER. EMBO J. 2011, 30, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, D.G.; Stojanovic, M.; Marcellus, R.C.; Shore, G.C. Caspase Cleavage Product of BAP31 Induces Mitochondrial Fission through Endoplasmic Reticulum Calcium Signals, Enhancing Cytochrome c Release to the Cytosol. J. Cell Biol. 2003, 160, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Yoon, Y.; Bonekamp, N.A.; McNiven, M.A.; Schrader, M. A Role for Fis1 in Both Mitochondrial and Peroxisomal Fission in Mammalian Cells. Mol. Biol. Cell 2005, 16, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Ihenacho, U.K.; Meacham, K.A.; Harwig, M.C.; Widlansky, M.E.; Hill, R.B. Mitochondrial Fission Protein 1: Emerging Roles in Organellar Form and Function in Health and Disease. Front. Endocrinol. 2021, 12, 660095. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Dang, S. Mitochondrial Dynamics Related Genes -MFN1, MFN2 and DRP1 Polymorphisms Are Associated with Risk of Lung Cancer. Pharmacogenom. Pers. Med. 2021, 14, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Zorzano, A.; Liesa, M.; Sebastián, D.; Segalés, J.; Palacín, M. Mitochondrial Fusion Proteins: Dual Regulators of Morphology and Metabolism. Semin. Cell Dev. Biol. 2010, 21, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L. Mitochondrial Dynamics. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–139. [Google Scholar] [CrossRef]
- Hoppins, S.; Lackner, L.; Nunnari, J. The Machines That Divide and Fuse Mitochondria. Annu. Rev. Biochem. 2007, 76, 751–780. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, S.-Y.; Karbowski, M.; Smith, C.L.; Youle, R.J. Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis. Mol. Biol. Cell 2004, 15, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Pyakurel, A.; Savoia, C.; Hess, D.; Scorrano, L. Extracellular Regulated Kinase Phosphorylates Mitofusin 1 to Control Mitochondrial Morphology and Apoptosis. Mol. Cell 2015, 58, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Boulton, D.P.; Caino, M.C. Mitochondrial Fission and Fusion in Tumor Progression to Metastasis. Front. Cell Dev. Biol. 2022, 10, 849962. [Google Scholar] [CrossRef]
- Roberts, E.R.; Thomas, K.J. The Role of Mitochondria in the Development and Progression of Lung Cancer. Comput. Struct. Biotechnol. J. 2013, 6, e201303019. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, C.; Wei, G.; Kong, W.; Meng, H.; Du, Y.; Li, J. Mitofusin1 Is a Major Mediator in Glucose-Induced Epithelial-to-Mesenchymal Transition in Lung Adenocarcinoma Cells. OncoTargets Ther. 2020, 13, 3511–3523. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Zhang, H.J.; Toth, P.T.; Zhang, Y.; Marsboom, G.; Hong, Z.; Salgia, R.; Husain, A.N.; Wietholt, C.; Archer, S.L. Inhibition of Mitochondrial Fission Prevents Cell Cycle Progression in Lung Cancer. FASEB J. 2012, 26, 2175–2186. [Google Scholar] [CrossRef]
- Liu, D.; Sun, Z.; Ye, T.; Li, J.; Zeng, B.; Zhao, Q.; Wang, J.; Xing, H.R. The Mitochondrial Fission Factor FIS1 Promotes Stemness of Human Lung Cancer Stem Cells via Mitophagy. FEBS Open Bio 2021, 11, 1997–2007. [Google Scholar] [CrossRef] [PubMed]

| Clinical and Pathological Features | Total |
|---|---|
| Sample number | 68 |
| Control group | 21 |
| Patients with lung cancer | 47 |
| Median age (years) | 72 yrs [IQR: 66–74] |
| Gender | |
| Women | 35 |
| Men | 33 |
| NSCLC cancer subtype | |
| AC | 29 |
| SCC | 15 |
| LCC | 3 |
| NSCLC cancer characteristics (histopathology) | |
| TNM scale | |
| Tumor size | |
| pT1 | 5 |
| pT2 | 14 |
| pT3 | 11 |
| pT4 | 16 |
| Node involvement | |
| N0 | 18 |
| N1 | 7 |
| N2 | 18 |
| N3 | 3 |
| Cancer metastasis | |
| M0 | 33 |
| M1 | 13 |
| AJCC classification | |
| AJCC I | 6 |
| AJCC II | 8 |
| AJCC III | 21 |
| AJCC IV | 12 |
| Groups | MFN1 | FIS1 |
|---|---|---|
| Lung cancer patients/NSCLC patients (n = 47) | 19.8 pg/mL ± 31.5 (IQR: 8.500–21.250) | 0.5 pg/mL ± 0.5 (IQR: 0.253–0.512) |
| Control group (n = 21) | 10.2 pg/mL ± 6.9 (IQR: 6.400–9.925) | 2.2 pg/mL ± 4.4 (IQR: 0.436–1.699) |
| The significance level | p = 0.007 | p = 0.00006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutkowska, A.; Domańska-Senderowska, D.; Czarnecka-Chrebelska, K.H.; Pikus, E.; Zielińska, A.; Biskup, L.; Kołodziejska, A.; Madura, P.; Możdżan, M.; Załuska, U.; et al. Mitochondrial Dynamics in Non-Small Cell Lung Cancer. Cancers 2024, 16, 2823. https://doi.org/10.3390/cancers16162823
Dutkowska A, Domańska-Senderowska D, Czarnecka-Chrebelska KH, Pikus E, Zielińska A, Biskup L, Kołodziejska A, Madura P, Możdżan M, Załuska U, et al. Mitochondrial Dynamics in Non-Small Cell Lung Cancer. Cancers. 2024; 16(16):2823. https://doi.org/10.3390/cancers16162823
Chicago/Turabian StyleDutkowska, Agata, Daria Domańska-Senderowska, Karolina H. Czarnecka-Chrebelska, Ewa Pikus, Aleksandra Zielińska, Laura Biskup, Agata Kołodziejska, Paulina Madura, Maria Możdżan, Urszula Załuska, and et al. 2024. "Mitochondrial Dynamics in Non-Small Cell Lung Cancer" Cancers 16, no. 16: 2823. https://doi.org/10.3390/cancers16162823
APA StyleDutkowska, A., Domańska-Senderowska, D., Czarnecka-Chrebelska, K. H., Pikus, E., Zielińska, A., Biskup, L., Kołodziejska, A., Madura, P., Możdżan, M., Załuska, U., Zheng, E., Adamczyk, E., Kędzia, K., Wcisło, S., Wawrzycki, M., Brzeziańska-Lasota, E., Jabłoński, S., Antczak, A., & Poznański, M. (2024). Mitochondrial Dynamics in Non-Small Cell Lung Cancer. Cancers, 16(16), 2823. https://doi.org/10.3390/cancers16162823






