Efficacy and Safety of Sorafenib or Lenvatinib for Advanced Hepatocellular Carcinoma after Failure of First-Line Atezolizumab Plus Bevacizumab: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment of Included Studies
2.5. Statistical Analyses
3. Results
3.1. Selection of Studies
3.2. Characteristics of Eligible Studies
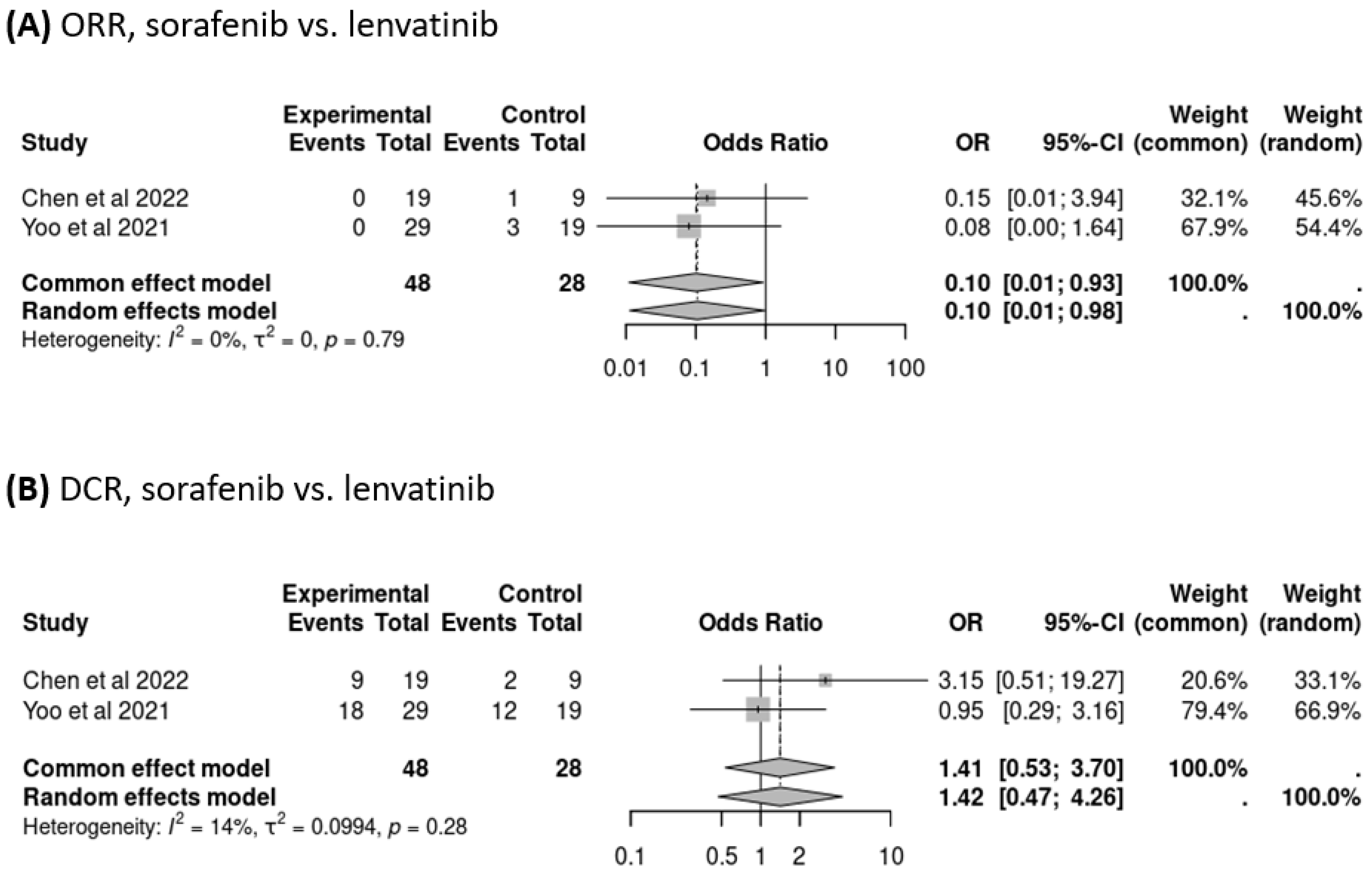
3.3. Response to Treatment Following Failure of Atezolizumab–Bevacizumab Therapy
3.4. Treatment Survival Outcomes Following the Failure of Atezolizumab–Bevacizumab Therapy
3.5. AEs
3.6. Sensitivity Analysis
3.7. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Wspolczesna Onkol. Oncol. 2018, 22, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology 2021, 73 (Suppl. S1), 158–191. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Lee, M.S.; Ryoo, B.Y.; Stein, S.; Lee, K.H.; Verret, W.; Spahn, J.; Shao, H.; Liu, B.; Pishvaian, M.J. Clinical Safety, Tolerability and Adverse Events of Special Interest in a Phase IB Study of Atezolizumab and Bevacizumab; APASL: Manila, Philippines, 2019. [Google Scholar]
- Rimini, M.; Rimassa, L.; Ueshima, K.; Burgio, V.; Shigeo, S.; Tada, T.; Suda, G.; Yoo, C.; Cheon, J.; Pinato, D.J.; et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open 2022, 7, 100591. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L. A new era in systemic therapy for hepatocellular carcinoma: Atezolizumab plus bevacizumab combination therapy. Liver Cancer 2020, 9, 119–137. [Google Scholar]
- Shao, Y.-Y.; Wang, S.-Y.; Lin, S.-M. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2021, 120, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, 238–255. [Google Scholar] [CrossRef]
- Bouattour, M.; Mehta, N.; He, A.R.; Cohen, E.I.; Nault, J.-C. Systemic Treatment for Advanced Hepatocellular Carcinoma. Liver Cancer 2019, 8, 341–358. [Google Scholar] [CrossRef]
- Rimassa, L.; Pressiani, T.; Merle, P. Systemic Treatment Options in Hepatocellular Carcinoma. Liver Cancer 2019, 8, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, J.H.; Ryu, M.H.; Park, S.R.; Lee, D.; Kim, K.M.; Shim, J.H.; Lim, Y.S.; Lee, H.C.; Lee, J.; et al. Clinical Outcomes with Multikinase Inhibitors after Progression on First-Line Atezolizumab plus Bevacizumab in Patients with Advanced Hepatocellular Carcinoma: A Multinational Multicenter Retrospective Study. Liver Cancer 2021, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; E McKenzie, J.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; A Akl, E.; E Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Tufanaru, C.; Qureshi, R.; Mattis, P.; Mu, P. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int. J. Evid. Based Healthc. 2015, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, N. Conducting Meta-analyses of Proportions in R. J. Behav. Data Sci. 2023, 3, 64–126. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Chen, C.-T.; Feng, Y.-H.; Yen, C.-J.; Chen, S.-C.; Lin, Y.-T.; Lu, L.-C.; Hsu, C.-H.; Cheng, A.-L.; Shao, Y.-Y. Prognosis and treatment pattern of advanced hepatocellular carcinoma after failure of first-line atezolizumab and bevacizumab treatment. Hepatol. Int. 2022, 16, 1199–1207. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Wang, J.-H.; Hung, C.-H. Efficacy and Safety of Lenvatinib After Progression on First-line Atezolizumab Plus Bevacizumab Treatment in Advanced Hepatocellular Carcinoma Patients. Anticancer Res. 2023, 43, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Falette-Puisieux, M.; Nault, J.-C.; Bouattour, M.; Lequoy, M.; Amaddeo, G.; Decaens, T.; Di Fiore, F.; Manfredi, S.; Merle, P.; Baron, A.; et al. Beyond atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: Overall efficacy and safety of tyrosine kinase inhibitors in a real-world setting. Ther. Adv. Med. Oncol. 2023, 15, 17588359231189425. [Google Scholar] [CrossRef] [PubMed]
- Muto, H.; Kuzuya, T.; Kawabe, N.; Ohno, E.; Funasaka, K.; Nagasaka, M.; Nakagawa, Y.; Miyahara, R.; Shibata, T.; Hashimoto, S.; et al. Clinical Outcomes With Lenvatinib in Patients Previously Treated With Atezolizumab/Bevacizumab for Advanced Hepatocellular Carcinoma. Anticancer Res. 2023, 43, 4673–4682. [Google Scholar] [CrossRef]
- Persano, M.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Presa, J.; et al. Sequential therapies after atezolizumab plus bevacizumab or lenvatinib first-line treatments in hepatocellular carcinoma patients. Eur. J. Cancer 2023, 189, 112933. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Real- life Practice Experts for HCC (RELPEC) Study Group; and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Lenvatinib as Second-Line Treatment after Atezolizumab plus Bevacizumab for Unresectable Hepatocellular Carcinoma: Clinical Results Show Importance of Hepatic Reserve Function. Oncology 2023, 101, 624–633. [Google Scholar] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Sequential Therapy for Hepatocellular Carcinoma after Failure of Atezolizumab plus Bevacizumab Combination Therapy. Liver Cancer 2021, 10, 85–93. [Google Scholar] [CrossRef]
- Osa, A.; Uenami, T.; Koyama, S.; Fujimoto, K.; Okuzaki, D.; Takimoto, T.; Hirata, H.; Yano, Y.; Yokota, S.; Kinehara, Y.; et al. Clinical implications of monitoring nivolumab immunokinetics in non–small cell lung cancer patients. JCI Insight 2018, 3, e59125. [Google Scholar] [CrossRef]






| Author/Year | Study Design | Period | Country | No. of Centers | No. of Patients | Intervention (Patients) | Comparator (Patients) | ORR/DCR | OS, Months (Median, 95% CI) | PFS, Month (Median, 95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al., 2022 [21] | Retrospective | January 2018–May 2021 | Taiwan | 4 | 41 | Sorafenib (19), Lenvatinib (9), Others (2), Not received second-line therapy (11) | NA | ORR S: 0/19; L: 1/9 DCR S: 9/19; L: 2/9 | S: 8.3 L: 3.8 | S: 2.6 L: 2.0 |
| Chen et al., 2023 [22] | Retrospective | January 2020–September 2022 | Taiwan | 1 | 27 | Lenvatinib (14), Others (13) | NA | ORR L: 3/14 DCR L: 8/14 | L: 3.3 | L: 4.2 |
| Manon et al., 2023 [23] | Retrospective | April 2020–June 2022 | France | 11 | 82 | Regorafenib (29) | Sorafenib (41), Lenvatinib (8), Cabozantinib (4) | NA | S: 7.0 (4.4–11.6) L: 5.7 (4.7–9.1) | S: 2.6 (2.2–3.4) L: 4.4 (1.8–5.7) |
| Muto et al., 2023 [24] | Retrospective | February 2021–May 2023 | Japan | 1 | 20 | Lenvatinib (20) | NA | ORR L: 12/20 DCR L: 19/20 | L: 10.5 (6.9–NR) | L: 6.0 (3.0–7.8) |
| Yoo et al., 2021 [13] | Retrospective | July 2016–April 2019 | Korea, Hong Kong, Singapore | 3 | 49 | Sorafenib (29), Lenvatinib (19), Cabozantinib (1) | NA | ORR S: 0/29; L: 3/19 DCR S: 18/29; L: 12/19 | S: 11.2 (2.7–19.6) L: 16.6 (3.6–29.6) | S: 2.5 (1.3–3.8) L: 6.1 (1.6–10.5) |
| Persano et al., 2023 [25] | Retrospective | July 2010–May 2022 | Multi-Country | Multi-Center | 206 | NA | Sorafenib (43), Lenvatinib (84), Cabozantinib (23), Others (56) | NA | S: 14.2 (8.8–15.7) L: 17.0 (14.8–18.9) | NA |
| Hiraoka et al., 2023 [26] | Retrospective | 2020–2022 | Japan | 1 | 130 | Lenvatinib (101) | Others (29) | ORR L: 17/71 DCR L: 50/71 | L: 13.6 | L: 3.5 |
| The JBI Critical Appraisal Checklist for Case Series’ Included Retrospective Single-Arm Studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall Appraisal |
| Chen et al., 2022 [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Chen et al., 2023 [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Manon et al., 2023 [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Muto et al., 2023 [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Yoo et al., 2021 [13] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Persano et al., 2023 [24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Hiraoka et al., 2023 [25] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Adverse Event | All Grade | ≥Grade 3 | ||
|---|---|---|---|---|
| ES, % (95% CI) | I2, % | ES, % (95% CI) | I2, % | |
| Hand–foot syndrome | 43 (27–59) | 72.7 | 8 (2–15) | 0 |
| Fatigue | 41 (23–59) | 87.7 | 6 (2–9) | 0 |
| Elevated aspartate or alanine aminotransferase | 35 (1–69) | 96.1 | N | N |
| Hypertension | 23 (13–34) | 56.8 | 4 (0–8) | 0 |
| Diarrhea | 22 (15–30) | 95.5 | 5 (−1–1) | 0 |
| Nausea | 12 (2–22) | 19.4 | 2 (−2–7) | 100 |
| Rash | 10 (4–15) | 0 | 5 (−2–11) | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.-R.; Weng, Y.-F.; Wu, T.-W.; Wu, C.-C.; Chou, Y.-C.; Hsu, C.-S. Efficacy and Safety of Sorafenib or Lenvatinib for Advanced Hepatocellular Carcinoma after Failure of First-Line Atezolizumab Plus Bevacizumab: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2813. https://doi.org/10.3390/cancers16162813
Peng T-R, Weng Y-F, Wu T-W, Wu C-C, Chou Y-C, Hsu C-S. Efficacy and Safety of Sorafenib or Lenvatinib for Advanced Hepatocellular Carcinoma after Failure of First-Line Atezolizumab Plus Bevacizumab: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(16):2813. https://doi.org/10.3390/cancers16162813
Chicago/Turabian StylePeng, Tzu-Rong, Yi-Fang Weng, Ta-Wei Wu, Chao-Chuan Wu, Yi-Chun Chou, and Ching-Sheng Hsu. 2024. "Efficacy and Safety of Sorafenib or Lenvatinib for Advanced Hepatocellular Carcinoma after Failure of First-Line Atezolizumab Plus Bevacizumab: A Systematic Review and Meta-Analysis" Cancers 16, no. 16: 2813. https://doi.org/10.3390/cancers16162813
APA StylePeng, T.-R., Weng, Y.-F., Wu, T.-W., Wu, C.-C., Chou, Y.-C., & Hsu, C.-S. (2024). Efficacy and Safety of Sorafenib or Lenvatinib for Advanced Hepatocellular Carcinoma after Failure of First-Line Atezolizumab Plus Bevacizumab: A Systematic Review and Meta-Analysis. Cancers, 16(16), 2813. https://doi.org/10.3390/cancers16162813







