Sorcin in Cancer Development and Chemotherapeutic Drug Resistance
Abstract
Simple Summary
Abstract
1. Introduction
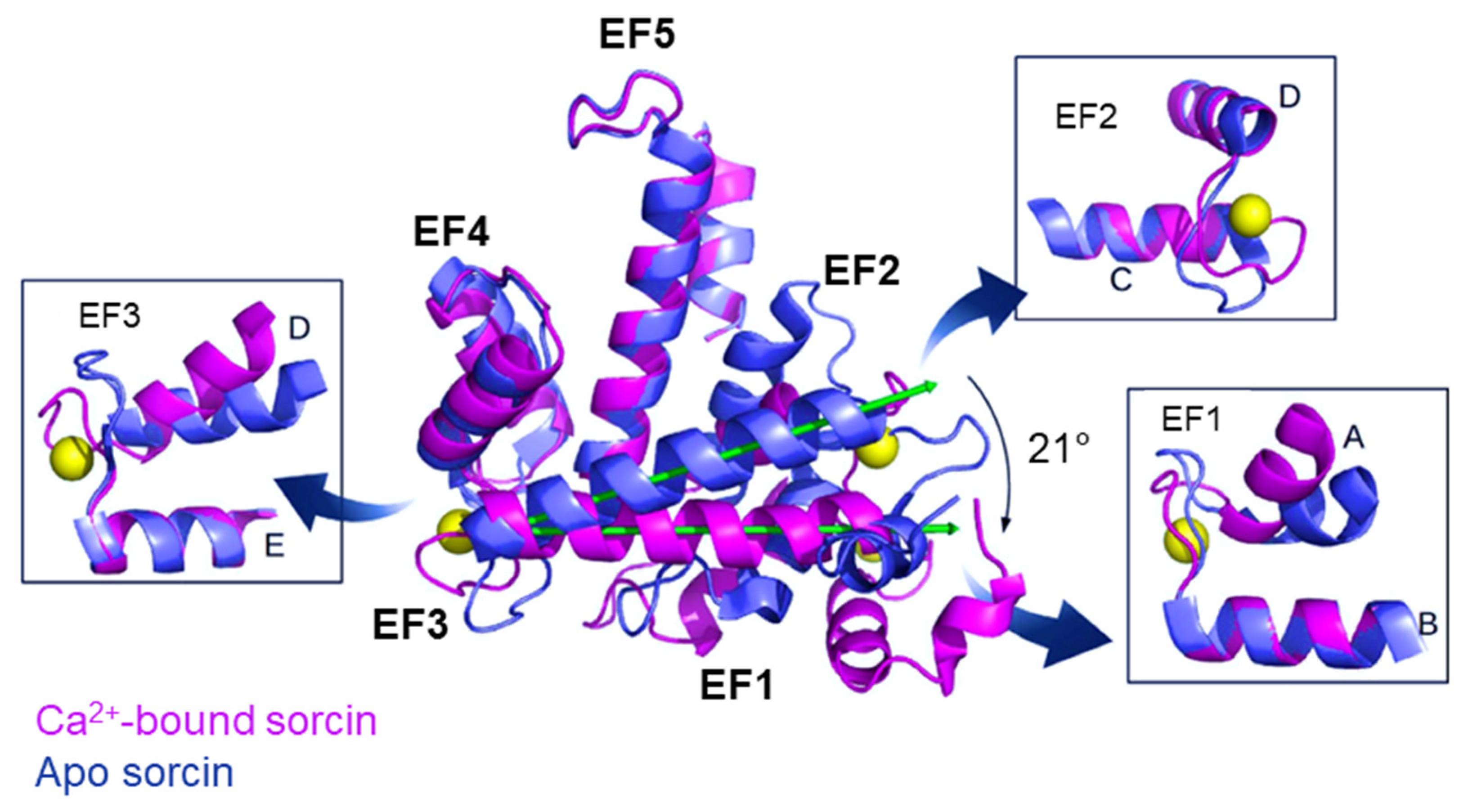
2. Sorcin Structure and Activation
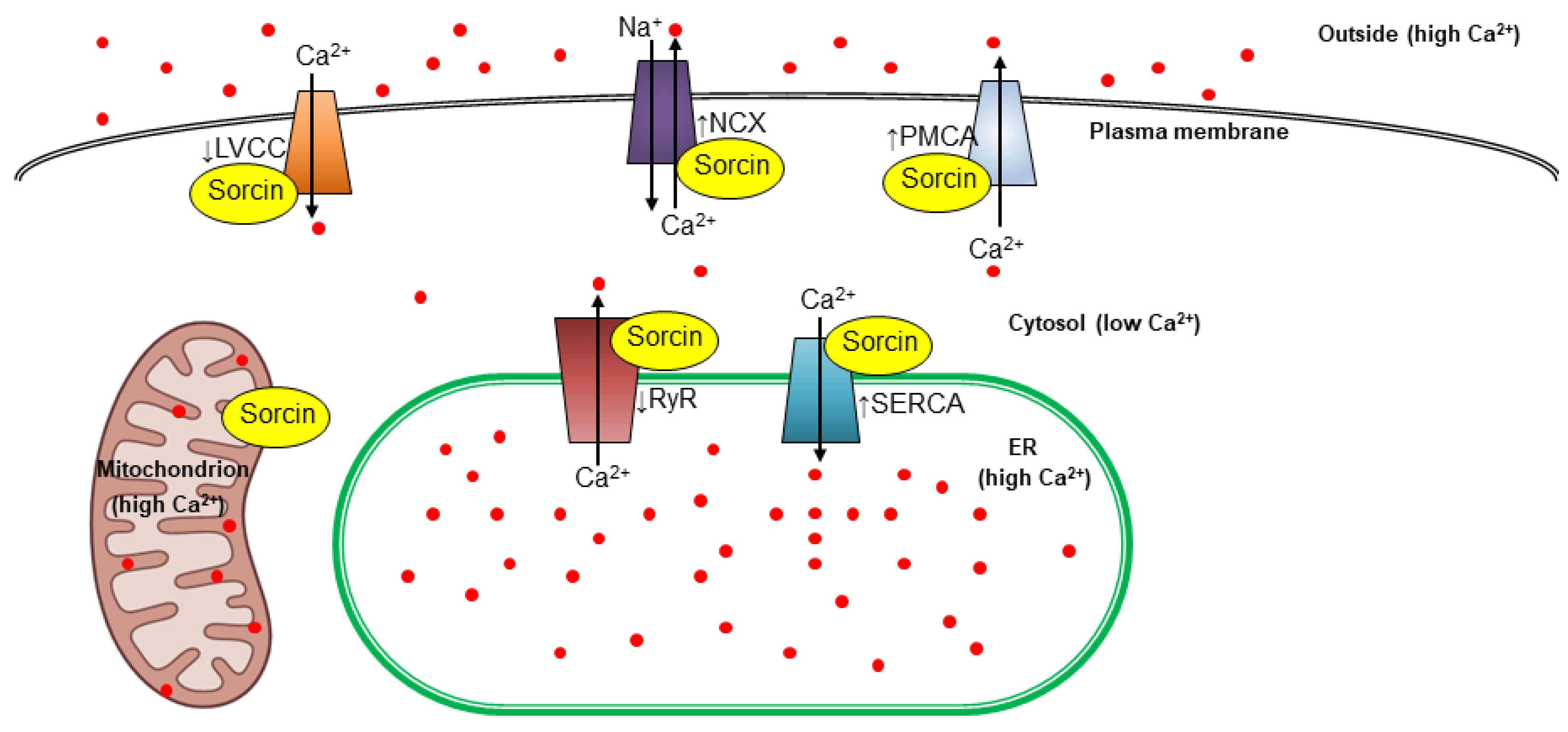
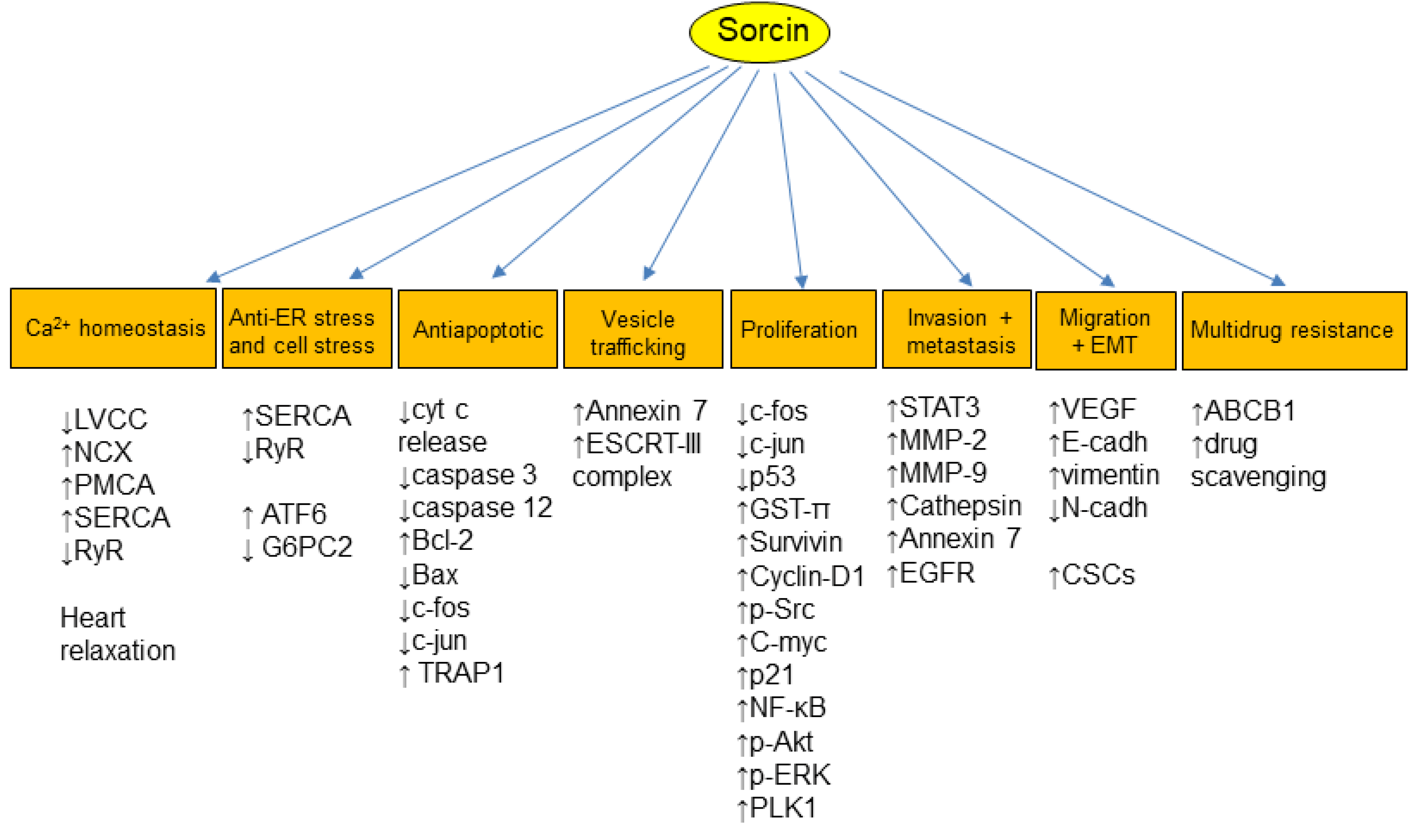
3. Sorcin Regulates Several Physiological Processes
4. Sorcin and Cancer
5. Multidrug Resistance
6. Sorcin in Multidrug Resistance
7. Targeting Sorcin: Promising Strategies for Overcoming Cancer and MDR
8. Conclusions
9. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Van der Bliek, A.M.; Meyers, M.B.; Biedler, J.L.; Hes, E.; Borst, P. A 22-kd protein (sorcin/V19) encoded by an amplified gene in multidrug-resistant cells, is homologous to the calcium-binding light chain of calpain. EMBO J. 1986, 5, 3201. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Ilari, A.; Battista, T.; Chiarini, V.; Fazi, F.; Fiorillo, A.; Colotti, G. Molecular bases of Sorcin-dependent resistance to chemotherapeutic agents. Cancer Drug Resist. 2018, 1, 164–180. [Google Scholar] [CrossRef]
- Maki, M.; Kitaura, Y.; Satoh, H.; Ohkouchi, S.; Shibata, H. Structures, functions and molecular evolution of the penta-EF-hand Ca 2+-binding proteins. Biochim. Biophys. Acta Proteins Proteom. 2002, 1600, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zamparelli, C.; Ilari, A.; Verzili, D.; Giangiacomo, L.; Colotti, G.; Pascarella, S.; Chiancone, E. Structure-function relationships in sorcin, a member of the penta EF-hand family. Interaction of sorcin fragments with the ryanodine receptor and an Escherichia coli model system. Biochemistry 2000, 39, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Mella, M.; Colotti, G.; Zamparelli, C.; Verzili, D.; Ilari, A.; Chiancone, E. Information transfer in the penta-EF-hand protein sorcin does not operate via the canonical structural/functional pairing. A study with site-specific mutants. J. Biol. Chem. 2003, 278, 24921–24928. [Google Scholar] [CrossRef] [PubMed]
- Ilari, A.; Johnson, K.A.; Nastopoulos, V.; Verzili, D.; Zamparelli, C.; Colotti, G.; Tsernoglou, D.; Chiancone, E. The crystal structure of the sorcin calcium binding domain provides a model of Ca2+-dependent processes in the full-length protein. J. Mol. Biol. 2002, 317, 447–458. [Google Scholar] [CrossRef]
- Ilari, A.; Fiorillo, A.; Poser, E.; Lalioti, V.S.; Sundell, G.N.; Ivarsson, Y.; Genovese, I.; Colotti, G. Structural basis of Sorcin-mediated calcium-dependent signal transduction. Sci. Rep. 2015, 5, 16828. [Google Scholar] [CrossRef]
- Meyers, M.B.; Zamparelli, C.; Verzili, D.; Dicker, A.P.; Blanck, T.J.J.; Chiancone, E. Calcium-dependent translocation of sorcin to membranes: Functional relevance in contractile tissue. FEBS Lett. 1995, 357, 230–234. [Google Scholar] [CrossRef]
- Nastopoulos, V.; Ilari, A.; Colotti, G.; Zamparelli, C.; Verzili, D.; Chiancone, E.; Tsernoglou, D. Two different crystal forms of sorcin, a penta-EF-hand Ca2+-binding protein. Acta Crystallogr. D Biol. Crystallogr. 2001, 57 Pt 6, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Colotti, G.; Zamparelli, C.; Verzili, D.; Mella, M.; Loughrey, C.M.; Smith, G.L.; Chiancone, E. The W105G and W99G sorcin mutants demonstrate the role of the D helix in the Ca2+-dependent interaction with annexin VII and the cardiac ryanodine receptor. Biochemistry 2006, 45, 12519–12529. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Ilari, A.; Verzili, D.; Zamparelli, C.; Antaramian, A.; Rueda, A.; Valdivia, H.H.; Chiancone, E.; Colotti, G. Molecular basis for the impaired function of the natural F112L sorcin mutant: X-ray crystal structure, calcium affinity, and interaction with annexin VII and the ryanodine receptor. FASEB J. 2008, 22, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Zamparelli, C.; Macquaide, N.; Colotti, G.; Verzili, D.; Seidler, T.; Smith, G.L.; Chiancone, E. Activation of the cardiac Na+–Ca2+ exchanger by sorcin via the interaction of the respective Ca2+-binding domains. J. Mol. Cell Cardiol. 2010, 49, 132. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Carotti, A.; Ilari, A.; Fiorillo, A.; Battista, T.; Colotti, G.; Ivarsson, Y. Profiling calcium-dependent interactions between Sorcin and intrinsically disordered regions of human proteome. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129618. [Google Scholar] [CrossRef] [PubMed]
- Lalioti, V.S.; Ilari, A.; O’Connell, D.J.; Poser, E.; Sandoval, I.V.; Colotti, G. Sorcin links calcium signaling to vesicle trafficking, regulates Polo-like kinase 1 and is necessary for mitosis. PLoS ONE 2014, 9, e85438. [Google Scholar] [CrossRef] [PubMed]
- Battista, T.; Fiorillo, A.; Chiarini, V.; Genovese, I.; Ilari, A.; Colotti, G. Roles of Sorcin in Drug Resistance in Cancer: One Protein, Many Mechanisms, for a Novel Potential Anticancer Drug Target. Cancers 2020, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.B.; Pickel, V.M.; Sheu, S.S.; Sharma, V.K.; Scotto, K.W.; Fishman, G.I. Association of sorcin with the cardiac ryanodine receptor. J. Biol. Chem. 1995, 270, 26411–26418. [Google Scholar] [CrossRef] [PubMed]
- Lokuta, A.J.; Meyers, M.B.; Sander, P.R.; Fishman, G.I.; Valdivia, H.H. Modulation of cardiac ryanodine receptors by Sorcin. J. Biol. Chem. 1997, 272, 25333–25338. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.F.; Antaramian, A.; Rueda, A.; Gómez, A.M.; Valdivia, H.H. Sorcin Inhibits Calcium Release and Modulates Excitation-Contraction Coupling in the Heart. J. Biol. Chem. 2003, 278, 34660–34666. [Google Scholar] [CrossRef]
- Valdivia, H.H.; Farrell, E.F.; Antaramian, A.; Benkusky, N.; Zhu, X.; Rueda, A.; Gómez, A.M. Sorcin and ryanodine receptors in heart failure. J. Muscle Res. Cell Motil. 2004, 25, 605–607. [Google Scholar] [PubMed]
- Rueda, A.; Song, M.; Toro, L.; Stefani, E.; Valdivia, H.H. Sorcin modulation of Ca2+ sparks in rat vascular smooth muscle cells. J. Physiol. 2006, 576, 887–901. [Google Scholar] [CrossRef]
- Matsumoto, T.; Hisamatsu, Y.; Ohkusa, T.; Inoue, N.; Sato, T.; Suzuki, S.; Ikeda, Y.; Matsuzaki, M. Sorcin interacts with sarcoplasmic reticulum Ca2+-ATPase and modulates excitation-contraction coupling in the heart. Basic. Res. Cardiol. 2005, 100, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.R.; Colotti, G.; Chiancone, E.; Smith, G.L.; Fearon, I.M. Sorcin modulates cardiac L-type Ca2+ current by functional interaction with the α1C subunit in rabbits. Exp. Physiol. 2008, 93, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.R.; Colotti, G.; Chiancone, E.; Higuchi, Y.; Seidler, T.; Smith, G.L. Complex modulation of L-type Ca2+ current inactivation by sorcin in isolated rabbit cardiomyocytes. Pflug. Arch. 2009, 457, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Seidler, T.; Miller, S.L.; Loughrey, C.M.; Kania, A.; Burow, A.; Kettlewell, S.; Teucher, N.; Wagner, S.; Kögler, H.; Meyers, M.B.; et al. Effects of adenovirus-mediated sorcin overexpression on excitation-contraction coupling in isolated rabbit cardiomyocytes. Circ. Res. 2003, 93, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, M.; Saez, L.; Mata, A.M. Sorcin activates the brain PMCA and blocks the inhibitory effects of molecular markers of alzheimer’s disease on the pump activity. Int. J. Mol. Sci. 2021, 22, 6055. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Weber, C.; Farrell, E.T.; Alvarado, F.J.; Zhao, Y.T.; Gómez, A.M.; Valdivia, H.H. Sorcin ablation plus β-adrenergic stimulation generate an arrhythmogenic substrate in mouse ventricular myocytes. J. Mol. Cell Cardiol. 2018, 114, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Belke, D.D.; Gloss, B.; Dieterle, T.; McDonough, P.M.; Kim, Y.K.; Brunton, L.L.; Dillmann, W.H. In vivo adenoviral transfer of sorcin reverses cardiac contractile abnormalities of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H68–H75. [Google Scholar] [CrossRef]
- Suarez, J.; McDonough, P.M.; Scott, B.T.; Suarez-Ramirez, A.; Wang, H.; Fricovsky, E.S.; Dillmann, W.H. Sorcin modulates mitochondrial Ca2+ handling and reduces apoptosis in neonatal rat cardiac myocytes. Am. J. Physiol. Cell Physiol. 2013, 304, C248–C256. [Google Scholar] [CrossRef]
- Kawakami, M.; Nakamura, T.; Okamura, N.; Komoto, C.; Markova, S.; Kobayashi, H.; Hashimoto, N.; Okumura, K.; Sakaeda, T. Knock-down of sorcin induces up-regulation of MDR1 in HeLa cells. Biol. Pharm. Bull. 2007, 30, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, F.; Laudiero, G.; Piscazzi, A.; Secondo, A.; Scorziello, A.; Lombardi, V.; Matassa, D.S.; Fersini, A.; Neri, V.; Esposito, F.; et al. Sorcin induces a drug-resistant phenotype in human colorectal cancer by modulating Ca2+ homeostasis. Cancer Res. 2011, 71, 7659–7669. [Google Scholar] [CrossRef]
- Marmugi, A.; Parnis, J.; Chen, X.; Carmichael, L.; Hardy, J.; Mannan, N.; Marchetti, P.; Piemonti, L.; Bosco, D.; Johnson, P.; et al. Sorcin links pancreatic β-cell lipotoxicity to ER Ca2+ stores. Diabetes 2016, 65, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.Z.; Gao, T.; Jimenez Awuapura, N.; Ayathamattam, J.; Chabosseau, P.L.; Kalvakolanu, D.V.; Valdivia, H.H.; Rutter, G.A.; Leclerc, I. The Ca2+-binding protein sorcin stimulates transcriptional activity of the unfolded protein response mediator ATF6. FEBS Lett. 2021, 595, 1782–1796. [Google Scholar] [CrossRef] [PubMed]
- Noordeen, N.A.; Meur, G.; Rutter, G.A.; Leclerc, I. Glucose-induced nuclear shuttling of ChREBP is mediated by sorcin and Ca2+ ions in pancreatic β-cells. Diabetes 2012, 61, 574–585. [Google Scholar] [CrossRef]
- Daniel, P.V.; Dogra, S.; Rawat, P.; Choubey, A.; Khan, A.S.; Rajak, S.; Kamthan, M.; Mondal, P. NF-κB p65 regulates hepatic lipogenesis by promoting nuclear entry of ChREBP in response to a high carbohydrate diet. J. Biol. Chem. 2021, 296, 100714. [Google Scholar] [CrossRef]
- Canela-Xandri, O.; Rawlik, K.; Tenesa, A. An atlas of genetic associations in UK Biobank. Nat. Genet. 2018, 50, 1593–1599. [Google Scholar] [CrossRef]
- Andreev, V.P.; Petyuk, V.A.; Brewer, H.M.; Karpievitch, Y.V.; Xie, F.; Clarke, J.; Camp, D.; Smith, R.D.; Lieberman, A.P.; Albin, R.L.; et al. Label-free quantitative LC-MS proteomics of Alzheimer’s disease and normally aged human brains. J. Proteome Res. 2012, 11, 3053–3067. [Google Scholar] [CrossRef]
- Tsuji, T.; Shiozaki, A.; Kohno, R.; Yoshizato, K.; Shimohama, S. Proteomic profiling and neurodegeneration in Alzheimer’s disease. Neurochem. Res. 2002, 27, 1245–1253. [Google Scholar] [CrossRef]
- Seyfried, N.T.; Dammer, E.B.; Swarup, V.; Nandakumar, D.; Duong, D.M.; Yin, L.; Deng, Q.; Nguyen, T.; Hales, C.M.; Wingo, T.; et al. A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer’s Disease. Cell Syst. 2017, 4, 60–72. [Google Scholar] [CrossRef]
- Drummond, E.; Nayak, S.; Faustin, A.; Pires, G.; Hickman, R.A.; Askenazi, M.; Cohen, M.; Haldiman, T.; Kim, C.; Han, X.; et al. Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer’s disease. Acta Neuropathol. 2017, 133, 933–954. [Google Scholar] [CrossRef] [PubMed]
- Umoh, M.E.; Dammer, E.B.; Dai, J.; Duong, D.M.; Lah, J.J.; Levey, A.I.; Gearing, M.; Glass, J.D.; Seyfried, N.T. A proteomic network approach across the ALS—FTD disease spectrum resolves clinical phenotypes and genetic vulnerability in human brain. EMBO Mol. Med. 2018, 10, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hulette, C.; Wang, Y.; Zhang, T.; Pan, C.; Wadhwa, R.; Zhang, J. Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: Relevance to Parkinson disease. Mol. Cell. Proteom. 2006, 5, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Singh, S.; Lokappa, S.B.; Varkey, J. Gene co-expression network analysis for identifying genetic markers in Parkinson’s disease-a three-way comparative approach. Genomics 2019, 111, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Kalathur, R.K.; Giner-Lamia, J.; Machado, S.; Barata, T.; Ayasolla, K.R.; Futschik, M.E. The unfolded protein response and its potential role in Huntington’s disease elucidated by a systems biology approach. F1000Res 2015, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Hondius, D.C.; Eigenhuis, K.N.; Morrema, T.H.J.; van der Schors, R.C.; van Nierop, P.; Bugiani, M.; Li, K.W.; Hoozemans, J.J.M.; Smit, A.B.; Rozemuller, A.J.M. Proteomics analysis identifies new markers associated with capillary cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Giamogante, F.; Barazzuol, L.; Battista, T.; Fiorillo, A.; Vicario, M.; D’Alessandro, G.; Cipriani, R.; Limatola, C.; Rossi, D.; et al. Sorcin is an early marker of neurodegeneration, Ca2+ dysregulation and endoplasmic reticulum stress associated to neurodegenerative diseases. Cell Death Dis. 2020, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.J.; Heyny-von Haussen, R.; Mall, G.; Wolf, S. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteome Sci. 2008, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Lee, H.J.; Kim, S.S.; Kwon, Y.S.; Chun, W. Sequestration of sorcin by aberrant forms of tau results in the defective calcium homeostasis. Korean J. Physiol. Pharmacol. 2016, 20, 387–397. [Google Scholar] [CrossRef][Green Version]
- Pack-Chung, E.; Meyers, M.B.; Pettingell, W.P.; Moir, R.D.; Brownawell, A.M.; Cheng, I.; Tanzi, R.E.; Kim, T.W. Presenilin 2 interacts with sorcin, a modulator of the ryanodine receptor. J. Biol. Chem. 2000, 275, 14440–14445. [Google Scholar] [CrossRef]
- Woods, W.S.; Boettcher, J.M.; Zhou, D.H.; Kloepper, K.D.; Hartman, K.L.; Ladror, D.T.; Qi, Z.; Rienstra, C.M.; George, J.M. Conformation-specific binding of alpha-synuclein to novel protein partners detected by phage display and NMR spectroscopy. J. Biol. Chem. 2007, 282, 34555–34567. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sirohi, V.K.; Kumari, S.; Shukla, V.; Manohar, M.; Popli, P.; Dwivedi, A. Sorcin is involved during embryo implantation via activating VEGF/PI3K/Akt pathway in mice. J. Mol. Endocrinol. 2018, 60, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Salzer, U.; Hinterdorfer, P.; Hunger, U.; Borken, C.; Prohaska, R. Ca++-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood 2002, 99, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen. Med. 2011, 6, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Buschow, S.I.; van Balkom, B.W.; Aalberts, M.; Heck, A.J.; Wauben, M.; Stoorvogel, W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 2010, 88, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Demory Beckler, M.; Higginbotham, J.N.; Franklin, J.L.; Ham, A.J.; Halvey, P.J.; Imasuen, I.E.; Whitwell, C.; Li, M.; Liebler, D.C.; Coffey, R.J. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol. Cell Proteom. 2013, 12, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Gangoda, L.; Liem, M.; Fonseka, P.; Atukorala, I.; Ozcitti, C.; Mechler, A.; Adda, C.G.; Ang, C.S.; Mathivanan, S. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget 2015, 6, 15375–15396. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, P.; Chioureas, D.; Rutishauser, D.; Baltatzis, G.; Lennartsson, L.; Fonseca, P.; Azimi, A.; Hultenby, K.; Zubarev, R.; Ullén, A.; et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget 2015, 6, 21740–21754. [Google Scholar] [CrossRef]
- Pienimaeki-Roemer, A.; Kuhlmann, K.; Böttcher, A.; Konovalova, T.; Black, A.; Orsó, E.; Liebisch, G.; Ahrens, M.; Eisenacher, M.; Meyer, H.E.; et al. Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets. Transfusion 2015, 55, 507–521. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Joseph, J. Proteomic profiling of extracellular vesicles derived from ARPE-19 cells challenged with Aspergillus flavus and Candida albicans: Application in fungal endophthalmitis. Pathog. Dis. 2022, 80, ftac042. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Kim, Y.J.; Choi, D.A.; Kang, D.W.; Kim, J.; Yoo, H.S.; Shahriyar, S.A.; Mustajab, T.; Kim, J.; Han, K.R.; et al. Role of Gasdermin E in the Biogenesis of Apoptotic Cell–Derived Exosomes. J. Immunol. 2023, 210, 1974–1989. [Google Scholar] [CrossRef]
- de Oliveira Junior, G.P.; Welsh, J.A.; Pinckney, B.; Palu, C.C.; Lu, S.; Zimmerman, A.; Barbosa, R.H.; Sahu, P.; Noshin, M.; Gummuluru, S.; et al. Human red blood cells release microvesicles with distinct sizes and protein composition that alter neutrophil phagocytosis. J. Extracell. Biol. 2023, 2, e107. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.B.; Biedler, J.L. Increased synthesis of a low molecular weight protein in vincristine-resistant cells. Biochem. Biophys. Res. Commun. 1981, 99, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Van der Bliek, A.M.; Baas, F.; Van der Velde-Koerts, T.; Biedler, J.L.; Meyers, M.B.; Ozols, R.F.; Hamilton, T.C.; Joenje, H.; Borst, P. Genes Amplified and Overexpressed in Human Multidrug-resistant Cell Lines. [Online]. Available online: http://aacrjournals.org/cancerres/article-pdf/48/21/5927/2433354/cr0480215927.pdf (accessed on 23 December 2022).
- Bouchelouche, P.; Friche, E.; Sehested, M.; Jensen, P.B.; Skovsgaard, T. Cytosolic free Ca2+ in daunorubicin and vincristine resistant Ehrlich ascites tumor cells. Drug accumulation is independent of intracellular Ca2+ changes. Biochem. Pharmacol. 1991, 41, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, S.L.; Tamayo, P.; Gaasenbeek, M.; Sturla, L.M.; Angelo, M.; McLaughlin, M.E.; Kim, J.Y.; Goumnerova, L.C.; Black, P.M.; Lau, C.; et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 2002, 415, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, G.; Zhao, C.; Wang, J.; Zhao, H.; Xue, Y.; Han, M.; Yang, C. Expression of sorcin predicts poor outcome in acute myeloid leukemia. Leuk. Res. 2003, 27, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Shai, R.; Shi, T.; Kremen, T.J.; Horvath, S.; Liau, L.M.; Cloughesy, T.F.; Mischel, P.S.; Nelson, S.F. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene 2003, 22, 4918–4923. [Google Scholar] [CrossRef]
- Padar, S.; van Breemen, C.; Thomas, D.W.; Uchizono, J.A.; Livesey, J.C.; Rahimian, R. Differential regulation of calcium homeostasis in adenocarcinoma cell line A549 and its Taxol-resistant subclone. Br. J. Pharmacol. 2004, 142, 305–316. [Google Scholar] [CrossRef]
- French, P.J.; Swagemakers, S.M.; Nagel, J.H.; Kouwenhoven, M.C.; Brouwer, E.; van der Spek, P.; Luider, T.M.; Kros, J.M.; van den Bent, M.J.; Sillevis Smitt, P.A. Gene expression profiles associated with treatment response in oligodendrogliomas. Cancer Res. 2005, 24, 11335–11344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.; Tan, Y.; Qi, J.; Xiao, Y.; Yang, C.; Zhu, Z.; Xiong, D. Sorcin, an important gene associated with multidrug-resistance in human leukemia cells. Leuk. Res. 2006, 30, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Kouno, J.; Adachi, K.; Takahashi, H.; Teramoto, A.; Matsumoto, K.; Sugisaki, Y.; Onda, M.; Tsunoda, T. Identification of histological markers for malignant glioma by genome-wide expression analysis: Dynein, alpha-PIX and sorcin. Acta Neuropathol. 2006, 111, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, N.; Zhou, Y.; Tan, Y.; Cheng, Y.; Yang, C.; Zhu, Z.; Xiong, D. Overexpression of sorcin in multidrug resistant human leukemia cells and its role in regulating cell apoptosis. Biochem. Biophys. Res. Commun. 2006, 349, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, J.K.; Das, B.R. Identification of differentially expressed genes in tobacco chewing-mediated oral cancer by differential display–polymerase chain reaction. Eur. J. Clin. Investig. 2007, 37, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Watanabe, M.; Huang, P.; Sakaguchi, M.; Ochiai, K.; Nasu, Y.; Ouchida, M.; Huh, N.H.; Shimizu, K.; Kashiwakura, Y.; et al. REIC/Dkk-3 stable transfection reduces the malignant phenotype of mouse prostate cancer RM9 cells. Int. J. Mol. Med. 2009, 24, 789–794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qu, Y.; Yang, Y.; Liu, B.; Xiao, W. Comparative proteomic profiling identified sorcin being associated with gemcitabine resistance in non-small cell lung cancer. Med. Oncol. 2010, 27, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Su, T.; Leng, A.; Zhang, X.; Xu, M.; Yan, L.; Gu, H.; Zhang, G. Upregulation of soluble resistance-related calcium-binding protein (sorcin) in gastric cancer. Med. Oncol. 2010, 27, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, N.; Nakao, R.; Kondo, R.; Nishitsuji, M.; Saito, Y.; Kuga, T.; Hatayama, T.; Nakayama, Y. Increased expression of sorcin is associated with multidrug resistance in leukemia cells via up-regulation of MDR1 expression through cAMP response element-binding protein. Biochem. Biophys. Res. Commun. 2014, 448, 430–436. [Google Scholar] [CrossRef]
- Tong, W.; Sun, D.; Wang, Q.; Suo, J. Sorcin Enhances Metastasis and Promotes Epithelial-to-Mesenchymal Transition of Colorectal Cancer. Cell Biochem. Biophys. 2015, 72, 453–459. [Google Scholar] [CrossRef]
- Gao, Y.; Li, W.; Liu, X.; Gao, F.; Zhao, X. Reversing effect and mechanism of soluble resistance-relatedcalcium-binding protein on multidrug resistance in human lung cancer A549/DDP cells. Mol. Med. Rep. 2015, 11, 2118–2124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dabaghi, M.; Rahgozar, S.; Moshtaghian, J.; Moafi, A.; Abedi, M.; Pourabutaleb, E. Overexpression of SORCIN is a Prognostic Biomarker for Multidrug-Resistant Pediatric Acute Lymphoblastic Leukemia and Correlates with Upregulated MDR1/P-gp. Genet. Test. Mol. Biomarkers 2016, 20, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Meng, Q.; Liu, Z.; Huo, X.; Sun, P.; Sun, H.; Ma, X.; Peng, J.; Liu, K. Targeting P-glycoprotein and SORCIN: Dihydromyricetin strengthens anti-proliferative efficiency of adriamycin via MAPK/ERK and Ca2+-mediated apoptosis pathways in MCF-7/ADR and K562/ADR. J. Cell Physiol. 2018, 233, 3066–3079. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhu, S.; Tong, Y.; Huang, G.; Tan, B.; Yang, L. Antitumor activity of triptolide in SKOV3 cells and SKOV3/DDP in vivo and in vitro. Anticancer Drugs 2020, 31, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, W.; Xu, X.; Wang, F.; Li, X.; Xu, G. A novel homeostatic loop of sorcin drives paclitaxel-resistance and malignant progression via Smad4/ZEB1/miR-142-5p in human ovarian cancer. Oncogene 2021, 40, 4906–4918. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Akhtar, J.; Priya, R.; Sakhuja, P.; Goyal, S.; Agarwal, A.K.; Ghose, V.; Polisetty, R.V.; Sirdeshmukh, R.; Siraj, F.; et al. Tissue proteome analysis for profiling proteins associated with lymph node metastasis in gallbladder cancer. BMC Cancer 2023, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Z.; Zhu, Y.; Fu, C.; Li, N.; Peng, F. Sorcin regulate pyroptosis by interacting with NLRP3 inflammasomes to facilitate the progression of hepatocellular carcinoma. Cell Death Dis. 2023, 14, 678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, S.; Sanches, J.G.P.; Li, Y.; Wei, Y.; Pu, C.; Zhang, J. Sorcin promotes proliferation of hepatocellular carcinoma by regulating VEGFA/B via PI3K pathway. J. Physiol. Biochem. 2024, 80, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Chen, Z.C.; Zhang, G.Y.; Yi, H.; Xiao, Z.Q. A subcelluar proteomic investigation into vincristine-resistant gastric cancer cell line. J. Cell Biochem. 2008, 104, 1010–1021. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Shan, B.; Lin, L.; Dong, J.; Sun, Q.; Zhou, Q.; Han, X. Clinical Significance and Prognostic Value of Human Soluble Resistance-Related Calcium-Binding Protein: A Pan-Cancer Analysis. Front. Med. 2021, 8, 752619. [Google Scholar] [CrossRef]
- Romito, O.; Guéguinou, M.; Raoul, W.; Champion, O.; Robert, A.; Trebak, M.; Goupille, C.; Potier-Cartereau, M. Calcium signaling: A therapeutic target to overcome resistance to therapies in cancer. Cell Calcium 2022, 108, 102673. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, S.K.; Kim, J.K.; Park, H.K.; Lee, E.; Jang, J.; Lee, Y.H.; Khim, K.W.; Hyun, J.M.; Eom, H.J.; et al. Flightless-1 inhibits ER stress-induced apoptosis in colorectal cancer cells by regulating Ca2+ homeostasis. Exp. Mol. Med. 2020, 52, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, X.; Li, S.; Zhou, Y.; Wang, J.; Cheng, T.; Yang, M.; Xiong, D. Inhibition of sorcin reverses multidrug resistance of K562/A02 cells and MCF-7/A02 cells via regulating apoptosis-related proteins. Cancer Chemother. Pharmacol. 2013, 72, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, L.; Feng, B.; Liu, G. Reversing effect of sorcin in the drug resistance of human nasopharyngeal carcinoma. Anat. Rec. 2014, 297, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Jiang, Y.F.; Wang, J.H. shRNA-mediated silencing of sorcin increases drug chemosensitivity in myeloma KM3/DDP and U266/ADM cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 2300–2310. [Google Scholar] [PubMed]
- Anthony, D.F.; Beattie, J.; Paul, A.; Currie, S. Interaction of calcium/calmodulin-dependent protein kinase IIδC with sorcin indirectly modulates ryanodine receptor function in cardiac myocytes. J. Mol. Cell Cardiol. 2007, 43, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Laudiero, G.; Maddalena, F.; Amoroso, M.R.; Piscazzi, A.; Cozzolino, F.; Monti, M.; Garbi, C.; Fersini, A.; Pucci, P.; et al. Mitochondrial chaperone Trap1 and the calcium binding protein sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res. 2010, 70, 6577–6586. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Yang, M.; Yan, C.; Fan, D.; Zhou, Y.; Zhang, Y.; Yagüe, E.; Xiong, D. Sorcin silencing inhibits epithelial-to-mesenchymal transition and suppresses breast cancer metastasis in vivo. Breast Cancer Res. Treat. 2014, 143, 287–299. [Google Scholar] [CrossRef]
- Tuo, H.; Shu, F.; She, S.; Yang, M.; Zou, X.Q.; Huang, J.; Hu, H.D.; Hu, P.; Ren, H.; Peng, S.F.; et al. Sorcin induces gastric cancer cell migration and invasion contributing to STAT3 activation. Oncotarget 2017, 8, 104258–104271. [Google Scholar] [CrossRef]
- Ling, F.; Zhang, H.; Sun, Y.; Meng, J.; Sanches, J.G.P.; Huang, H.; Zhang, Q.; Yu, X.; Wang, B.; Hou, L.; et al. AnnexinA7 promotes epithelial–mesenchymal transition by interacting with Sorcin and contributes to aggressiveness in hepatocellular carcinoma. Cell Death Dis. 2021, 12, 1018. [Google Scholar] [CrossRef]
- Tito, C.; Genovese, I.; Giamogante, F.; Benedetti, A.; Miglietta, S.; Barazzuol, L.; Cristiano, L.; Iaiza, A.; Carolini, S.; De Angelis, L.; et al. Sorcin promotes migration in cancer and regulates the EGF-dependent EGFR signaling pathways. Cell. Mol. Life Sci. 2023, 80. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Zhang, P.; Xiong, S.; Yuan, J.; Gao, X.; Guan, W.; Wang, F.; Li, X.; Dou, H.; et al. Lipid-coated albumin-paclitaxel nanoparticles loaded with sorcin-siRNA reverse cancer chemoresistance via restoring intracellular calcium ion homeostasis. J. Nanobiotechnol. 2022, 20, 319. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Demidova, N.S.; Ilyinskaya, G.V.; Shiryaeva, O.A.; Chernova, O.B.; Goncharova, S.A.; Kopnin, B.P. Decreased sensitivity of multidrug-resistant tumor cells to cisplatin is correlated with sorcin gene co-amplification. Neoplasma 1995, 42, 195–201. [Google Scholar]
- Parekh, H.K.; Deng, H.B.; Choudhary, K.; Houser, S.R.; Simpkins, H. Overexpression of sorcin, a calcium-binding protein, induces a low level of paclitaxel resistance in human ovarian and breast cancer cells. Biochem. Pharmacol. 2002, 63, 1149–1158. [Google Scholar] [CrossRef]
- He, Q.; Zhang, G.; Hou, D.; Leng, A.; Xu, M.; Peng, J.; Liu, T. Overexpression of sorcin results in multidrug resistance in gastric cancer cells with up-regulation of P-gp. Oncol. Rep. 2011, 25, 237–243. [Google Scholar] [CrossRef]
- Genovese, I.; Fiorillo, A.; Ilari, A.; Masciarelli, S.; Fazi, F.; Colotti, G. Binding of doxorubicin to Sorcin impairs cell death and increases drug resistance in cancer cells. Cell Death Dis. 2017, 8, e2950. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.; Wang, C.; Meng, Q.; Liu, Z.; Huo, X.; Yang, X.; Sun, P.; Sun, H.; Ma, X.; et al. Combination of dihydromyricetin and ondansetron strengthens antiproliferative efficiency of adriamycin in K562/ADR through downregulation of SORCIN: A new strategy of inhibiting P-glycoprotein. J. Cell Physiol. 2019, 234, 3685–3696. [Google Scholar] [CrossRef]
- Deng, L.M.; Tan, T.; Zhang, T.Y.; Xiao, X.F.; Gu, H. MiR-1 reverses multidrug resistance in gastric cancer cells via downregulation of sorcin through promoting the accumulation of intracellular drugs and apoptosis of cells. Int. J. Oncol. 2019, 55, 451–461. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Gu, A.M.; Li, Y.; Wang, T.; Li, C.; Gu, Y.; Lin, J.; Ding, X. Identification and Validation of a Metabolism-Related Prognostic Signature Associated with M2 Macrophage Infiltration in Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 10625. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updates 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Genovese, I.; Ilari, A.; Assaraf, Y.G.; Fazi, F.; Colotti, G. Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist. Updates 2017, 32, 23–46. [Google Scholar] [CrossRef]
- Limniatis, G.; Georges, E. Knockout of P-glycoprotein abolish the collateral sensitivity of CHORC5 multidrug resistant cells. Biochem. Biophys. Res. Commun. 2022, 608, 23–29. [Google Scholar] [CrossRef]
- Van der Bliek, A.M.; Van der Velde-Koerts, T.; Ling, V.; Borst, P. Overexpression and Amplification of Five Genes in a Multidrug-Resistant Chinese Hamster Ovary Cell Line. Mol. Cell Biol. 1986, 6, 1671–1678. [Google Scholar] [CrossRef]
- Torigoe, K.; Sato, S.; Kusaba, H.; Kohno, K.; Kuwano, M.; Okumura, K.; Green, E.D.; Tsui, L.C.; Scherer, S.W.; Schlessinger, D.; et al. A YAC-based contig of 1.5 mb spanning the human multidrug resistance gene region and delineating the amplification unit in three human multidrug-resistant cell lines. Genome Res. 1995, 5, 233–244. [Google Scholar] [CrossRef]
- Wang, Y.C.; Juric, D.; Francisco, B.; Yu, R.X.; Duran, G.E.; Chen, K.G.; Chen, X.; Sikic, B.I. Regional activation of chromosomal arm 7q with and without gene amplification in taxane-selected human ovarian cancer cell lines. Genes. Chromosomes Cancer 2006, 45, 365–374. [Google Scholar] [CrossRef]
- Flahaut, M.; Mühlethaler-Mottet, A.; Martinet, D.; Fattet, S.; Bourloud, K.B.; Auderset, K.; Meier, R.; Schmutz, N.B.; Delattre, O.; Joseph, J.M.; et al. Molecular cytogenetic characterization of doxorubicin-resistant neuroblastoma cell lines: Evidence that acquired multidrug resistance results from a unique large amplification of the 7q21 region. Genes. Chromosomes Cancer 2006, 45, 495–508. [Google Scholar] [CrossRef]
- Yabuki, N.; Sakata, K.; Yamasaki, T.; Terashima, H.; Mio, T.; Miyazaki, Y.; Fujii, T.; Kitada, K. Gene amplification and expression in lung cancer cells with acquired paclitaxel resistance. Cancer Genet. Cytogenet. 2007, 173, 1–9. [Google Scholar] [CrossRef]
- Kitada, K.; Yamasaki, T. The MDR1/ABCB1 regional amplification in large inverted repeats with asymmetric sequences and microhomologies at the junction sites. Cancer Genet. Cytogenet. 2007, 178, 120–127. [Google Scholar] [CrossRef]
- Finalet Ferreiro, J.; Rouhigharabaei, L.; Urbankova, H.; van der Krogt, J.A.; Michaux, L.; Shetty, S.; Krenacs, L.; Tousseyn, T.; De Paepe, P.; Uyttebroeck, A.; et al. Integrative genomic and transcriptomic analysis identified candidate genes implicated in the pathogenesis of hepatosplenic T-cell lymphoma. PLoS ONE 2014, 9, e102977. [Google Scholar] [CrossRef]
- Patch, A.M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef]
- Hansen, S.N.; Ehlers, N.S.; Zhu, S.; Thomsen, M.B.; Nielsen, R.L.; Liu, D.; Wang, G.; Hou, Y.; Zhang, X.; Xu, X.; et al. The stepwise evolution of the exome during acquisition of docetaxel resistance in breast cancer cells. BMC Genom. 2016, 17, 442. [Google Scholar] [CrossRef]
- Litviakov, N.V.; Cherdyntseva, N.V.; Tsyganov, M.M.; Slonimskaya, E.M.; Ibragimova, M.K.; Kazantseva, P.V.; Kzhyshkowska, J.; Choinzonov, E.L. Deletions of multidrug resistance gene loci in breast cancer leads to the down-regulation of its expression and predict tumor response to neoadjuvant chemotherapy. Oncotarget 2016, 7, 7829–7841. [Google Scholar] [CrossRef]
- Januchowski, R.; Sterzyńska, K.; Zawierucha, P.; Ruciński, M.; Świerczewska, M.; Partyka, M.; Bednarek-Rajewska, K.; Brązert, M.; Nowicki, M.; Zabel, M.; et al. Microarray-based detection and expression analysis of new genes associated with drug resistance in ovarian cancer cell lines. Oncotarget 2017, 8, 49944–49958. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.; Ho, J.N.; Jin, H.; Byun, S.S.; Lee, E. Analysis of resistance-associated gene expression in docetaxel-resistant prostate cancer cells. Oncol. Lett. 2017, 14, 3011–3018. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Karim, S.; Abusamra, H.; Pushparaj, P.N.; Khan, J.A.; Abuzenadah, A.M.; Gari, M.A.; Bakhashab, S.; Ahmed, F.; Al-Qahtani, M.H. Genomic amplification of chromosome 7 in the Doxorubicin resistant K562 cell line. Bioinformation 2018, 14, 587–593. [Google Scholar] [CrossRef]
- Lombard, A.P.; Lou, W.; Armstrong, C.M.; D’Abronzo, L.S.; Ning, S.; Evans, C.P.; Gao, A.C. Activation of the ABCB1 Amplicon in Docetaxel- And Cabazitaxel-Resistant Prostate Cancer Cells. Mol. Cancer Ther. 2021, 20, 2061–2070. [Google Scholar] [CrossRef]
- Bergonzini, C.; Gregori, A.; Hagens, T.M.S.; van der Noord, V.E.; van de Water, B.; Zweemer, A.J.M.; Coban, B.; Capula, M.; Mantini, G.; Botto, A.; et al. ABCB1 overexpression through locus amplification represents an actionable target to combat paclitaxel resistance in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2024, 43, 4. [Google Scholar] [CrossRef]
- Huang, J.F.; Wen, C.J.; Zhao, G.Z.; Dai, Y.; Li, Y.; Wu, L.X.; Zhou, H.H. Overexpression of ABCB4 contributes to acquired doxorubicin resistance in breast cancer cells in vitro. Cancer Chemother. Pharmacol. 2018, 82, 199–210. [Google Scholar] [CrossRef]
- Han, L.; Long, Q.; Li, S.; Xu, Q.; Zhang, B.; Dou, X.; Qian, M.; Jiramongkol, Y.; Guo, J.; Cao, L.; et al. Senescent stromal cells promote cancer resistance through SIRT1 loss-potentiated overproduction of small extracellular vesicles. Cancer Res. 2020, 80, 3383–3398. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Luo, L.; You, Z.; Chen, H.; Wang, J.; Zhang, F.; Liu, Y.; Ke, Y. Glioblastoma stem cells deliver ABCB4 transcribed by ATF3 via exosomes conferring glioblastoma resistance to temozolomide. Cell Death Dis. 2024, 15, 318. [Google Scholar] [CrossRef]
- Nambiar, S.; Mirmohammadsadegh, A.; Hassan, M.; Mota, R.; Marini, A.; Alaoui, A.; Tannapfel, A.; Hegemann, J.H.; Hengge, U.R. Identification and functional characterization of ASK/Dbf4, a novel cell survival gene in cutaneous melanoma with prognostic relevance. Carcinogenesis 2007, 28, 2501–2510. [Google Scholar] [CrossRef]
- Bonte, D.; Lindvall, C.; Liu, H.; Dykema, K.; Furge, K.; Weinreich, M. Cdc7-Dbf4 kinase overexpression in multiple cancers and tumor cell lines is correlated with p53 inactivation. Neoplasia 2008, 10, 920–931. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Kingsbury, S.R.; Tudzarova, S.; Hong, H.K.; Loddo, M.; Rashid, M.; Rodriguez-Acebes, S.; Prevost, A.T.; Ledermann, J.A.; Stoeber, K.; et al. Cdc7 kinase is a predictor of survival and a novel therapeutic target in epithelial ovarian carcinoma. Clin. Cancer Res. 2009, 15, 2417–2425. [Google Scholar] [CrossRef]
- Choschzick, M.; Lebeau, A.; Marx, A.H.; Tharun, L.; Terracciano, L.; Heilenkötter, U.; Jaenicke, F.; Bokemeyer, C.; Simon, R.; Sauter, G.; et al. Overexpression of cell division cycle 7 homolog is associated with gene amplification frequency in breast cancer. Hum. Pathol. 2010, 41, 358–365. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, H.Q.; Ba, Y. High expression of cell division cycle 7 protein correlates with poor prognosis in patients with diffuse large B-cell lymphoma. Med. Oncol. 2012, 29, 3498–3503. [Google Scholar] [CrossRef]
- Cheng, A.N.; Jiang, S.S.; Fan, C.C.; Lo, Y.K.; Kuo, C.Y.; Chen, C.H.; Liu, Y.L.; Lee, C.C.; Chen, W.S.; Huang, T.S.; et al. Increased Cdc7 expression is a marker of oral squamous cell carcinoma and overexpression of Cdc7 contributes to the resistance to DNA-damaging agents. Cancer Lett. 2013, 337, 218–225. [Google Scholar] [CrossRef]
- Sasi, N.K.; Bhutkar, A.; Lanning, N.J.; MacKeigan, J.P.; Weinreich, M. DDK Promotes Tumor Chemoresistance and Survival via Multiple Pathways. Neoplasia 2017, 19, 439–450. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, L.; Li, X.; Liu, L.; Kuang, T.; Qiu, Z.; Deng, W.; Wang, W. The prognostic significance and potential mechanism of DBF4 zinc finger in hepatocellular carcinoma. Sci. Rep. 2024, 14, 10662. [Google Scholar] [CrossRef]
- McCartan, D.; Bolger, J.C.; Fagan, A.; Byrne, C.; Hao, Y.; Qin, L.; McIlroy, M.; Xu, J.; Hill, A.D.; Gaora, P.Ó.; et al. Global characterization of the SRC-1 transcriptome identifies ADAM22 as an ER-independent mediator of endocrine-resistant breast cancer. Cancer Res. 2012, 72, 220–229. [Google Scholar] [CrossRef]
- Li, J.; Lu, M.; Jin, J.; Lu, X.; Xu, T.; Jin, S. MiR-449a Suppresses Tamoxifen Resistance in Human Breast Cancer Cells by Targeting ADAM22. Cell. Physiol. Biochem. 2018, 50, 66–78. [Google Scholar] [CrossRef]
- Xing, B.; Lei, Z.; Wang, Z.; Wang, Q.; Jiang, Q.; Zhang, Z.; Liu, X.; Qi, Y.; Li, S.; Guo, X.; et al. A disintegrin and metalloproteinase 22 activates integrin β1 through its disintegrin domain to promote the progression of pituitary adenoma. Neuro Oncol. 2024, 26, 137–152. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, P.; Chen, Z.; Gu, X.; Zhang, T. ADAM22 acts as a novel predictive biomarker for unfavorable prognosis and facilitates metastasis via PI3K/AKT signaling pathway in nasopharyngeal carcinoma. Pathol. Res. Pract. 2024, 256, 155264. [Google Scholar] [CrossRef]
- Ali, R.; Huang, Y.; Maher, S.E.; Kim, R.W.; Giordano, F.J.; Tellides, G.; Geirsson, A. MiR-1 mediated suppression of Sorcin regulates myocardial contractility through modulation of Ca2+ signaling. J. Mol. Cell Cardiol. 2012, 52, 1027–1037. [Google Scholar] [CrossRef]
- Lin, X.Q.; Wu, W.; Chen, X.; Chen, R.P.; Wu, F.; Chen, Z.F.; Huang, E.J.; Chen, C. MiR-1 inhibits migration of gastric cancer cells. Front. Biosci.–Landmark 2020, 25, 452–462. [Google Scholar] [CrossRef]
- Shan, Z.X.; Lin, Q.X.; Deng, C.Y.; Zhu, J.N.; Mai, L.P.; Liu, J.L.; Fu, Y.H.; Liu, X.Y.; Li, Y.X.; Zhang, Y.Y.; et al. MiR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010, 584, 3592–3600. [Google Scholar] [CrossRef]
- Wei, W.; Hu, Z.; Fu, H.; Tie, Y.; Zhang, H.; Wu, Y.; Zheng, X. MicroRNA-1 and microRNA-499 downregulate the expression of the ets1 proto-oncogene in HepG2 cells. Oncol. Rep. 2012, 28, 701–706. [Google Scholar] [CrossRef]
- Li, J.; Chen, B.A.; Zhu, M.S.; Gao, F.; Ding, J.H.; Gao, C.; Sun, Y.Y.; Cheng, J.; Wang, J.; Zhao, G.; et al. Influence of tetrandrine on SORCIN gene expression in K562/A02 cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi / Zhongguo Bing Li Sheng Li Xue Hui = J. Exp. Hematol./Chin. Assoc. Pathophysiol. 2008, 16, 65–69. [Google Scholar]
- Tan, Y.H.; Qi, J.; Liu, W.J.; Yang, C.Z. Preliminary studies on the mechanisms of a new anti-tumor agent PH II-7 with special preference to multidrug resistant tumor cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2002, 24, 134–139. [Google Scholar]
- Li, G.Y.; Liu, J.Z.; Zhang, B.; Yang, M.; Chen, S.G.; Hou, M.; Wang, L.X. Tegillarca granosa extract Haishengsu (HSS) suppresses expression of mdr1, BCR/ABL and sorcin in drug-resistant K562/ADM tumors in mice. Adv. Med. Sci. 2013, 58, 112–117. [Google Scholar] [CrossRef]
- Li, G.Y.; Zhang, L.; Liu, J.Z.; Chen, S.G.; Xiao, T.W.; Liu, G.Z.; Wang, J.X.; Wang, L.X.; Hou, M. Marine drug Haishengsu increases chemosensitivity to conventional chemotherapy and improves quality of life in patients with acute leukemia. Biomed. Pharmacother. 2016, 81, 160–165. [Google Scholar] [CrossRef]
- Wood, R.J.; Tchack, L.; Angelo, G.; Pratt, R.E.; Sonna, L.A. DNA microarray analysis of vitamin D-induced gene expression in a human colon carcinoma cell line. Physiol. Genom. 2004, 17, 122–129. [Google Scholar] [CrossRef]
- Groebe, K.; Cen, J.; Schvartz, D.; Sargsyan, E.; Chowdhury, A.; Roomp, K.; Schneider, R.; Alderborn, A.; Sanchez, J.C.; Bergsten, P. Palmitate-Induced Insulin Hypersecretion and Later Secretory Decline Associated with Changes in Protein Expression Patterns in Human Pancreatic Islets. J. Proteome Res. 2018, 17, 3824–3836. [Google Scholar] [CrossRef]
- Cen, J.; Sargsyan, E.; Forslund, A.; Bergsten, P. Mechanisms of beneficial effects of metformin on fatty acid-treated human islets. J. Mol. Endocrinol. 2018, 61, 91–99. [Google Scholar] [CrossRef]
- Rutti, S.; Arous, C.; Schvartz, D.; Timper, K.; Sanchez, J.C.; Dermitzakis, E.; Donath, M.Y.; Halban, P.A.; Bouzakri, K. Fractalkine (CX3CL1), a new factor protecting β-cells against TNFα. Mol. Metab. 2014, 3, 731–741. [Google Scholar] [CrossRef]
- Liu, H.; Shen, M.; Zhao, D.; Ru, D.; Duan, Y.; Ding, C.; Li, H. The Effect of Triptolide-Loaded Exosomes on the Proliferation and Apoptosis of Human Ovarian Cancer SKOV3 Cells. Biomed. Res. Int. 2019, 2019, 2595801. [Google Scholar] [CrossRef]

| Category of Molecules | Molecule | Mechanism of Regulation | Effect |
|---|---|---|---|
| MicroRNAs | miR-1 | Binding to 3′-UTR of SRI gene | ↓ sorcin |
| miR-142-5p | Binding to 3′-UTR of SRI gene | ↓ sorcin | |
| siRNAs | siRNA vs. sorcinin nanocarriers | Binding to sorcin mRNA | ↓ sorcin |
| Growth factors | TGF-β1 | ↑ SMAD2 phosphorylation | ↓ sorcin |
| TNFα | ↑ mTOR, NFκB phosphoryl. | ↓ sorcin | |
| Chemokine | Fractalkine | ↓ mTOR, NFκB phosphoryl. | ↑ sorcin |
| Small molecules | Tetrandrine | Calcium channel blocking | ↓ sorcin |
| PH II-7 | ? | ↓ sorcin | |
| Dihydromyricetin | ↓ ERK-Akt phosphorylation | ↓ sorcin | |
| Ondansetron | Sorcin binding | ↓ sorcin | |
| Calcitriol | ? | ↓ sorcin | |
| Palmitate | ↓ AMPK- pEIF2α phosphoryl. | ↓ sorcin | |
| Triptolide | ? | ↓ sorcin | |
| Protein extracts | Haishengsu | ? | ↓ sorcin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Exertier, C.; Antonelli, L.; Fiorillo, A.; Bernardini, R.; Colotti, B.; Ilari, A.; Colotti, G. Sorcin in Cancer Development and Chemotherapeutic Drug Resistance. Cancers 2024, 16, 2810. https://doi.org/10.3390/cancers16162810
Exertier C, Antonelli L, Fiorillo A, Bernardini R, Colotti B, Ilari A, Colotti G. Sorcin in Cancer Development and Chemotherapeutic Drug Resistance. Cancers. 2024; 16(16):2810. https://doi.org/10.3390/cancers16162810
Chicago/Turabian StyleExertier, Cécile, Lorenzo Antonelli, Annarita Fiorillo, Roberta Bernardini, Beatrice Colotti, Andrea Ilari, and Gianni Colotti. 2024. "Sorcin in Cancer Development and Chemotherapeutic Drug Resistance" Cancers 16, no. 16: 2810. https://doi.org/10.3390/cancers16162810
APA StyleExertier, C., Antonelli, L., Fiorillo, A., Bernardini, R., Colotti, B., Ilari, A., & Colotti, G. (2024). Sorcin in Cancer Development and Chemotherapeutic Drug Resistance. Cancers, 16(16), 2810. https://doi.org/10.3390/cancers16162810







