Nutritional Intervention for the Elderly during Chemotherapy: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
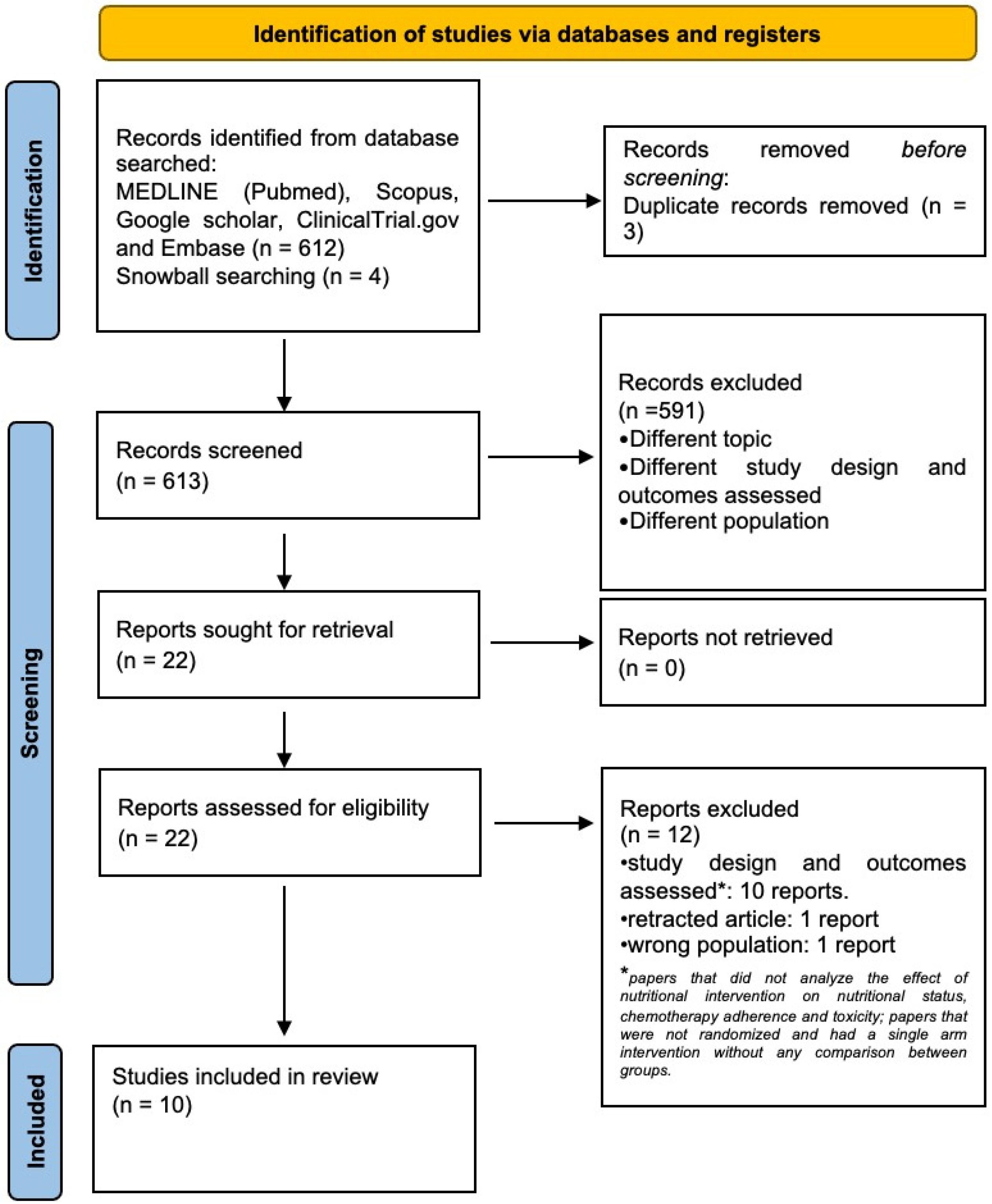
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Selection and Data Collection Processes
2.4. Data Items
2.5. Outcomes Measured
2.5.1. Primary Outcomes
- ONS-related chemotherapy adherence: Defined as the completion of chemotherapy treatments according to the study protocol.
- ONS-related chemotherapy toxicity: Measured by the incidence of chemotherapy side effects.
- ONS-related overall survival: Defined as the time from chemotherapy initiation to the date of death from any cause or the last follow-up visit.
2.5.2. Secondary Outcome
- ONS-related nutritional status: evaluated through anthropometric measurements (e.g., body weight gain and BMI) and/or body composition analysis (e.g., Bioelectrical Impedance Analysis—BIA or radiologic imaging). The prevalence of cachexia, defined as weight loss greater than 5% or weight loss greater than 2% in individuals already showing depletion [15], was also retrieved.
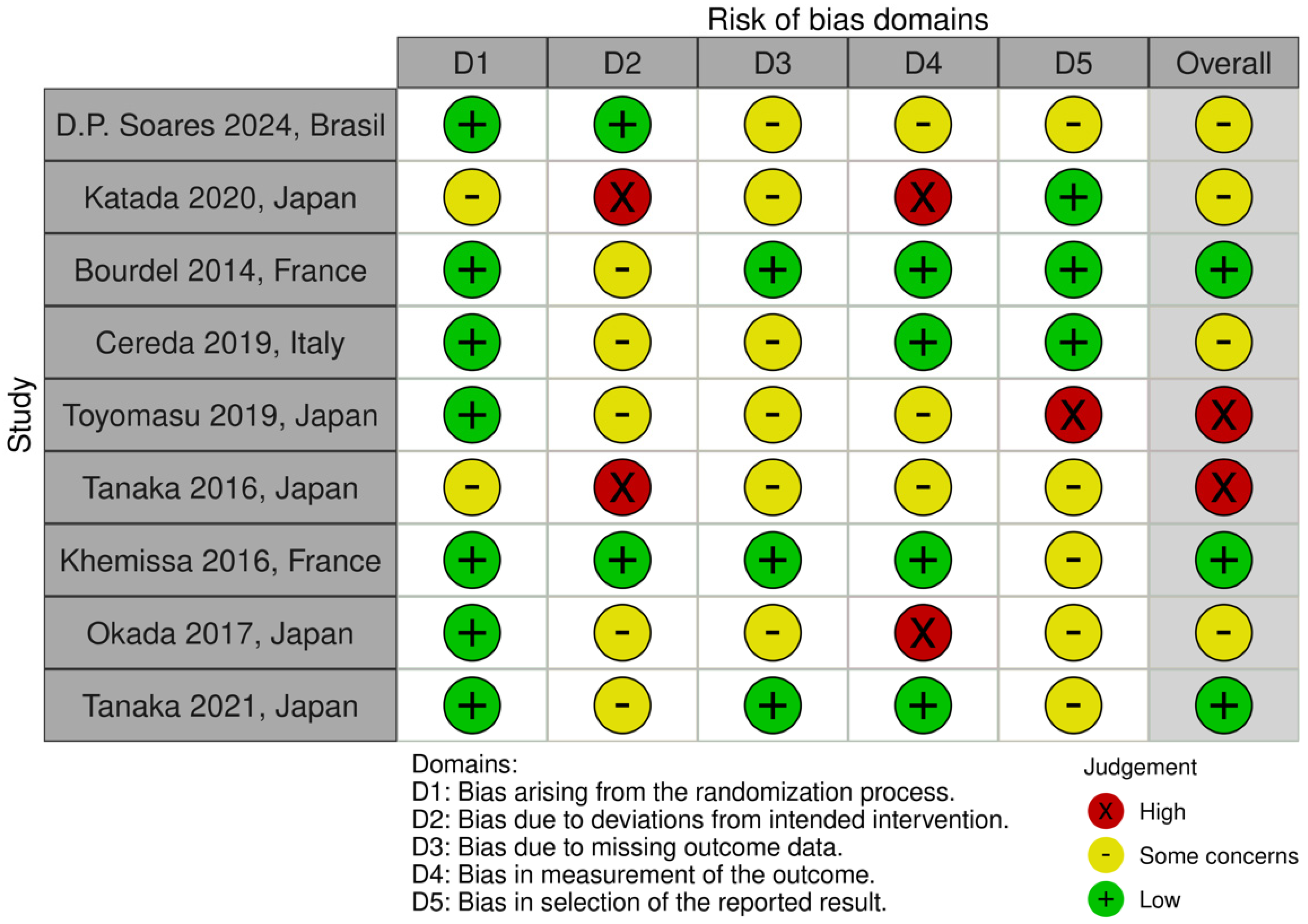
2.6. Study Risk of Bias Assessment
3. Results
3.1. Quality of Included Studies
3.2. Primary Outcomes: Chemotherapy Adherence, Chemotherapy Toxicity, and Overall Survival
3.3. Secondary Outcome: Nutritional Status
3.4. Types of Nutritional Intervention
3.4.1. Multimodal Interventions
3.4.2. Whey Protein Supplements
3.4.3. Amino Acid Supplements
3.4.4. Fish Oil Omega-3-Enriched Supplements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Search Strategy for Medline (Pubmed)
Appendix B
| Author | Year | Reason for Exclusion | Comments |
|---|---|---|---|
| Hasegawa et al. [42] | 2021 | study design and outcomes assessed | Nutritional intervention with pancreatic enzyme replacement therapy and nutritional supplements (not specified) was not provided in all patients and the effect of nutritional intervention on overall survival, chemotherapy adherence and toxicity was not assessed |
| Pimental et al. [55] | 2021 | retracted article | |
| Regueme et al. [56] | 2021 | study design and outcomes assessed | post hoc analysis of the same dataset of patients of the study of Bourdel Marchason et al. [25] |
| Van der werf et al. [57] | 2020 | wrong population | mean age in the intervention group is <65 years |
| Naito T et al. [58] | 2019 | study design and outcomes assessed | prospective single-cohort study, only assessed feasibility of multimodal intervention without assessing chemotherapy adherence, toxicity, and effects on body composition |
| Iyikesici et al. [53] | 2019 | study design and outcomes assessed | prospective single-cohort study which assessed the feasibility of nutritional intervention with ketogenic diet combined with hyperthermia without a comparison with a control group |
| Kostecka et al. [59] | 2019 | study design and outcomes assessed | |
| Eltweri et al. [60] | 2016 | study design and outcomes assessed | nutritional intervention is provided through parental nutrition; the study assessed only the composition of fatty acid on cell membranes; measures of OS, PFS, lean muscle mass, bmi, etc., are lacking |
| Gavazzi et al. [61] | 2016 | study design and outcomes assessed | wrong outcomes assessed (measures of OS, PFS, lean muscle mass, chemotherapy adherence and toxicity, bmi, etc., are lacking) |
| Zy chen et al. [62] | 2015 | study design and outcomes assessed | wrong population (not all patients have tumors and they are not on chemotherapy), wrong study design, wrong outcomes assessed |
| Qin et al. [63] | 2015 | study design and outcomes assessed | oral nutritional intervention not provided |
| Ogata et al. [64] | 2016 | study design and outcomes assessed | case series without comparison |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Malka, D.; Sabaté, J.M.; Bonnetain, F.; Lecomte, T.; Aparicio, T.; Locher, C.; Laharie, D.; Ezenfis, J.; Taieb, J. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: An AGEO prospective cross-sectional multicenter study. Nutr. Cancer 2012, 64, 535–542. [Google Scholar] [CrossRef] [PubMed]
- St Guily, J.L.; Bouvard, É.; Raynard, B.; Goldwasser, F.; Maget, B.; Prevost, A.; Seguy, D.; Romano, O.; Narciso, B.; Couet, C.; et al. NutriCancer: A French observational multicentre cross-sectional study of malnutrition in elderly patients with cancer. J. Geriatr. Oncol. 2018, 9, 74–80. [Google Scholar] [CrossRef]
- Arends, J. Malnutrition in cancer patients: Causes, consequences and treatment options. Eur. J. Surg. Oncol. 2024, 50, 107074. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Van Soom, T.; El Bakkali, S.; Gebruers, N.; Verbelen, H.; Tjalma, W.; van Breda, E. The effects of chemotherapy on energy metabolic aspects in cancer patients: A systematic review. Clin. Nutr. 2020, 39, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Martinez-Tapia, C.; Heitz, D.; Geiss, R.; Albrand, G.; Falandry, C.; Gisselbrecht, M.; Couderc, A.; Boulahssass, R.; Liuu, E.; et al. Prevalence and prognostic impact of cachexia among older patients with cancer: A nationwide cross-sectional survey (NutriAgeCancer). J. Cachexia Sarcopenia Muscle 2021, 12, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Antoun, S.; Sawyer, M.B.; Baracos, V.E. Two faces of drug therapy in cancer: Drug-related lean tissue loss and its adverse consequences to survival and toxicity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. Cancer 2020, 122, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Montégut, L.; López-Otín, C.; Kroemer, G. Aging and cancer. Mol. Cancer 2024, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Katada, C.; Fukazawa, S.; Sugawara, M.; Sakamoto, Y.; Takahashi, K.; Takahashi, A.; Watanabe, A.; Wada, T.; Ishido, K.; Furue, Y.; et al. Randomized study of prevention of gastrointestinal toxicities by nutritional support using an amino acid-rich elemental diet during chemotherapy in patients with esophageal cancer (KDOG 1101). Esophagus 2021, 18, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.D.P.; Siqueira, J.M.; Brito, F.D.S.B.; Pimentel, G.D. A Randomized Controlled Trial on the Effects of Leucine-Supplement Combined with Nutritional Counseling on Body Composition in Mix Cancer Older Men. Nutrients 2024, 16, 210. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, Y.; Mochiki, E.; Yanai, M.; Suzuki, M.; Yanoma, T.; Kimura, A.; Kogure, N.; Ogata, K.; Kuwano, H. A prospective pilot study of an elemental nutritional supplement for prevention of oral mucositis during S-1 adjuvant chemotherapy for gastric cancer. Surg. Oncol. 2019, 29, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Takahashi, T.; Yamaguchi, K.; Osada, S.; Shimokawa, T.; Yoshida, K. Elemental diet plus glutamine for the prevention of mucositis in esophageal cancer patients receiving chemotherapy: A feasibility study. Support. Care Cancer 2016, 24, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Nakajima, Y.; Nishikage, T.; Ryotokuji, T.; Miyawaki, Y.; Hoshino, A.; Tokairin, Y.; Kawada, K.; Nagai, K.; Kawano, T. A prospective study of nutritional supplementation for preventing oral mucositis in cancer patients receiving chemotherapy. Asia Pac. J. Clin. Nutr. 2017, 26, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Khemissa, F.; Mineur, L.; Amsellem, C.; Assenat, E.; Ramdani, M.; Bachmann, P.; Janiszewski, C.; Cristiani, I.; Collin, F.; Courraud, J.; et al. A phase III study evaluating oral glutamine and transforming growth factor-beta 2 on chemotherapy-induced toxicity in patients with digestive neoplasm. Dig. Liver Dis. 2016, 48, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Okugawa, Y.; Hishida, A.; Ogawa, A.; Okamoto, K.; Shintani, M.; Morimoto, Y.; Nishikawa, R.; Yokoe, T.; Tanaka, K.; et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci. Rep. 2017, 7, 4826. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Takeuchi, H.; Nakashima, Y.; Nagano, H.; Ueno, T.; Tomizuka, K.; Morita, S.; Emi, Y.; Hamai, Y.; Hihara, J.; et al. Effects of an elemental diet to reduce adverse events in patients with esophageal cancer receiving docetaxel/cisplatin/5-fluorouracil: A phase III randomized controlled trial—EPOC 2 (JFMC49-1601-C5). ESMO Open 2021, 6, 100277. [Google Scholar] [CrossRef] [PubMed]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.-F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J.; et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: A two-year randomized controlled trial. PLoS ONE 2014, 9, e108687. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef] [PubMed]
- Mcmillan, D.C.; Crozier, J.E.M.; Canna, K.; Angerson, W.J.; Mcardle, C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Color. Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.; Brearley, S.G.; Pilling, M.; Molassiotis, A. The impact of chemotherapy-related nausea on patients’ nutritional status, psychological distress and quality of life. Support. Care Cancer 2013, 21, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Zhu, X.; Szumowski, M.; Scott, G.D.; Grossberg, A.J.; Levasseur, P.R.; Graham, K.; Khan, S.; Damaraju, S.; Colmers, W.F.; et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. J. Exp. Med. 2011, 208, 2449–2463. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann. Palliat. Med. 2019, 8, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pamoukdjian, F.; Laurent, M.; Martinez-Tapia, C.; Rolland, Y.; Paillaud, E.; Canoui-Poitrine, F. Clinical Medicine Frailty Parameters, Morbidity and Mortality in Older Adults with Cancer: A Structural Equation Modelling Approach Based on the Fried Phenotype. J. Clin. Med. 2020, 9, 1826. [Google Scholar] [CrossRef] [PubMed]
- Aversa, Z.; Costelli, P.; Muscaritoli, M. Cancer-induced muscle wasting: Latest findings in prevention and treatment. Ther. Adv. Med. Oncol. 2017, 9, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Milne, A.C.; Potter, J.; Vivanti, A.; Avenell, A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst. Rev. 2009, 2009, CD003288. [Google Scholar] [CrossRef] [PubMed]
- Almuradova, E.; Menekse, S. Survival outcomes and prognostic nutritional index in very elderly small-cell lung cancer patients: Importance of active treatment and nutritional support. Aging Male 2023, 26, 2251573. [Google Scholar] [CrossRef] [PubMed]
- Cintoni, M.; Grassi, F.; Palombaro, M.; Rinninella, E.; Pulcini, G.; Di Donato, A.; Salvatore, L.; Quero, G.; Tortora, G.; Alfieri, S.; et al. Nutritional Interventions during Chemotherapy for Pancreatic Cancer: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grupińska, J.; Budzyń, M.; Maćkowiak, K.; Brzeziński, J.J.; Kycler, W.; Leporowska, E.; Gryszczyńska, B.; Kasprzak, M.P.; Iskra, M.; Formanowicz, D. Beneficial Effects of Oral Nutritional Supplements on Body Composition and Biochemical Parameters in Women with Breast Cancer Undergoing Postoperative Chemotherapy: A Propensity Score Matching Analysis. Nutrients 2021, 13, 3549. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Deal, A.M.; Shachar, S.S.; Walko, C.M.; Patel, J.N.; O’neil, B.; McLeod, H.L.; Weinberg, M.S.; Choi, S.K.; Muss, H.B.; et al. The impact of skeletal muscle on the pharmacokinetics and toxicity of 5-fluorouracil in colorectal cancer. Cancer Chemother. Pharmacol. 2018, 81, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef] [PubMed]
- Antoun, S.; Borget, I.; Lanoy, E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr. Opin. Support. Palliat. Care 2013, 7, 383–389. [Google Scholar] [CrossRef]
- Dingemans, A.; van Walree, N.; Schramel, F.; Soud, M.Y.-E.; Baltruškevičienė, E.; Lybaert, W.; Veldhorst, M.; Berg, C.V.D.; Kaasa, S. High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet ESPEN Protein Recommendations During Systemic Treatment. Clin. Nutr. ESPEN 2023, 36, 11–48. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ijichi, H.; Saito, K.; Ishigaki, K.; Takami, M.; Sekine, R.; Usami, S.; Nakai, Y.; Koike, K.; Kubota, N. Protein intake after the initiation of chemotherapy is an independent prognostic factor for overall survival in patients with unresectable pancreatic cancer: A prospective cohort study. Clin. Nutr. 2021, 40, 4792–4798. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guideline ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2015, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K.; Samak, G. Role of Glutamine in Protection of Intestinal Epithelial Tight Junctions. J. Epithel. Biol. Pharmacol. 2012, 5, 47. [Google Scholar] [CrossRef]
- Sornsuvit, C.; Komindr, S.; Chuncharunee, S.; Wanikiat, P.; Archararit, N.; Santanirand, P. Pilot Study: Effects of parenteral glutamine dipeptide supplementation on neutrophil functions and prevention of chemotherapy-induced side-effects in acute myeloid leukaemia patients. J. Int. Med. Res. 2008, 36, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Lee, S.S.; Oh, S.J.; Lim, S.Y.; Lim, S.Y.; Jeon, W.K.; Oh, T.Y.; Kim, J.W. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin. Nutr. 2007, 26, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Leung HW, C.; Chan AL, F. Glutamine in Alleviation of Radiation-Induced Severe Oral Mucositis: A Meta-Analysis. Nutr. Cancer 2016, 68, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, L.; Panda, N.; Dash, M.K.; Mohanty, S.; Samantaray, S. Management of Chemoradiation-Induced Mucositis in Head and Neck Cancers with Oral Glutamine. J. Glob. Oncol. 2016, 2, 200–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satoh, Y.; Kotani, H.; Iida, Y.; Taniura, T.; Notsu, Y.; Harada, M. Supplementation of l-arginine boosts the therapeutic efficacy of anticancer chemoimmunotherapy. Cancer Sci. 2020, 111, 2248–2258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.; Bajorek, B. Defining “elderly” in clinical practice guidelines for pharmacotherapy. Pharm. Pract. 2014, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Prolonged fasting/refeeding promotes hematopoietic stem cell regeneration and rejuvenation. Rejuvenation Res. 2014, 17, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Iyikesici, M.S. Feasibility study of metabolically supported chemotherapy with weekly carboplatin/paclitaxel combined with ketogenic diet, hyperthermia and hyperbaric oxygen therapy in metastatic non-small cell lung cancer. Int. J. Hyperth. 2019, 36, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Ligorio, F.; Dieci, M.V.; Lambertini, M.; De Placido, S.; Iorfida, M.; Botticelli, A.; Strina, C.; Vingiani, A.; Provenzano, L.; et al. Targeting Triple Negative BREAst Cancer Metabolism with a Combination of Chemoimmunotherapy and a FASTing-like Approach in the Preoperative Setting: The BREAKFAST 2 Trial (BREAKFAST-2). Ann. Oncol. 2023, 34, S322–S323. [Google Scholar] [CrossRef]
- Pimentel, G.D.; Pichard, C.; Laviano, A.; Fernandes, R.C. High protein diet improves the overall survival in older adults with advanced gastrointestinal cancer. Clin. Nutr. 2021, 40, 1376–1380, Erratum in Clin. Nutr. 2021, 40, 1441. [Google Scholar] [CrossRef] [PubMed]
- Regueme, S.C.; Echeverria, I.; Monéger, N.; Durrieu, J.; Becerro-Hallard, M.; Duc, S.; Lafargue, A.; Mertens, C.; Laksir, H.; Ceccaldi, J.; et al. Protein intake, weight loss, dietary intervention, and worsening of quality of life in older patients during chemotherapy for cancer. Support. Care Cancer 2021, 29, 687–696. [Google Scholar] [CrossRef]
- van der Werf, A.; Langius, J.A.E.; Beeker, A.; Ten Tije, A.J.; Vulink, A.J.; Haringhuizen, A.; Berkhof, J.; van der Vliet, H.J.; Verheul, H.M.W.; de van der Schueren, M.A.E. The effect of nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: A randomized controlled trial. Clin. Nutr. 2020, 39, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Mitsunaga, S.; Miura, S.; Tatematsu, N.; Inano, T.; Mouri, T.; Tsuji, T.; Higashiguchi, T.; Inui, A.; Okayama, T.; et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kostecka, M. The Potential Influence of Dietary Counseling on Nutritional Status and Dietary Supplement Consumption in Breast Cancer Patients: A Pilot Study. Nutr. Cancer 2019, 71, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Eltweri, A.M.; Thomas, A.L.; Fisk, H.L.; Arshad, A.; Calder, P.C.; Dennison, A.R.; Bowrey, D.J. Plasma and erythrocyte uptake of omega-3 fatty acids from an intravenous fish oil based lipid emulsion in patients with advanced oesophagogastric cancer. Clin. Nutr. 2017, 36, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, C.; Colatruglio, S.; Valoriani, F.; Mazzaferro, V.; Sabbatini, A.; Biffi, R.; Mariani, L.; Miceli, R. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: A multicentre randomised clinical trial. Eur. J. Cancer 2016, 64, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Gao, C.; Ye, T.; Zuo, X.Z.; Wang, G.H.; Xu, X.S.; Yao, Y. Association between nutritional risk and routine clinical laboratory measurements and adverse outcomes: A prospective study in hospitalized patients of Wuhan Tongji Hospital. Eur. J. Clin. Nutr. 2015, 69, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, D. Nutritional Support of Tumor Patients with Chemotherapy. Cell Biochem. Biophys. 2015, 72, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Ishibashi, N.; Yamaguchi, K.; Uchida, S.; Kamei, H.; Nakayama, G.; Hirakawa, H.; Tanigawa, M.; Akagi, Y. Preventive effects of amino-acid-rich elemental diet Elental® on chemotherapy-induced oral mucositis in patients with colorectal cancer: A prospective pilot study. Support. Care Cancer 2016, 24, 783–789. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Location | Study Design | Aim | Inclusion Criteria | Exclusion Criteria | Intervention Group | Control Group |
|---|---|---|---|---|---|---|---|---|
| Studies evaluating the effect of multimodal intervention | ||||||||
| Bourdel-Marchasson et al. [25] | 2014 | France | RCT | Evaluate the effect of tailored nutritional counseling on mortality, toxicities, and chemotherapy outcomes | Patients older than 70 years with lymphoma or carcinoma and undergoing chemotherapy | Patients with cerebral metastasis and patients unable to take part in follow-up | Tailored nutritional counseling to achieve a daily protein and caloric goal of 1.2 g/kg/d and 30 kCal/kg body weight/d, respectively. Amino acid supplements were provided if pertinent | Usual dietary advice without daily protein and caloric intake goal |
| Studies evaluating the effect of whey protein supplementation | ||||||||
| Cereda et al. [26] | 2019 | Italy | RCT | Evaluate the effect of whey protein supplements on nutritional status and chemotherapy toxicity | Adult patients, malnourished (6-month unintentional weight loss ≥ 10%), and candidate to or undergoing chemotherapy | Patients aged <18 years and undergoing artificial nutrition (enteral or parenteral) | Whey protein supplementation in addition to usual nutritional counseling | Nutritional counseling without whey protein supplementation |
| Studies evaluating the effect of amino acids supplementation | ||||||||
| Katada et al. [17] | 2020 | Japan | RCT | Evaluate the effect of amino acid supplementation in form of “elemental diet” on chemotherapy toxicity | Adult patients with esophageal carcinoma, candidate to receive chemotherapy, and able to orally intake an elemental diet | Patients with a history of hypersensitivity to elemental diet | Mixture of amino acid supplements for 9 weeks after the start of chemotherapy | Patients in the control group did not receive any oral supplementation |
| D.P. Soares et al. [18] | 2024 | Brazil | RCT | Evaluate the effect of L-Leucine supplementation on nutritional status evaluated with body weight and body composition | Patients older than 60 years and candidates to receive chemotherapy | Patients younger than 60 years old, with mental disorders or cognitive or walking disabilities; patients undergoing artificial nutrition (enteral or parenteral) | Amino acid supplements (L-leucine) and nutritional counseling | Placebo |
| Toyomasu et al. [19] | 2019 | Japan | RCT | Evaluate the effect of amino acid supplementation on chemotherapy-induced oral mucositis or diarrhea | Adult patients with gastric cancer who had curative resection and are candidates to receive chemotherapy | Patients with severe heart disease, interstitial pneumonia or pulmonary fibrosis, bleeding tendency, liver cirrhosis or active hepatitis, chronic renal failure, severe diabetes and severe drug allergy | Mixture of amino acid supplements | Patients in the control group did not receive any oral supplementation |
| Tanaka et al., 2016 [20] | 2016 | Japan | RCT | Evaluate the effect of amino acid supplementation in form of “elemental diet” with glutamine for prevention of chemotherapy-induced oral mucositis | Adult patients with esophageal carcinoma and candidates to receive chemotherapy | Patients previously treated with chemotherapy for malignant disease or irradiation to major bone areas, patients with serious concomitant illness, symptomatic infectious disease, severe drug allergy, symptomatic peripheral neuropathy, or uncontrolled diabetes mellitus | Mixture of amino acid supplements with glutamine; glutamine supplements alone | Patients in the control group did not receive any oral supplementation |
| Khemissa et al. [22] | 2016 | France | RCT | Evaluate the effect of amino acid supplementation and transforming growth factor-beta 2 (the so-called “immune nutrition”) for chemotherapy-induced non-hematological toxicities | Adult patients with esophageal carcinoma and candidate to chemotherapy | Patients previously treated with chemotherapy for malignant disease or irradiation to major bone areas, patients with serious concomitant illness, symptomatic infectious disease, severe drug allergy, symptomatic peripheral neuropathy, or uncontrolled diabetes mellitus. | Amino acids supplements in form of “immunonutrition” containing TGF-beta and glutamine | Placebo |
| Okada et al. [21] | 2017 | Japan | RCT | Evaluate the effect of amino acid supplementation in form of “elemental diet” on chemotherapy-induced oral mucositis and diarrhea | Adult patients with esophagus carcinoma candidate to receive chemotherapy and without history of oral complications or immunodeficiency before chemotherapy | Patients simultaneously undergoing chemotherapy and radiation therapy | Amino acid supplements for 14 days and during chemotherapy | Patients in the control group did not receive any oral supplementation |
| Tanaka et al., 2021 [24] | 2021 | Japan | RCT | Evaluate the effect of amino acid supplementation in form of “elemental diet” on chemotherapy-induced oral mucositis | Adult patients with esophagus carcinoma and candidate to receive chemotherapy | Patients with symptomatic infectious disease, symptomatic peripheral neuropathy, patients with serious concomitant illness, patients with symptomatic bone or brain metastases, patients diagnosed with oral mucositis at registration | Mixture of amino acid supplements for 56 days during chemotherapy | Patients in the control group did not receive any oral supplementation |
| Studies evaluating the effect of fish oil omega-3-enriched oral supplements | ||||||||
| Shirai et al. [23] | 2017 | Japan | Retrospective cohort study | Evaluate the effect of fish oil omega-3-enriched oral supplements on chronological alterations in biochemical and physiological status during chemotherapeutic treatment | Adult patients with a clinical diagnosis of gastrointestinal cancer and candidate to receive chemotherapy | Not available | One or two packs of fish oil omega-3-enriched oral supplements per day for six months during chemotherapy | No additional nutritional treatment |
| Author | Age (Years) | Gender Male (%) | BMI | Weight Gain | Cancer Site | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control Group | Intervention Group | Control Group | Intervention Group | Control Group | Intervention Group | Control Group | Intervention Group | ||
| Studies evaluating the effect of multimodal intervention | |||||||||
| Bourdel-Marchasson et al. [25] | 78.3 ± 4.7 | 77.7 ± 5.2 | 91 (54.5) | 81 (47.9) | NA | NA | 4 | 5 | Lymphoma or carcinoma (not specified) |
| Studies evaluating the effect of whey protein supplementation | |||||||||
| Cereda et al. [26] | 65.7 ± 11.4 | 65.1 ± 11.7 | 49 (58.3) | 47 (57.3) | 22.3 ± 3.9 | 22 ± 4.1 | 0.7 (4.2) | 1 (4.1) | Lung, stomach, esophagus, pancreas, colon, blood, breast, and head–neck cancer |
| Studies evaluating the effect of amino acid supplementation | |||||||||
| Katada et al. [17] | 66.7 ± 5.0 | 67.8 ± 4.8 | 29 (82.9) | 30 (83.3) | 20.0 ± 2.9 | 20.7 ± 2.3 | 0.97 (5.4) | 0.99 (3.9) | Esophageal cancer |
| D.P. Soares et al. [18]. | 65.00 ± 7.23 | 65.22 ± 8.19 | 18 (100%) | 18 (100%) | 22.41 ± 3.64 | 22.34 ± 2.79 | 0.22 | 2.27 | Gastrointestinal and hepato-biliary-pancreatic cancer |
| Toyomasu et al. [19] | 67.1 (59–80) | 68.4 (61–80) | 8 (73%) | 9 (82%) | NA | NA | NA | NA | Gastric cancer |
| Tanaka et al. [20] | 68 (49–82) | 75 (58–83) | 9 (90%) | 10 (100%) | 21.75 (18.37–25.59) | 21.08 (14.60–24.20) | -5.40 | 1.70 | Esophageal cancer |
| Khemissa et al. [22] | 66 (60–75) | 68 (61–74) | 67 (66%) | 63 (64%) | 24.2 (22.0–27.2) | 24 (21.0–26.1) | NA | NA | Gastrointestinal and hepato-biliary-pancreatic cancer |
| Okada et al. [21] | 67.1 | 65.3 | 8 (80%) | 9 (90%) | 22.1 | 21.4 | NA | NA | Esophageal cancer |
| Tanaka et al. [24] | 68 (44, 86) | 68 (34, 83) | 50 (86) | 43 (78) | NA | NA | NA | NA | Esophageal cancer |
| Studies evaluating the effect of fish oil omega-3-enriched oral supplements | |||||||||
| Shirai et al. [23] | 68.9 ± 10.3 | 72.3 ± 8.4 | 64 (70%) | 26 (70%) | 21.7 ± 3.4 | 21.2 ± 2.9 | NA | NA | Gastrointestinal and hepato-biliary-pancreatic cancer |
| Author | Chemotherapy Adherence | Chemotherapy Toxicity | Overall Survival | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group | Intervention Group | OR | IC | p Value | Control Group | Intervention Group | OR | IC | p Value | Chemotherapy Toxicity | Control Group | Intervention Group | OR | IC | p Value | |
| Studies evaluating the effect of multimodal intervention | ||||||||||||||||
| Bourdel-Marchasson et al. [25] | NA | NA | NA | NA | NA | 7 (10.4%) | 7 (4.2%) | NA | NA | 0.03 | Infectious toxicities | NA | NA | NA | NA | NA |
| Studies evaluating the effect of whey protein supplementation | ||||||||||||||||
| Cereda et al. [26] | NA | NA | NA | NA | NA | 83 (98.8%) | 73 (89%) | −9.8 | −16.9~−2.6 | 0.009 | Any chemotherapy toxicity | NA | NA | NA | NA | NA |
| Studies evaluating the effect of amino acid supplementation | ||||||||||||||||
| Katada et al. [17] | NA | NA | NA | NA | NA | 25 (71.4%) | 30 (83.3%) | NA | NA | NA | Gastrointestinal toxicities | NA | NA | NA | NA | NA |
| D.P. Soares et al. [18] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Toyomasu et al. [19] | NA | NA | NA | NA | NA | 3 (27.3%) | 1 (9.1%) | NA | NA | NA | Gastrointestinal toxicities | NA | NA | NA | NA | NA |
| Tanaka et al. [20] | 8 (80%) | 8 (80%) | NA | NA | NA | 6 (60%) | 1 (10%) | 0.1 | 0.0–0.6 | 0.02 | Gastrointestinal toxicities | NA | NA | NA | NA | NA |
| Khemissa et al. [22] | 88 (86%) | 83 (84%) | NA | NA | NA | 92 (90%) | 88 (89%) | NA | NA | 0.82 | Any chemotherapy toxicity | NA | NA | NA | NA | NA |
| Okada et al. [21] | NA | NA | NA | NA | NA | 8 (80%) | 4 (40%) | NA | NA | 0.02 | Gastrointestinal toxicities | NA | NA | NA | NA | NA |
| Tanaka et al. [24] | NA | NA | NA | NA | NA | 20 (34%) | 8 (15%) | NA | NA | 0.0141 | Gastrointestinal toxicities | NA | NA | NA | NA | NA |
| Studies evaluating the effect of fish oil omega-3-enriched oral supplements | ||||||||||||||||
| Shirai et al. [23] | 5 | 8 | NA | NA | 0.05 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vella, R.; Pizzocaro, E.; Bannone, E.; Gualtieri, P.; Frank, G.; Giardino, A.; Frigerio, I.; Pastorelli, D.; Gruttadauria, S.; Mazzali, G.; et al. Nutritional Intervention for the Elderly during Chemotherapy: A Systematic Review. Cancers 2024, 16, 2809. https://doi.org/10.3390/cancers16162809
Vella R, Pizzocaro E, Bannone E, Gualtieri P, Frank G, Giardino A, Frigerio I, Pastorelli D, Gruttadauria S, Mazzali G, et al. Nutritional Intervention for the Elderly during Chemotherapy: A Systematic Review. Cancers. 2024; 16(16):2809. https://doi.org/10.3390/cancers16162809
Chicago/Turabian StyleVella, Roberta, Erica Pizzocaro, Elisa Bannone, Paola Gualtieri, Giulia Frank, Alessandro Giardino, Isabella Frigerio, Davide Pastorelli, Salvatore Gruttadauria, Gloria Mazzali, and et al. 2024. "Nutritional Intervention for the Elderly during Chemotherapy: A Systematic Review" Cancers 16, no. 16: 2809. https://doi.org/10.3390/cancers16162809
APA StyleVella, R., Pizzocaro, E., Bannone, E., Gualtieri, P., Frank, G., Giardino, A., Frigerio, I., Pastorelli, D., Gruttadauria, S., Mazzali, G., di Renzo, L., & Butturini, G. (2024). Nutritional Intervention for the Elderly during Chemotherapy: A Systematic Review. Cancers, 16(16), 2809. https://doi.org/10.3390/cancers16162809









