Patients with TP53-Mutated Acute Myeloid Leukemia Receiving Intensive Induction Therapy Have Superior Outcomes Due to a Higher Rate of Salvage Therapy: A Single Institution, Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design, Patients, and Treatment Characteristics
2.2. Outcomes and Statistical Analyses
3. Results
3.1. Demographic, Disease, and Clinical Characteristics
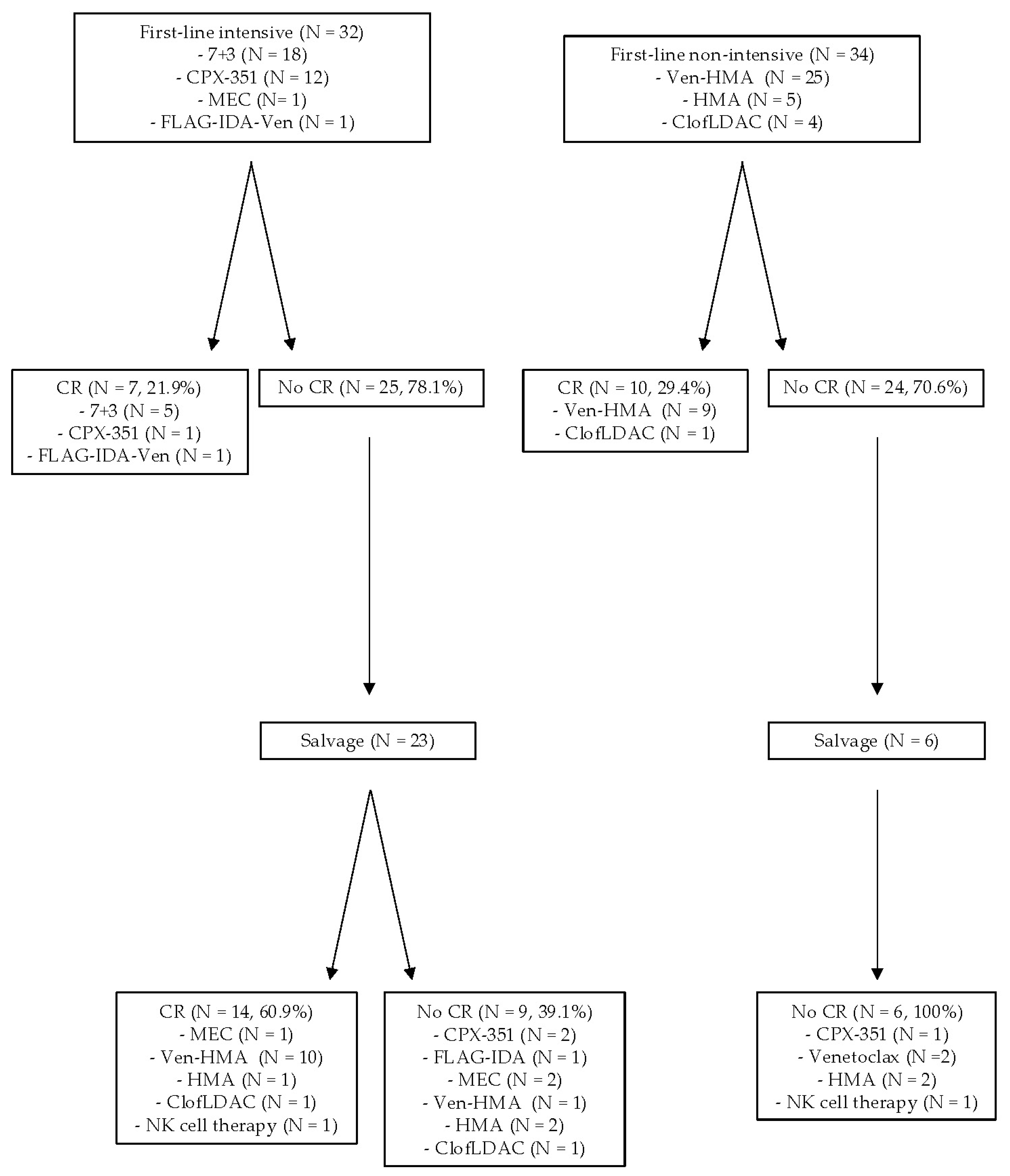
3.2. Treatment Patterns, Outcomes, and Survival of TP53m AML
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbosa, K.; Li, S.; Adams, P.D.; Deshpande, A.J. The role of TP53 in acute myeloid leukemia: Challenges and opportunities. Genes Chromosomes Cancer 2019, 58, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Kadia, T.M.; Jain, P.; Ravandi, F.; Garcia-Manero, G.; Andreef, M.; Takahashi, K.; Borthakur, G.; Jabbour, E.; Konopleva, M.; Daver, N.G.; et al. TP53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer 2016, 122, 3484–3491. [Google Scholar] [CrossRef]
- Metzeler, K.H.; Herold, T.; Rothenberg-Thurley, M.; Amler, S.; Sauerland, M.C.; Görlich, D.; Schneider, S.; Konstandin, N.P.; Dufour, A.; Bräundl, K.; et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 2016, 128, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.R.; Holland, J.F.; Glidewell, O.J.; Weinberg, V.; Brunner, K.; Obrecht, J.P.; Preisler, H.D.; Nawabi, I.W.; Prager, D.; Carey, R.W.; et al. Treatment of acute myelocytic leukemia: A study by cancer and leukemia group B. Blood 1981, 58, 1203–1212. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; Bixby, D.L.; et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021, 8, e481–e491. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Maiti, A.; Rausch, C.R.; Pemmaraju, N.; Naqvi, K.; Daver, N.G.; Kadia, T.M.; Borthakur, G.; Ohanian, M.; Alvarado, Y.; et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: A single-centre, phase 2 trial. Lancet Haematol. 2020, 7, e724–e736. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, C.D.; Pratz, K.W.; Letai, A.; Jonas, B.A.; Wei, A.H.; Thirman, M.; Arellano, M.; Frattini, M.G.; Kantarjian, H.; Popovic, R.; et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol. 2018, 19, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Arcese, W.; Isacchi, G.; Meloni, G.; Petti, M.C.; Monarca, B.; Testi, A.M.; Mandelli, F. Mitoxantrone, etoposide, and intermediate-dose cytarabine: An effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J. Clin. Oncol. 1991, 9, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Lachowiez, C.A.; Takahashi, K.; Loghavi, S.; Xiao, L.; Kadia, T.; Daver, N.; Adeoti, M.; Short, N.J.; Sasaki, K.; et al. Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2021, 39, 2768–2778. [Google Scholar] [CrossRef]
- Parker, J.E.; Pagliuca, A.; Mijovic, A.; Cullis, J.O.; Czepulkowski, B.; Rassam, S.M.; Samaratunga, I.R.; Grace, R.; Gover, P.A.; Mufti, G.J. Fludarabine, cytarabine, G-CSF and idarubicin (FLAG-IDA) for the treatment of poor-risk myelodysplastic syndromes and acute myeloid leukaemia. Br. J. Haematol. 1997, 99, 939–944. [Google Scholar] [CrossRef]
- Faderl, S.; Ravandi, F.; Huang, X.; Wang, X.; Jabbour, E.; Garcia-Manero, G.; Kadia, T.; Ferrajoli, A.; Konopleva, M.; Borthakur, G.; et al. Clofarabine plus low-dose cytarabine followed by clofarabine plus low-dose cytarabine alternating with decitabine in acute myeloid leukemia frontline therapy for older patients. Cancer 2012, 118, 4471–4477. [Google Scholar] [CrossRef]
- Daver, N.G.; Iqbal, S.; Renard, C.; Chan, R.J.; Hasegawa, K.; Hu, H.; Tse, P.; Yan, J.; Zoratti, M.J.; Xie, F.; et al. Treatment outcomes for newly diagnosed, treatment-naïve TP53-mutated acute myeloid leukemia: A systematic review and meta-analysis. J. Hematol. Oncol. 2023, 16, 19. [Google Scholar] [CrossRef]
- Lindsley, R.C.; Gibson, C.J.; Murdock, H.M.; Stone, R.M.; Cortes, J.E.; Uy, G.L.; Lin, T.L.; Ritchie, E.K.; Prebet, T.; Ryan, R.J.; et al. Genetic Characteristics and Outcomes By Mutation Status in a Phase 3 Study of CPX-351 Versus 7+3 in Older Adults with Newly Diagnosed, High-Risk/Secondary Acute Myeloid Leukemia (AML). Blood 2019, 134, 15. [Google Scholar] [CrossRef]
- Daver, N.G.; Iqbal, S.; Huang, J.; Renard, C.; Lin, J.; Pan, Y.; Williamson, M.; Ramsingh, G. Clinical characteristics and overall survival among acute myeloid leukemia patients with TP53 gene mutation or chromosome 17p deletion. Am. J. Hematol. 2023, 98, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zarif, M.; Zhou, Q.; Capo-Chichi, J.M.; Schuh, A.; Minden, M.D.; Atenafu, E.G.; Kumar, R.; Chang, H. TP53 Mutations in AML Patients Are Associated with Dismal Clinical Outcome Irrespective of Frontline Induction Regimen and Allogeneic Hematopoietic Cell Transplantation. Cancers 2023, 15, 3210. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Tashakori, M.; Kadia, T.; Loghavi, S.; Daver, N.; Kanagal-Shamanna, R.; Pierce, S.; Sui, D.; Wei, P.; Khodakarami, F.; Tang, Z.; et al. TP53 copy number and protein expression inform mutation status across risk categories in acute myeloid leukemia. Blood 2022, 140, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Haferlach, T.; Baer, C.; Hutter, S.; Meggendorfer, M.; Kern, W.; Haferlach, C. Specific subtype distribution with impact on prognosis of TP53 single-hit and double-hit events in AML and MDS. Blood Adv. 2023, 7, 2952–2956. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Baughn, L.B.; Myers, C.L.; Sachs, Z. Machine learning analysis of gene expression reveals TP53 Mutant-like AML with wild type TP53 and poor prognosis. Blood Cancer J. 2024, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Grob, T.; Al Hinai, A.S.A.; Sanders, M.A.; Kavelaars, F.G.; Rijken, M.; Gradowska, P.L.; Biemond, B.J.; Breems, D.A.; Maertens, J.; van Marwijk Kooy, M.; et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 2022, 139, 2347–2354. [Google Scholar] [CrossRef]
- Fernandez, H.F.; Sun, Z.; Yao, X.; Litzow, M.R.; Luger, S.M.; Paietta, E.M.; Racevskis, J.; Dewald, G.W.; Ketterling, R.P.; Bennett, J.M.; et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.S.; Petti, A.A.; Miller, C.A.; Fronick, C.C.; O’Laughlin, M.; Fulton, R.S.; Wilson, R.K.; Baty, J.D.; Duncavage, E.J.; Tandon, B.; et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N. Engl. J. Med. 2016, 375, 2023–2036. [Google Scholar] [CrossRef]
- Short, N.J.; Montalban-Bravo, G.; Hwang, H.; Ning, J.; Franquiz, M.J.; Kanagal-Shamanna, R.; Patel, K.P.; DiNardo, C.D.; Ravandi, F.; Garcia-Manero, G.; et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv. 2020, 4, 5681–5689. [Google Scholar] [CrossRef]
- Badar, T.; Atallah, E.; Shallis, R.M.; Goldberg, A.D.; Patel, A.; Abaza, Y.; Bewersdorf, J.P.; Saliba, A.N.; Correia, G.S.C.; Murthy, G.; et al. Outcomes of TP53-mutated AML with evolving frontline therapies: Impact of allogeneic stem cell transplantation on survival. Am. J. Hematol. 2022, 97, E232–E235. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Pratz, K.W.; Wei, A.H.; Pullarkat, V.; Jonas, B.A.; Recher, C.; Babu, S.; Schuh, A.C.; Dail, M.; Sun, Y.; et al. Outcomes in Patients with Poor-Risk Cytogenetics with or without TP53 Mutations Treated with Venetoclax and Azacitidine. Clin. Cancer Res. 2022, 28, 5272–5279. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Shoukier, M.; Konopleva, M.; Dinardo, C.D.; Ravandi, F.; Short, N.J.; Andreeff, M.; Borthakur, G.; Daver, N.; Pemmaraju, N.; et al. Outcomes in patients with newly diagnosed TP53-mutated acute myeloid leukemia with or without venetoclax-based therapy. Cancer 2021, 127, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Badar, T.; Atallah, E.; Shallis, R.; Saliba, A.N.; Patel, A.; Bewersdorf, J.P.; Grenet, J.; Stahl, M.; Duvall, A.; Burkart, M.; et al. Survival of TP53-mutated acute myeloid leukemia patients receiving allogeneic stem cell transplantation after first induction or salvage therapy: Results from the Consortium on Myeloid Malignancies and Neoplastic Diseases (COMMAND). Leukemia 2023, 37, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Gabdoulline, R.; Löffeld, P.; Dobbernack, V.; Kreimeyer, H.; Pankratz, M.; Flintrop, M.; Liebich, A.; Klesse, S.; Panagiota, V.; et al. Individual outcome prediction for myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia from MDS after allogeneic hematopoietic cell transplantation. Ann. Hematol. 2017, 96, 1361–1372. [Google Scholar] [CrossRef]
- Chua, C.C.; Gómez-De León, A. Time to define and refine maintenance strategies in acute myeloid leukaemia. Lancet Haematol. 2024, 11, e246–e247. [Google Scholar] [CrossRef]

| All Groups | TP53m | TP53wt | p-Value | |
|---|---|---|---|---|
| N | 423 | 86 (20.3%) | 337 (79.7%) | |
| Age at diagnosis | 65.3 (18.7–92.9) | 67.3 (28.9–87.1) | 64.4 (18.7–92.9) | 0.01 |
| Gender | ||||
| Male | 252 (59.6%) | 57 (66.3%) | 195 (57.9%) | 0.16 |
| Female | 171 (40.4%) | 29 (33.7%) | 142 (42.1%) | |
| Therapy-related AML | 42 (9.9%) | 19 (22.1%) | 23 (6.8%) | <0.01 |
| Secondary AML | 142 (33.6%) | 44 (51.2%) | 98 (29.1%) | <0.01 |
| ELN2022 risk category (N = 302) | ||||
| Favorable | 11 (2.6%) | 0 | 11 (3.3%) | <0.01 |
| Intermediate | 59 (14.0%) | 0 | 59 (17.5%) | |
| Adverse | 232 (54.9%) | 86 (100%) | 146 (43.3%) | |
| TP53m VAF, median (N = 70) | 0.65 (0.18–0.94) | NA | ||
| 0.1–0.4 | 23 (32.9%) | |||
| >0.4 | 47 (67.1%) | |||
| TP53 allelic state (N = 69) | ||||
| Biallelic TP53 alteration * | 56 (81.2%) | NA | ||
| Biallelic TP53 alteration (normalized) ** | 65 (94.2%) | |||
| TP53 mutations (total = 96) | ||||
| DNA-binding domains (DBD) | 78 (81.2%) | NA | ||
| Missense | 70 (72.9%) | |||
| Truncation, Frameshift, Deletion | 8 (8.3%) | |||
| Non-DBD (other domains) | 18 (18.8%) | |||
| Splice donor/acceptor sites | 6 (6.3%) | |||
| Cytogenetics, TP53m AML (N = 84) | NA | |||
| Normal cytogenetics | 16 (19%) | |||
| Complex karyotype | 56 (66.7%) | |||
| Monosomal karyotype | 43 (51.2%) | |||
| −5 or del(5q) | 33 (39.3%) | |||
| −7 | 22 (26.2%) | |||
| −17 or abn(17p) | 20 (23.8%) | |||
| ELN2022 cytogenetic risk category, TP53m AML (N = 84) | NA | |||
| Favorable | 0 | |||
| Intermediate | 27 (32.1%) | |||
| Adverse | 57 (67.9%) | |||
| Co-mutations | ||||
| FLT3 | 61 (14.4%) | 3 (3.5%) | 58 (17.2%) | <0.01 |
| IDH1/IDH2 | 76 (18.0%) | 4 (4.7%) | 72 (21.4%) | <0.01 |
| NPM1 | 57 (13.5%) | 1 (1.2%) | 56 (16.6%) | <0.01 |
| RUNX1 | 37 (8.8%) | 0 (0%) | 37 (11.0%) | 0.01 |
| ASXL1 | 102 (24.1%) | 14 (16.3%) | 88 (26.1%) | <0.01 |
| KRAS/NRAS | 83 (19.6%) | 10 (11.6%) | 73 (21.7%) | 0.47 |
| KIT | 13 (3.1%) | 1 (1.2%) | 12 (3.6%) | 0.31 |
| Baseline BM blast (%) | 50 (1–98) | 33 (1–94) | 56 (9–98) | <0.01 |
| Baseline Hgb (g/dL) | 8.5 (2.2–14.6) | 8.3 (5.0–14.5) | 8.6 (2.2–14.6) | 0.48 |
| Baseline WBC (109 cells/L) | 3.2 (0.1–329.9) | 3.1 (0.1–82.8) | 3.2 (0.1–329.9) | 0.60 |
| Baseline peripheral blast (%) | 13.8 (0–100) | 9 (0–78) | 15.2 (0.7–100) | 0.051 |
| Peripheral blast count (109 cells/L) | 0.6 (0–300.2) | 0.2 (0–31.7) | 0.7 (0–300.2) | 0.08 |
| Baseline ANC (109 cells/L) | 0.2 (0–38.2) | 0.4 (0–23.8) | 0.5 (0–38.2) | 0.41 |
| Baseline platelet count (109 cells/L) | 61 (4–1019) | 47 (7–1019) | 64 (4–867) | 0.26 |
| DIC at presentation | 18/227 (7.9%) | 5/45 (11.1%) | 13/182 (7.1%) | 0.38 |
| TLS at presentation | 11/181 (6%) | 2/36 (5.6%) | 7/145 (4.8%) | 0.25 |
| Hyperleukocytosis at presentation | 5/334 (1.5%) | 0/63 (0%) | 5/271 (1.8%) | 0.28 |
| Hazard Ratio (95%CI) | p-Value | |
|---|---|---|
| First-line intensive | 1.00 | 0.07 |
| First-line non-intensive | 1.76 (0.96–3.23) | |
| ECOG 0–1 | 1.00 | 0.83 |
| ECOG ≥ 2 | 1.07 (0.57–2.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumransub, N.; Steinwand, G.K.; Cordner, K.; Lee, Y.; Cao, Q.; Allred, J.; Bachanova, V.; Juckett, M.; Eckfeldt, C.; Maakaron, J.E.; et al. Patients with TP53-Mutated Acute Myeloid Leukemia Receiving Intensive Induction Therapy Have Superior Outcomes Due to a Higher Rate of Salvage Therapy: A Single Institution, Retrospective Study. Cancers 2024, 16, 2784. https://doi.org/10.3390/cancers16162784
Sumransub N, Steinwand GK, Cordner K, Lee Y, Cao Q, Allred J, Bachanova V, Juckett M, Eckfeldt C, Maakaron JE, et al. Patients with TP53-Mutated Acute Myeloid Leukemia Receiving Intensive Induction Therapy Have Superior Outcomes Due to a Higher Rate of Salvage Therapy: A Single Institution, Retrospective Study. Cancers. 2024; 16(16):2784. https://doi.org/10.3390/cancers16162784
Chicago/Turabian StyleSumransub, Nuttavut, Gabriel K. Steinwand, Keith Cordner, Yoonkyu Lee, Qing Cao, Jeremy Allred, Veronika Bachanova, Mark Juckett, Craig Eckfeldt, Joseph E. Maakaron, and et al. 2024. "Patients with TP53-Mutated Acute Myeloid Leukemia Receiving Intensive Induction Therapy Have Superior Outcomes Due to a Higher Rate of Salvage Therapy: A Single Institution, Retrospective Study" Cancers 16, no. 16: 2784. https://doi.org/10.3390/cancers16162784
APA StyleSumransub, N., Steinwand, G. K., Cordner, K., Lee, Y., Cao, Q., Allred, J., Bachanova, V., Juckett, M., Eckfeldt, C., Maakaron, J. E., Tracy, S. I., Ramesh, V., Nelson, A. C., Yohe, S., & Sachs, Z. (2024). Patients with TP53-Mutated Acute Myeloid Leukemia Receiving Intensive Induction Therapy Have Superior Outcomes Due to a Higher Rate of Salvage Therapy: A Single Institution, Retrospective Study. Cancers, 16(16), 2784. https://doi.org/10.3390/cancers16162784





