Therapeutic Senolysis of Axitinib-Induced Senescent Human Lung Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Reagents, and Mouse Model
2.2. Cell Viability Assay
2.3. Flow Cytometric Analysis
2.4. Measurement of Reactive Oxygen Species (ROS)
2.5. Photography
2.6. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Cell Proliferation Assay
2.8. Immunoblot Assay
2.9. In Vivo Mouse Model
2.10. Apoptosis Detection in Tumor Tissue
2.11. Detection of Senescence-Associated β-Gal on Tumor Tissues
2.12. Statistical Analyses
3. Results
3.1. Variable Sensitivity to TKIs among the Three Human Lung Cancer Cell Lines
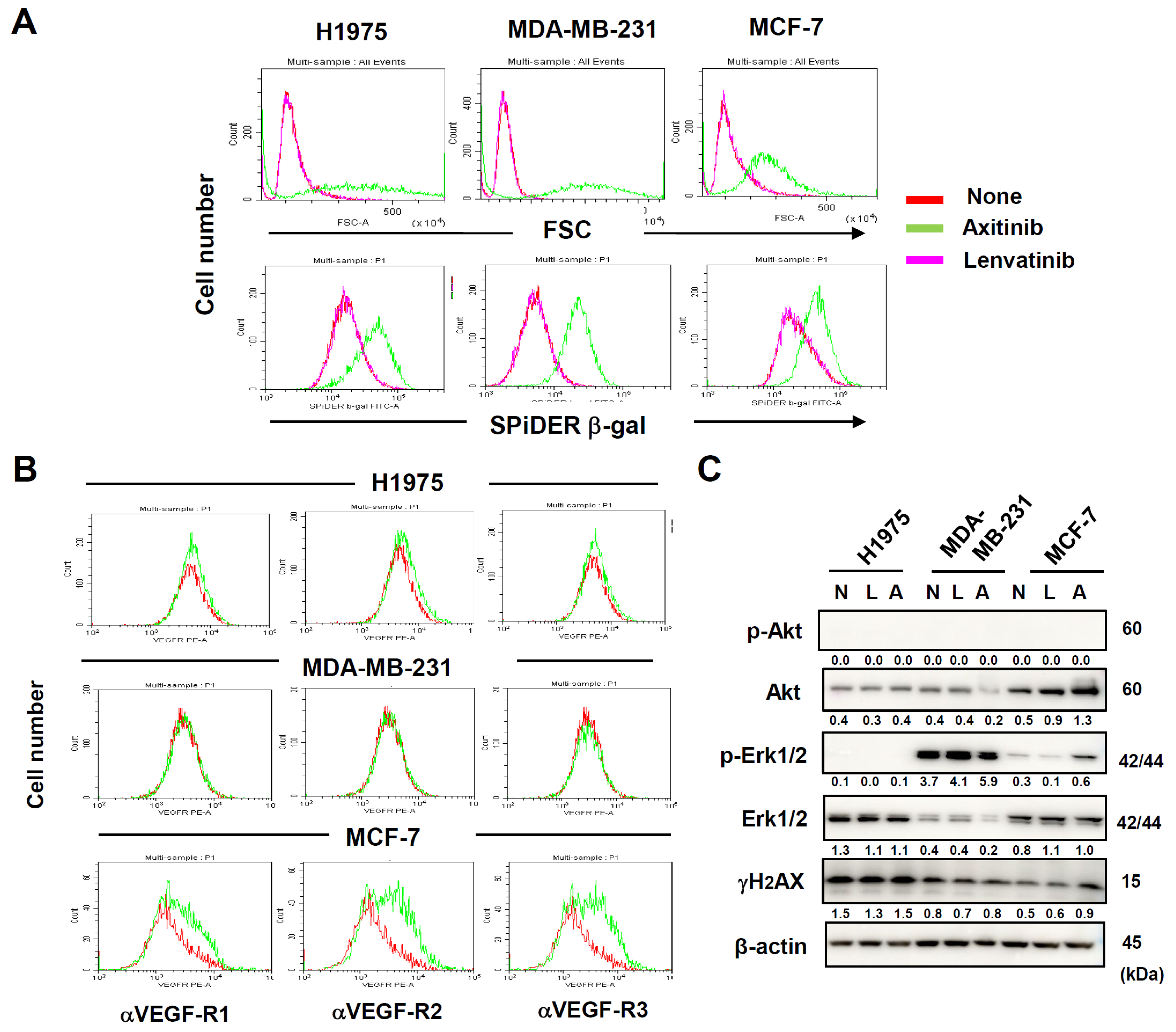
3.2. Axitinib-Induced Senescence in Human Cancer Cell Lines Independently of Their Expression of VEGFRs
3.3. Axitinib Induced SASP and Growth Arrest in A549 Cells
3.4. Drastic Senolysis of Axitinib-Induced Senescent A549 Cells
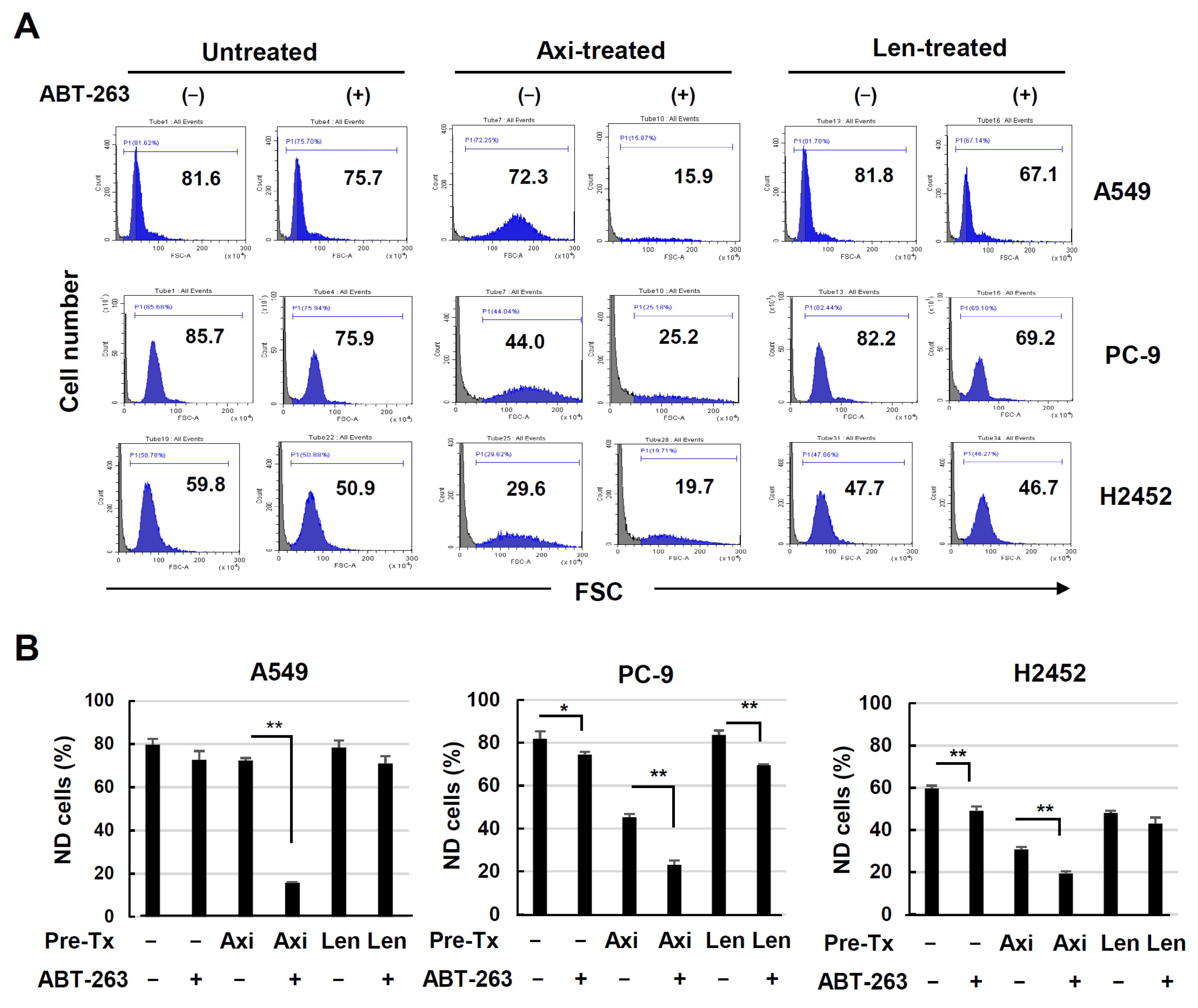
3.5. Senolysis of ABT-263 in Axitinib- or Lenvatinib-Treated Lung Cancer Cells
3.6. Senolysis of Axitinib-Induced Senescent A549 Cells Is Dependent on Apoptosis and Bcl-xL Inhibition
3.7. Combined Effects of Axitinib and ABT-263 in A549-Xenografted Nude Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wu, J.; Shang, B.; Bi, X.; Jiang, W.; Cao, C.; Zhou, A.; Shi, H.; Shou, J. Optimizing targeted drug selection in combination therapy for patients with advanced or metastatic renal cell carcinoma: A systematic review and network meta-analysis of safety. Cancer Med. 2023, 12, 7051–7064. [Google Scholar] [CrossRef] [PubMed]
- Sonpavde, G.; Hutson, T.E.; Rini, B.I. Axitinib for renal cell carcinoma. Expert Opin. Investig. Drugs 2008, 17, 741–748. [Google Scholar]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Robbins, P.B.; Powles, T.; Albiges, L.; Haanen, J.B.; Larkin, J.; Mu, X.J.; Ching, K.A.; Uemura, M.; Pal, S.K.; et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat. Med. 2020, 26, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Yan, X.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Li, S.; Mao, L.; Lian, B.; Wang, X.; et al. Axitinib in combination with toripalimab, a humanized immunoglobulin G4 monoclonal antibody against programmed cell death-1, in patients with metastatic mucosal melanoma: An open-label phase IB trial. J. Clin. Oncol. 2019, 37, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells aging and cancer. Nat. Rev. Mol. Cell. Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Fridman, J.S.; Yang, M.; Lee, S.; Baranov, E.; Hoffman, R.M.; Lowe, S.W. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002, 109, 335–346. [Google Scholar] [CrossRef]
- Chakradeo, S.; Elmore, L.W.; Gewirtz, D.A. Is senescence reversible? Curr. Drug Targets 2016, 17, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Tyutyunyk-Massey, L.; Gewirtz, D.A. Tumor Cell Escape from Therapy-Induced Senescence as a Model of Disease Recurrence after Dormancy. Cancer Res. 2019, 79, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Bloukh, S.; Carpenter, V.J.; Alwohoush, E.; Bakeer, J.; Darwish, S.; Azab, B.; Gewirtz, D.A. Therapy-Induced Senescence: An “Old” Friend Becomes the Enemy. Cancers 2020, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gil, J. Mechanisms and Functions of Cellular Senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Battram, A.M.; Bachiller, M.; Beatriz, M.-A.B. Senescence in the Development and Response to Cancer with Immunotherapy: A Double-Edged Sword. Int. J. Mol. Sci. 2020, 21, 4346. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, V.J.; Saleh, T.; Gewirtz, D.A. Senolytics for cancer therapy: Is all that glitters really gold? Cancers 2021, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Short, S.; Fielder, E.; Miwa, S.; von Zglinicki, T. Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine 2019, 41, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Sieben, C.J.; Sturmlechner, I.; Van de Sluis, B.; van Deursen, J.M. Two-step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018, 28, 723–737. [Google Scholar] [CrossRef]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef]
- Saleh, T.; Carpenter, V.J.; Tyutyunyk-Massey, L.; Murray, G.; Leverson, J.D.; Souers, A.J.; Alotaibi, M.R.; Faber, A.C.; Reed, J.; Harada, H.; et al. Clearance of therapy-induced senescent tumor cells by the senolytic ABT-263 via interference with BCL-XL-BAX interaction. Mol. Oncol. 2020, 14, 2504–2519. [Google Scholar] [CrossRef] [PubMed]
- Kartika, I.D.; Kotani, H.; Iida, Y.; Koyanagi, A.; Tanino, R.; Harada, M. Protective role of cytoplasmic p21Cip1/Waf1 in apoptosis of CDK4/6 inhibitor-treated senescence in breast cancer cells. Cancer Med. 2021, 10, 8988–8999. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, A.; Kotani, H.; Iida, Y.; Tanino, R.; Kartika, I.D.; Kishimoto, K.; Harada, M. Protective roles of cytoplasmic p21Cip1/Waf1 in senolysis and ferroptosis of lung cancer cells. Cell Prolif. 2022, 30, e13326. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Heukamp, L.C.; Siobal, M.; Schöttle, J.; Wieczorek, C.; Peifer, M.; Frasca, D.; Koker, M.; König, K.; Meder, L.; et al. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J. Clin. Investig. 2013, 123, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhou, X.; Liang, C.; Li, X.; Ge, M.; Chen, Y.; Yin, J.; Zhu, J.; Zhong, C. Apatinib triggers autophagic and apoptotic cell death via VEGFR2/STAT3/PD-L1 and ROS/Nrf2/p62 signaling in lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 266. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, T.; Kotani, H.; Iida, Y.; Koyanagi, A.; Tanino, R.; Tsubata, Y.; Isobe, T.; Harada, M. Pemetrexed sensitizes human lung cancer cells to cytotoxic immune cells. Cancer Sci. 2020, 111, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lowe, D.D.; Zou, H.Y.; Grazzini, M.L.; Hallin, M.E.; Wickman, G.R.; Amundson, K.; Chen, J.H.; Rewolinski, D.A.; Yamazaki, S.; Wu, E.Y.; et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin. Cancer Res. 2008, 14, 7272–7283. [Google Scholar] [CrossRef] [PubMed]
- Babaei, Z.; Panjehpour, M.; Parsian, H.; Aghaei, M. SAR131675 exhibits anticancer activity on human ovarian cancer cells through inhibition of VEGFR-3/ERK1/2/AKT signaling pathway. Cell Signal 2023, 111, 110856. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Santoni, M.; Soriani, A.; Nabissi, M.; Cardinali, C.; Santoni, A.; Santoni, G. Axitinib induces DNA damage response leading to senescence, mitotic catastrophe, and increased NK cell recognition in human renal carcinoma cells. Oncotarget 2015, 6, 36245–36259. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Nabissi, M.; Cardinali, C.; Santoni, M.; Bernardini, G.; Santoni, A.; Santoni, G. Axitinib induces senescence-associated cell death and necrosis in glioma cell lines: The proteasome inhibitor, bortezomib, potentiates axitinib-induced cytotoxicity in a p21(Waf/Cip1) dependent manner. Oncotarget 2017, 8, 3380–3395. [Google Scholar] [CrossRef] [PubMed]
- Mongiardi, M.P.; Radice, G.; Piras, M.; Stagni, V.; Pacioni, S.; Re, A.; Putti, S.; Ferrè, F.; Farsetti, A.; Pallini, R.; et al. Axitinib exposure triggers endothelial cells senescence through ROS accumulation and ATM activation. Oncogene 2019, 38, 5413–5424. [Google Scholar] [CrossRef] [PubMed]
- Sausville, E.A.; Peisach, J.; Horwitz, S.B. Effect of chelating agents and metal ions on the degradation of DNA by bleomycin. Biochemistry 1978, 17, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Decker, A.; Chow, M.S.; Kemsley, J.N.; Lehnert, N.; Solomon, E.I. Direct hydrogen-atom abstraction by activated bleomycin: An experimental and computational study. J. Am. Chem. Soc. 2006, 128, 4719–4733. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-asso ciated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Congiargiu, A.; Chen, Z.; Zinellu, A.; Carru, C.; Medici, S. Ferroptosis and Senescence: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3658. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Trends Cancer Biol. 2016, 26, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Larson, T.; Ou, S.-H.I.; Limentani, S.; Sandler, A.; Vokes, E.; Kim, S.; Liau, K.; Bycott, P.; Olszanski, A.J.; et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: Results from a phase II study. J. Clin. Oncol. 2009, 27, 3836–3841. [Google Scholar] [CrossRef] [PubMed]
- Kozloff, M.F.; Martin, L.P.; Krzakowski, M.; Samuel, T.A.; Rado, T.A.; Arriola, E.; De Castro Carpeño, J.; Herbst, R.S.; Tarazi, J.; Kim, S.; et al. Phase I trial of axitinib combined with platinum doublets in patients with advanced non-small cell lung cancer and other solid tumours. Br. J. Cancer 2012, 107, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P.; Yamamoto, N.; Bondarenko, I.M.; Poltoratskiy, A.; Novello, S.; Tang, J.; Bycott, P.; Niethammer, A.G.; Ingrosso, A.; Kim, S.; et al. Randomized phase II study of pemetrexed/cisplatin with or without axitinib for non-squamous non-small-cell lung cancer. BMC Cancer 2014, 14, 290. [Google Scholar] [CrossRef]
- Bondarenko, I.M.; Ingrosso, A.; Bycott, P.; Kim, S.; Cebotaru, C.L. Phase II study of axitinib with doublet chemotherapy in patients with advanced squamous non-small-cell lung cancer. BMC Cancer 2015, 15, 339. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotani, H.; Han, W.; Iida, Y.; Tanino, R.; Katakawa, K.; Okimoto, T.; Tsubata, Y.; Isobe, T.; Harada, M. Therapeutic Senolysis of Axitinib-Induced Senescent Human Lung Cancer Cells. Cancers 2024, 16, 2782. https://doi.org/10.3390/cancers16162782
Kotani H, Han W, Iida Y, Tanino R, Katakawa K, Okimoto T, Tsubata Y, Isobe T, Harada M. Therapeutic Senolysis of Axitinib-Induced Senescent Human Lung Cancer Cells. Cancers. 2024; 16(16):2782. https://doi.org/10.3390/cancers16162782
Chicago/Turabian StyleKotani, Hitoshi, Wei Han, Yuichi Iida, Ryosuke Tanino, Kazuaki Katakawa, Tamio Okimoto, Yukari Tsubata, Takeshi Isobe, and Mamoru Harada. 2024. "Therapeutic Senolysis of Axitinib-Induced Senescent Human Lung Cancer Cells" Cancers 16, no. 16: 2782. https://doi.org/10.3390/cancers16162782
APA StyleKotani, H., Han, W., Iida, Y., Tanino, R., Katakawa, K., Okimoto, T., Tsubata, Y., Isobe, T., & Harada, M. (2024). Therapeutic Senolysis of Axitinib-Induced Senescent Human Lung Cancer Cells. Cancers, 16(16), 2782. https://doi.org/10.3390/cancers16162782





