Screening and Surveillance of Colorectal Cancer: A Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. Screening and Surveillance for CRC
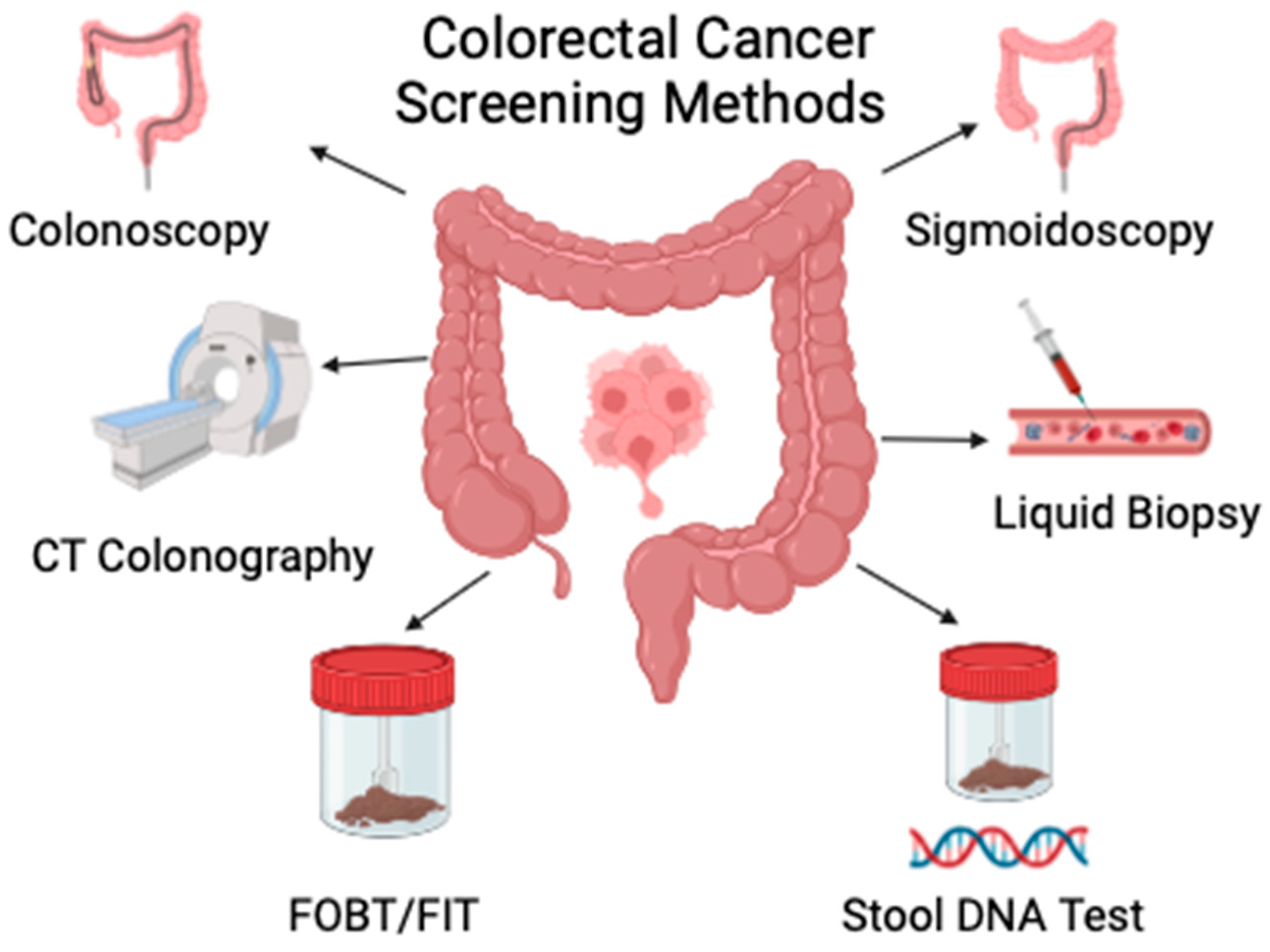
3. Current Available Tools, Efficacy and Cost-Effectiveness
3.1. Age-Appropriate Screening Guidelines
3.2. Stool or Blood-Based Testing
3.2.1. Guaiac Fecal Occult Blood Testing (gFOBT)
3.2.2. Fecal Immunohistochemical Test (FIT)
3.2.3. Multi-Target Stool DNA Test
3.3. Serum Tests for Colorectal Cancer Screening
3.4. Direct Visualization
3.4.1. Colonoscopy
3.4.2. Flexible Sigmoidoscopy
3.4.3. Computed Tomography Colonography (CTC)
3.4.4. Capsule Endoscopy
4. Recent Advances in Colonoscopy
5. Artificial Intelligence in Colonoscopy
6. Screening in Individuals with Baseline Risk of CRC
6.1. Family History
6.2. Familial Adenomatous Polyposis (FAP)
6.3. Hereditary Nonpolyposis Colorectal Cancer (HNPCC)
6.4. Colonic Inflammatory Bowel Disease
6.5. Abdominal Radiation
7. Surveillance Strategies
7.1. Surveillance after Endoscopy
7.1.1. Serrated Polyps
7.1.2. Adenomatous Polyps
7.1.3. Low-Risk Adenomas
7.1.4. Advanced Adenomas
8. Strategies for Clinical Practice
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2024. 1930. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html (accessed on 7 July 2024).
- O’Connell, J.B.; Maggard, M.A.; Ko, C.Y. Colon Cancer Survival Rates with the New American Joint Committee on Cancer Sixth Edition Staging. J. Natl. Cancer Inst. 2004, 96, 1420–1425. [Google Scholar] [CrossRef]
- Davis, D.M.; Marcet, J.E.; Frattini, J.C.; Prather, A.D.; Mateka, J.J.L.; Nfonsam, V.N. Is It Time to Lower the Recommended Screening Age for Colorectal Cancer? J. Am. Coll. Surg. 2011, 213, 352–361. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Dulskas, A.; Poskus, T.; Kildusiene, I.; Patasius, A.; Stulpinas, R.; Laurinavičius, A.; Mašalaitė, L.; Milaknytė, G.; Stundienė, I.; Venceviciene, L.; et al. National Colorectal Cancer Screening Program in Lithuania: Description of the 5-Year Performance on Population Level. Cancers 2021, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- PDQ Cancer Genetics Editorial Board. Genetics of Colorectal Cancer (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Fearon, E.R.; Vogelstein, B. A Genetic Model for Colorectal Tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Winawer, S.J.; Fletcher, R.H.; Miller, L.; Godlee, F.; Stolar, M.H.; Mulrow, C.D.; Woolf, S.H.; Glick, S.N.; Ganiats, T.G.; Bond, J.H.; et al. Colorectal Cancer Screening: Clinical Guidelines and Rationale. Gastroenterology 1997, 112, 594–642. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Waxman, I.; West, A.B.; Popnikolov, N.K.; Gatalica, Z.; Watari, J.; Obara, T.; Kohgo, Y.; Pasricha, P.J. Prevalence and Distinctive Biologic Features of Flat Colorectal Adenomas in a North American Population. Gastroenterology 2001, 120, 1657–1665. [Google Scholar] [CrossRef]
- Rembacken, B.J.; Fujii, T.; Cairns, A.; Dixon, M.F.; Yoshida, S.; Chalmers, D.M.; Axon, A.T. Flat and Depressed Colonic Neoplasms: A Prospective Study of 1000 Colonoscopies in the UK. Lancet Lond. Engl. 2000, 355, 1211–1214. [Google Scholar] [CrossRef]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F. Prevention of Colorectal Cancer by Colonoscopic Polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef]
- Song, M.; Emilsson, L.; Bozorg, S.R.; Nguyen, L.H.; Joshi, A.D.; Staller, K.; Nayor, J.; Chan, A.T.; Ludvigsson, J.F. Risk of Colorectal Cancer Incidence and Mortality after Polypectomy: A Swedish Record-Linkage Study. Lancet Gastroenterol. Hepatol. 2020, 5, 537–547. [Google Scholar] [CrossRef]
- Cheng, L.; Eng, C.; Nieman, L.Z.; Kapadia, A.S.; Du, X.L. Trends in Colorectal Cancer Incidence by Anatomic Site and Disease Stage in the United States from 1976 to 2005. Am. J. Clin. Oncol. 2011, 34, 573–580. [Google Scholar] [CrossRef]
- Schub, R.; Steinheber, F.U. Rightward Shift of Colon Cancer. A Feature of the Aging Gut. J. Clin. Gastroenterol. 1986, 8, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.E.; Cross, A.J.; Schoen, R.E.; Senore, C.; Pinsky, P.F.; Miller, E.A.; Segnan, N.; Wooldrage, K.; Wieszczy-Szczepanik, P.; Armaroli, P.; et al. Effectiveness of Colonoscopy Screening vs. Sigmoidoscopy Screening in Colorectal Cancer. JAMA Netw. Open 2024, 7, e240007. [Google Scholar] [CrossRef] [PubMed]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- Shaukat, A.; Levin, T.R. Current and Future Colorectal Cancer Screening Strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Colon and Rectum—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 13 May 2024).
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Off. J. Am. Coll. Gastroenterol. ACG 2021, 116, 458. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal Cancer Screening for Average-Risk Adults: 2018 Guideline Update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Harrod, C.S.; Crandall, C.J.; Wilt, T.J. Clinical Guidelines Committee of the American College of Physicians Screening for Colorectal Cancer in Asymptomatic Average-Risk Adults: A Guidance Statement from the American College of Physicians (Version 2). Ann. Intern. Med. 2023, 176, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Perdue, L.A.; Henrikson, N.B.; Bean, S.I.; Blasi, P.R. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 1978–1998. [Google Scholar] [CrossRef]
- Levin, B.; Lieberman, D.A.; McFarland, B.; Andrews, K.S.; Brooks, D.; Bond, J.; Dash, C.; Giardiello, F.M.; Glick, S.; Johnson, D.; et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008, 134, 1570–1595. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Turnbull, B.A.; Ross, M.E.; Colorectal Cancer Study Group. Fecal DNA versus Fecal Occult Blood for Colorectal-Cancer Screening in an Average-Risk Population. N. Engl. J. Med. 2004, 351, 2704–2714. [Google Scholar] [CrossRef]
- Ramdzan, A.R.; Abd Rahim, M.A.; Mohamad Zaki, A.; Zaidun, Z.; Mohammed Nawi, A. Diagnostic Accuracy of FOBT and Colorectal Cancer Genetic Testing: A Systematic Review & Meta-Analysis. Ann. Glob. Health 2019, 85, 70. [Google Scholar] [CrossRef]
- Scholefield, J.H.; Moss, S.M.; Mangham, C.M.; Whynes, D.K.; Hardcastle, J.D. Nottingham Trial of Faecal Occult Blood Testing for Colorectal Cancer: A 20-Year Follow-Up. Gut 2012, 61, 1036–1040. [Google Scholar] [CrossRef]
- Young, G.P.; Symonds, E.L.; Allison, J.E.; Cole, S.R.; Fraser, C.G.; Halloran, S.P.; Kuipers, E.J.; Seaman, H.E. Advances in Fecal Occult Blood Tests: The FIT Revolution. Dig. Dis. Sci. 2015, 60, 609–622. [Google Scholar] [CrossRef]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of Fecal Immunochemical Tests for Colorectal Cancer: Systematic Review and Meta-Analysis. Ann. Intern. Med. 2014, 160, 171. [Google Scholar] [CrossRef]
- D’Souza, N.; Brzezicki, A.; Abulafi, M. Faecal Immunochemical Testing in General Practice. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2019, 69, 60–61. [Google Scholar] [CrossRef]
- Jensen, C.D.; Corley, D.A.; Quinn, V.P.; Doubeni, C.A.; Zauber, A.G.; Lee, J.K.; Zhao, W.K.; Marks, A.R.; Schottinger, J.E.; Ghai, N.R.; et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening. Ann. Intern. Med. 2016, 164, 456–463. [Google Scholar] [CrossRef]
- van Rossum, L.G.; van Rijn, A.F.; Laheij, R.J.; van Oijen, M.G.; Fockens, P.; van Krieken, H.H.; Verbeek, A.L.; Jansen, J.B.; Dekker, E. Random Comparison of Guaiac and Immunochemical Fecal Occult Blood Tests for Colorectal Cancer in a Screening Population. Gastroenterology 2008, 135, 82–90. [Google Scholar] [CrossRef]
- Allison, J.E.; Tekawa, I.S.; Ransom, L.J.; Adrain, A.L. A Comparison of Fecal Occult-Blood Tests for Colorectal-Cancer Screening. N. Engl. J. Med. 1996, 334, 155–159. [Google Scholar] [CrossRef]
- Cheng, T.-I.; Wong, J.-M.; Hong, C.-F.; Cheng, S.H.; Cheng, T.-J.; Shieh, M.-J.; Lin, Y.-M.; Tso, C.Y.; Huang, A.T. Colorectal Cancer Screening in Asymptomaic Adults: Comparison of Colonoscopy, Sigmoidoscopy and Fecal Occult Blood Tests. J. Formos. Med. Assoc. Taiwan Yi Zhi 2002, 101, 685–690. [Google Scholar]
- Nakama, H.; Yamamoto, M.; Kamijo, N.; Li, T.; Wei, N.; Fattah, A.S.; Zhang, B. Colonoscopic Evaluation of Immunochemical Fecal Occult Blood Test for Detection of Colorectal Neoplasia. Hepatogastroenterology 1999, 46, 228–231. [Google Scholar]
- Nakama, H.; Kamijo, N.; Abdul Fattah, A.S.; Zhang, B. Validity of Immunological Faecal Occult Blood Screening for Colorectal Cancer: A Follow up Study. J. Med. Screen. 1996, 3, 63–65. [Google Scholar] [CrossRef]
- Parra-Blanco, A.; Gimeno-García, A.Z.; Quintero, E.; Nicolás, D.; Moreno, S.G.; Jiménez, A.; Hernández-Guerra, M.; Carrillo-Palau, M.; Eishi, Y.; López-Bastida, J. Diagnostic Accuracy of Immunochemical versus Guaiac Faecal Occult Blood Tests for Colorectal Cancer Screening. J. Gastroenterol. 2010, 45, 703–712. [Google Scholar] [CrossRef]
- Chiu, H.-M.; Lee, Y.-C.; Tu, C.-H.; Chen, C.-C.; Tseng, P.-H.; Liang, J.-T.; Shun, C.-T.; Lin, J.-T.; Wu, M.-S. Association between Early Stage Colon Neoplasms and False-Negative Results from the Fecal Immunochemical Test. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 832–838.e1-2. [Google Scholar] [CrossRef]
- Chiang, T.-H.; Lee, Y.-C.; Tu, C.-H.; Chiu, H.-M.; Wu, M.-S. Performance of the Immunochemical Fecal Occult Blood Test in Predicting Lesions in the Lower Gastrointestinal Tract. CMAJ Can. 2011, 183, 1474–1481. [Google Scholar] [CrossRef]
- Grobbee, E.J.; Wisse, P.H.; Schreuders, E.H.; van Roon, A.; van Dam, L.; Zauber, A.G.; Lansdorp-Vogelaar, I.; Bramer, W.; Berhane, S.; Deeks, J.J.; et al. Guaiac-Based Faecal Occult Blood Tests versus Faecal Immunochemical Tests for Colorectal Cancer Screening in Average-Risk Individuals. Cochrane Database Syst. Rev. 2022, 6, CD009276. [Google Scholar] [CrossRef]
- Song, L.-L.; Li, Y.-M. Current Noninvasive Tests for Colorectal Cancer Screening: An Overview of Colorectal Cancer Screening Tests. World J. Gastrointest. Oncol. 2016, 8, 793–800. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef]
- Binefa, G.; Rodríguez-Moranta, F.; Teule, A.; Medina-Hayas, M. Colorectal Cancer: From Prevention to Personalized Medicine. World J. Gastroenterol. 2014, 20, 6786–6808. [Google Scholar] [CrossRef]
- Cooper, G.S.; Markowitz, S.D.; Chen, Z.; Tuck, M.; Willis, J.E.; Berger, B.M.; Brenner, D.E.; Li, L. Evaluation of Patients with an Apparent False Positive Stool DNA Test: The Role of Repeat Stool DNA Testing. Dig. Dis. Sci. 2018, 63, 1449–1453. [Google Scholar] [CrossRef]
- Naber, S.K.; Knudsen, A.B.; Zauber, A.G.; Rutter, C.M.; Fischer, S.E.; Pabiniak, C.J.; Soto, B.; Kuntz, K.M.; Lansdorp-Vogelaar, I. Cost-Effectiveness of a Multitarget Stool DNA Test for Colorectal Cancer Screening of Medicare Beneficiaries. PLoS ONE 2019, 14, e0220234. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Mannalithara, A. Comparative Effectiveness and Cost Effectiveness of a Multitarget Stool DNA Test to Screen for Colorectal Neoplasia. Gastroenterology 2016, 151, 427–439.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid Biopsy at the Frontier of Detection, Prognosis and Progression Monitoring in Colorectal Cancer. Mol. Cancer 2022, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.C.; Gray, D.M.; Singh, H.; Issaka, R.B.; Raymond, V.M.; Eagle, C.; Hu, S.; Grady, W. A Cell-Free DNA Blood-Based Test for Colorectal Cancer Screening. N. Engl. J. Med. 2024, 390, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Lofton-Day, C.; Model, F.; Devos, T.; Tetzner, R.; Distler, J.; Schuster, M.; Song, X.; Lesche, R.; Liebenberg, V.; Ebert, M.; et al. DNA Methylation Biomarkers for Blood-Based Colorectal Cancer Screening. Clin. Chem. 2008, 54, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.D.; Xiong, W.; Bunker, A.M.; Vaughn, C.P.; Furtado, L.V.; Roberts, W.L.; Fang, J.C.; Samowitz, W.S.; Heichman, K.A. Septin 9 Methylated DNA Is a Sensitive and Specific Blood Test for Colorectal Cancer. BMC Med. 2011, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Circulating Methylated Septin 9 Nucleic Acid in the Plasma of Patients with Gastrointestinal Cancer in the Stomach and Colon—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23730408/ (accessed on 4 May 2024).
- Schoen, R.E.; Pinsky, P.F.; Weissfeld, J.L.; Yokochi, L.A.; Church, T.; Laiyemo, A.O.; Bresalier, R.; Andriole, G.L.; Buys, S.S.; Crawford, E.D.; et al. Colorectal-Cancer Incidence and Mortality with Screening Flexible Sigmoidoscopy. N. Engl. J. Med. 2012, 366, 2345–2357. [Google Scholar] [CrossRef]
- Newcomb, P.A.; Storer, B.E.; Morimoto, L.M.; Templeton, A.; Potter, J.D. Long-Term Efficacy of Sigmoidoscopy in the Reduction of Colorectal Cancer Incidence. J. Natl. Cancer Inst. 2003, 95, 622–625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-Term Colorectal-Cancer Incidence and Mortality after Lower Endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef]
- Long-Term Colorectal-Cancer Mortality after Adenoma Removal—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25162886/ (accessed on 4 May 2024).
- Doubeni, C.A.; Weinmann, S.; Adams, K.; Kamineni, A.; Buist, D.S.M.; Ash, A.S.; Rutter, C.M.; Doria-Rose, V.P.; Corley, D.A.; Greenlee, R.T.; et al. Screening Colonoscopy and Risk for Incident Late-Stage Colorectal Cancer Diagnosis in Average-Risk Adults: A Nested Case-Control Study. Ann. Intern. Med. 2013, 158, 312–320. [Google Scholar] [CrossRef]
- Kahi, C.J.; Myers, L.J.; Slaven, J.E.; Haggstrom, D.; Pohl, H.; Robertson, D.J.; Imperiale, T.F. Lower Endoscopy Reduces Colorectal Cancer Incidence in Older Individuals. Gastroenterology 2014, 146, 718–725.e3. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Chang-Claude, J.; Jansen, L.; Knebel, P.; Stock, C.; Hoffmeister, M. Reduced Risk of Colorectal Cancer up to 10 Years after Screening, Surveillance, or Diagnostic Colonoscopy. Gastroenterology 2014, 146, 709–717. [Google Scholar] [CrossRef]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Butterly, L.F. Colonoscopy: Quality Indicators. Clin. Transl. Gastroenterol. 2015, 6, e77. [Google Scholar] [CrossRef]
- Rex, D.K.; Bond, J.H.; Winawer, S.; Levin, T.R.; Burt, R.W.; Johnson, D.A.; Kirk, L.M.; Litlin, S.; Lieberman, D.A.; Waye, J.D.; et al. Quality in the Technical Performance of Colonoscopy and the Continuous Quality Improvement Process for Colonoscopy: Recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2002, 97, 1296–1308. [Google Scholar] [CrossRef]
- Mouchli, M.A.; Ouk, L.; Scheitel, M.R.; Chaudhry, A.P.; Felmlee-Devine, D.; Grill, D.E.; Rashtak, S.; Wang, P.; Wang, J.; Chaudhry, R.; et al. Colonoscopy Surveillance for High Risk Polyps Does Not Always Prevent Colorectal Cancer. World J. Gastroenterol. 2018, 24, 905–916. [Google Scholar] [CrossRef]
- Atkin, W.S.; Edwards, R.; Kralj-Hans, I.; Wooldrage, K.; Hart, A.R.; Northover, J.M.A.; Parkin, D.M.; Wardle, J.; Duffy, S.W.; Cuzick, J.; et al. Once-Only Flexible Sigmoidoscopy Screening in Prevention of Colorectal Cancer: A Multicentre Randomised Controlled Trial. Lancet Lond. Engl. 2010, 375, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Holme, Ø.; Løberg, M.; Kalager, M.; Bretthauer, M.; Hernán, M.A.; Aas, E.; Eide, T.J.; Skovlund, E.; Schneede, J.; Tveit, K.M.; et al. Effect of Flexible Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality: A Randomized Clinical Trial. JAMA 2014, 312, 606–615. [Google Scholar] [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of Screening Sigmoidoscopy and Screening Colonoscopy on Colorectal Cancer Incidence and Mortality: Systematic Review and Meta-Analysis of Randomised Controlled Trials and Observational Studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef]
- Castells, A.; Bessa, X.; Quintero, E.; Bujanda, L.; Cubiella, J.; Salas, D.; Lanas, A.; Carballo, F.; Morillas, J.D.; Hernández, C.; et al. Risk of Advanced Proximal Neoplasms According to Distal Colorectal Findings: Comparison of Sigmoidoscopy-Based Strategies. J. Natl. Cancer Inst. 2013, 105, 878–886. [Google Scholar] [CrossRef]
- Hoff, G.; Grotmol, T.; Skovlund, E.; Bretthauer, M.; Norwegian Colorectal Cancer Prevention Study Group. Risk of Colorectal Cancer Seven Years after Flexible Sigmoidoscopy Screening: Randomised Controlled Trial. BMJ 2009, 338, b1846. [Google Scholar] [CrossRef] [PubMed]
- Segnan, N.; Armaroli, P.; Bonelli, L.; Risio, M.; Sciallero, S.; Zappa, M.; Andreoni, B.; Arrigoni, A.; Bisanti, L.; Casella, C.; et al. Once-Only Sigmoidoscopy in Colorectal Cancer Screening: Follow-up Findings of the Italian Randomized Controlled Trial--SCORE. J. Natl. Cancer Inst. 2011, 103, 1310–1322. [Google Scholar] [CrossRef]
- Elmunzer, B.J.; Hayward, R.A.; Schoenfeld, P.S.; Saini, S.D.; Deshpande, A.; Waljee, A.K. Effect of Flexible Sigmoidoscopy-Based Screening on Incidence and Mortality of Colorectal Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS Med. 2012, 9, e1001352. [Google Scholar] [CrossRef]
- Senore, C.; Bonelli, L.; Sciallero, S.; Casella, C.; Santarelli, A.; Armaroli, P.; Zanetti, R.; Segnan, N. Assessing Generalizability of the Findings of Sigmoidoscopy Screening Trials: The Case of SCORE Trial. J. Natl. Cancer Inst. 2015, 107, 385. [Google Scholar] [CrossRef]
- Lieberman, D.A.; Weiss, D.G.; Veterans Affairs Cooperative Study Group. 380 One-Time Screening for Colorectal Cancer with Combined Fecal Occult-Blood Testing and Examination of the Distal Colon. N. Engl. J. Med. 2001, 345, 555–560. [Google Scholar] [CrossRef]
- Knudsen, A.B.; Zauber, A.G.; Rutter, C.M.; Naber, S.K.; Doria-Rose, V.P.; Pabiniak, C.; Johanson, C.; Fischer, S.E.; Lansdorp-Vogelaar, I.; Kuntz, K.M. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. JAMA 2016, 315, 2595–2609. [Google Scholar] [CrossRef]
- Johnson, C.D.; Chen, M.-H.; Toledano, A.Y.; Heiken, J.P.; Dachman, A.; Kuo, M.D.; Menias, C.O.; Siewert, B.; Cheema, J.I.; Obregon, R.G.; et al. Accuracy of CT Colonography for Detection of Large Adenomas and Cancers. N. Engl. J. Med. 2008, 359, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Heresbach, D.; Djabbari, M.; Riou, F.; Marcus, C.; Le Sidaner, A.; Pierredon-Foulogne, M.A.; Ponchon, T.; Boudiaf, M.; Seyrig, J.A.; Laumonier, H.; et al. Accuracy of Computed Tomographic Colonography in a Nationwide Multicentre Trial, and Its Relation to Radiologist Expertise. Gut 2011, 60, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Hassan, C.; Halligan, S.; Marmo, R. Colorectal Cancer: CT Colonography and Colonoscopy for Detection--Systematic Review and Meta-Analysis. Radiology 2011, 259, 393–405. [Google Scholar] [CrossRef]
- Knudsen, A.B.; Lansdorp-Vogelaar, I.; Rutter, C.M.; Savarino, J.E.; van Ballegooijen, M.; Kuntz, K.M.; Zauber, A.G. Cost-Effectiveness of Computed Tomographic Colonography Screening for Colorectal Cancer in the Medicare Population. J. Natl. Cancer Inst. 2010, 102, 1238–1252. [Google Scholar] [CrossRef]
- Leggett, B.; Whitehall, V. Role of the Serrated Pathway in Colorectal Cancer Pathogenesis. Gastroenterology 2010, 138, 2088–2100. [Google Scholar] [CrossRef]
- Lin, J.S.; Piper, M.A.; Perdue, L.A.; Rutter, C.M.; Webber, E.M.; O’Connor, E.; Smith, N.; Whitlock, E.P. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016, 315, 2576–2594. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Pickhardt, P.J.; Taylor, A.J.; Leung, W.K.; Winter, T.C.; Hinshaw, J.L.; Gopal, D.V.; Reichelderfer, M.; Hsu, R.H.; Pfau, P.R. CT Colonography versus Colonoscopy for the Detection of Advanced Neoplasia. N. Engl. J. Med. 2007, 357, 1403–1412. [Google Scholar] [CrossRef]
- PubMed. Colorectal and Extracolonic Cancers Detected at Screening CT Colonography in 10,286 Asymptomatic Adults. Available online: https://pubmed.ncbi.nlm.nih.gov/20308446/ (accessed on 4 May 2024).
- Pooler, B.D.; Kim, D.H.; Pickhardt, P.J. Indeterminate but Likely Unimportant Extracolonic Findings at Screening CT Colonography (C-RADS Category E3): Incidence and Outcomes Data from a Clinical Screening Program. AJR Am. J. Roentgenol. 2016, 207, 996–1001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Incidental Adnexal Masses Detected at Low-Dose Unenhanced CT in Asymptomatic Women Age 50 and Older: Implications for Clinical Management and Ovarian Cancer Screening—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20663974/ (accessed on 4 May 2024).
- Health Quality Ontario. Colon Capsule Endoscopy for the Detection of Colorectal Polyps: An Evidence-Based Analysis. Ont. Health Technol. Assess. Ser. 2015, 15, 1–39. [Google Scholar]
- Kobaek-Larsen, M.; Kroijer, R.; Dyrvig, A.-K.; Buijs, M.M.; Steele, R.J.C.; Qvist, N.; Baatrup, G. Back-to-Back Colon Capsule Endoscopy and Optical Colonoscopy in Colorectal Cancer Screening Individuals. Color. Dis. 2018, 20, 479–485. [Google Scholar] [CrossRef]
- Cash, B.D.; Fleisher, M.R.; Fern, S.; Rajan, E.; Haithcock, R.; Kastenberg, D.M.; Pound, D.; Papageorgiou, N.P.; Fernández-Urién, I.; Schmelkin, I.J.; et al. Multicentre, Prospective, Randomised Study Comparing the Diagnostic Yield of Colon Capsule Endoscopy versus CT Colonography in a Screening Population (the TOPAZ Study). Gut 2021, 70, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; May, F.P.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gross, S.A.; Jacobson, B.C.; Shaukat, A.; Robertson, D.J. Updates on Age to Start and Stop Colorectal Cancer Screening: Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2022, 162, 285–299. [Google Scholar] [CrossRef]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastrointest. Endosc. 2017, 86, 18–33. [Google Scholar] [CrossRef]
- NCCN Guidelines for Patients. Colorectal Cancer Screening; NCCN Foundation: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Maida, M.; Camilleri, S.; Manganaro, M.; Garufi, S.; Scarpulla, G. New Endoscopy Advances to Refine Adenoma Detection Rate for Colorectal Cancer Screening: None Is the Winner. World J. Gastrointest. Oncol. 2017, 9, 402–406. [Google Scholar] [CrossRef]
- Halpern, Z.; Gross, S.A.; Gralnek, I.M.; Shpak, B.; Pochapin, M.; Hoffman, A.; Mizrahi, M.; Rochberger, Y.S.; Moshkowitz, M.; Santo, E.; et al. Comparison of Adenoma Detection and Miss Rates between a Novel Balloon Colonoscope and Standard Colonoscopy: A Randomized Tandem Study. Endoscopy 2015, 47, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Spadaccini, M.; Mori, Y.; Foroutan, F.; Facciorusso, A.; Gkolfakis, P.; Tziatzios, G.; Triantafyllou, K.; Antonelli, G.; Khalaf, K.; et al. Real-Time Computer-Aided Detection of Colorectal Neoplasia During Colonoscopy. Ann. Intern. Med. 2023, 176, 1209–1220. [Google Scholar] [CrossRef]
- Young, E.; Edwards, L.; Singh, R. The Role of Artificial Intelligence in Colorectal Cancer Screening: Lesion Detection and Lesion Characterization. Cancers 2023, 15, 5126. [Google Scholar] [CrossRef]
- Maida, M.; Marasco, G.; Facciorusso, A.; Shahini, E.; Sinagra, E.; Pallio, S.; Ramai, D.; Murino, A. Effectiveness and Application of Artificial Intelligence for Endoscopic Screening of Colorectal Cancer: The Future Is Now. Expert Rev. Anticancer Ther. 2023, 23, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Impact of Artificial Intelligence on Colonoscopy Surveillance After Polyp Removal: A Pooled Analysis of Randomized Trials—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36038128/ (accessed on 7 July 2024).
- Areia, M.; Mori, Y.; Correale, L.; Repici, A.; Bretthauer, M.; Sharma, P.; Taveira, F.; Spadaccini, M.; Antonelli, G.; Ebigbo, A.; et al. Cost-Effectiveness of Artificial Intelligence for Screening Colonoscopy: A Modelling Study. Lancet Digit. Health 2022, 4, e436–e444. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; East, J.E.; Hassan, C.; Halvorsen, N.; Berzin, T.M.; Byrne, M.; von Renteln, D.; Hewett, D.G.; Repici, A.; Ramchandani, M.; et al. Benefits and Challenges in Implementation of Artificial Intelligence in Colonoscopy: World Endoscopy Organization Position Statement. Dig. Endosc. 2023, 35, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, A.S.; Higgins, J.P.T.; Pharoah, P. Relative and Absolute Risk of Colorectal Cancer for Individuals with a Family History: A Meta-Analysis. Eur. J. Cancer 2006, 42, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Giovannucci, E.L.; Colditz, G.A.; Hunter, D.J.; Speizer, F.E.; Willett, W.C. A Prospective Study of Family History and the Risk of Colorectal Cancer. N. Engl. J. Med. 1994, 331, 1669–1674. [Google Scholar] [CrossRef]
- Ahsan, H.; Neugut, A.I.; Garbowski, G.C.; Jacobson, J.S.; Forde, K.A.; Treat, M.R.; Waye, J.D. Family History of Colorectal Adenomatous Polyps and Increased Risk for Colorectal Cancer. Ann. Intern. Med. 1998, 128, 900–905. [Google Scholar] [CrossRef]
- Rex, D.K.; Johnson, D.A.; Anderson, J.C.; Schoenfeld, P.S.; Burke, C.A.; Inadomi, J.M. American College of Gastroenterology American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2009 [Corrected]. Am. J. Gastroenterol. 2009, 104, 739–750. [Google Scholar] [CrossRef]
- Qaseem, A.; Denberg, T.D.; Hopkins, R.H.; Humphrey, L.L.; Levine, J.; Sweet, D.E.; Shekelle, P.; Clinical Guidelines Committee of the American College of Physicians. Screening for Colorectal Cancer: A Guidance Statement from the American College of Physicians. Ann. Intern. Med. 2012, 156, 378–386. [Google Scholar] [CrossRef]
- West, N.J.; Boustière, C.; Fischbach, W.; Parente, F.; Leicester, R.J. Colorectal Cancer Screening in Europe: Differences in Approach; Similar Barriers to Overcome. Int. J. Colorectal Dis. 2009, 24, 731–740. [Google Scholar] [CrossRef]
- American Gastroenterological Association. American Gastroenterological Association Medical Position Statement: Hereditary Colorectal Cancer and Genetic Testing. Gastroenterology 2001, 121, 195–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Menon, G.; Carr, S.; Kasi, A. Familial Adenomatous Polyposis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vasen, H.F.A.; Möslein, G.; Alonso, A.; Aretz, S.; Bernstein, I.; Bertario, L.; Blanco, I.; Bülow, S.; Burn, J.; Capella, G.; et al. Guidelines for the Clinical Management of Familial Adenomatous Polyposis (FAP). Gut 2008, 57, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Smyrk, T.; McGinn, T.; Lanspa, S.; Cavalieri, J.; Lynch, J.; Slominski-Castor, S.; Cayouette, M.C.; Priluck, I.; Luce, M.C. Attenuated Familial Adenomatous Polyposis (AFAP). A Phenotypically and Genotypically Distinctive Variant of FAP. Cancer 1995, 76, 2427–2433. [Google Scholar] [CrossRef]
- Knudsen, A.L.; Bisgaard, M.L.; Bülow, S. Attenuated Familial Adenomatous Polyposis (AFAP): A Review of the Literature. Fam. Cancer 2003, 2, 43–55. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Hughes, K.S.; Parmigiani, G.; Braun, D. Penetrance of Colorectal Cancer Among Mismatch Repair Gene Mutation Carriers: A Meta-Analysis. JNCI Cancer Spectr. 2020, 4, pkaa027. [Google Scholar] [CrossRef]
- Lynch, H.T.; Smyrk, T.C.; Watson, P.; Lanspa, S.J.; Lynch, J.F.; Lynch, P.M.; Cavalieri, R.J.; Boland, C.R. Genetics, Natural History, Tumor Spectrum, and Pathology of Hereditary Nonpolyposis Colorectal Cancer: An Updated Review. Gastroenterology 1993, 104, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Mecklin, J.P.; Järvinen, H.J. Tumor Spectrum in Cancer Family Syndrome (Hereditary Nonpolyposis Colorectal Cancer). Cancer 1991, 68, 1109–1112. [Google Scholar] [CrossRef]
- Wilkins, T.; McMechan, D.; Talukder, A.; Herline, A. Colorectal Cancer Screening and Surveillance in Individuals at Increased Risk. Am. Fam. Physician 2018, 97, 111–116. [Google Scholar]
- Ojha, S.K.; Laslett, N. Hereditary Nonpolyposis Colon Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Van Assche, G.; Dignass, A.; Bokemeyer, B.; Danese, S.; Gionchetti, P.; Moser, G.; Beaugerie, L.; Gomollón, F.; Häuser, W.; Herrlinger, K.; et al. Second European Evidence-Based Consensus on the Diagnosis and Management of Ulcerative Colitis Part 3: Special Situations. J. Crohns Colitis 2013, 7, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The Risk of Colorectal Cancer in Ulcerative Colitis: A Meta-Analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- American Society for Gastrointestinal Endoscopy Standards of Practice Committee; Shergill, A.K.; Lightdale, J.R.; Bruining, D.H.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Evans, J.A.; et al. The Role of Endoscopy in Inflammatory Bowel Disease. Gastrointest. Endosc. 2015, 81, 1101–1121.e13. [Google Scholar] [CrossRef]
- Farraye, F.A.; Odze, R.D.; Eaden, J.; Itzkowitz, S.H. AGA Technical Review on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. Gastroenterology 2010, 138, 746–774.e4. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.T.; Feuerstein, J.D. Colorectal Cancer Surveillance in Inflammatory Bowel Disease: Practice Guidelines and Recent Developments. World J. Gastroenterol. 2019, 25, 4148–4157. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef] [PubMed]
- Melville, D.M.; Jass, J.R.; Morson, B.C.; Pollock, D.J.; Richman, P.I.; Shepherd, N.A.; Ritchie, J.K.; Love, S.B.; Lennard-Jones, J.E. Observer Study of the Grading of Dysplasia in Ulcerative Colitis: Comparison with Clinical Outcome. Hum. Pathol. 1989, 20, 1008–1014. [Google Scholar] [CrossRef]
- Claessen, M.M.H.; Lutgens, M.W.M.D.; van Buuren, H.R.; Oldenburg, B.; Stokkers, P.C.F.; van der Woude, C.J.; Hommes, D.W.; de Jong, D.J.; Dijkstra, G.; van Bodegraven, A.A.; et al. More Right-Sided IBD-Associated Colorectal Cancer in Patients with Primary Sclerosing Cholangitis. Inflamm. Bowel Dis. 2009, 15, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Connell, W.R.; Lennard-Jones, J.E.; Williams, C.B.; Talbot, I.C.; Price, A.B.; Wilkinson, K.H. Factors Affecting the Outcome of Endoscopic Surveillance for Cancer in Ulcerative Colitis. Gastroenterology 1994, 107, 934–944. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-Intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-Anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Sinagra, E.; Tomasello, G.; Raimondo, D.; Sturm, A.; Giunta, M.; Messina, M.; Damiano, G.; Palumbo, V.D.; Spinelli, G.; Rossi, F.; et al. Advanced Endoscopic Imaging for Surveillance for Dysplasia and Colorectal Cancer in Inflammatory Bowel Disease: Could the Pathologist Be Further Helped? Saudi J. Gastroenterol. 2014, 20, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, R.; East, J.E.; Hassan, C.; Hazewinkel, Y.; Kamiński, M.F.; Neumann, H.; Pellisé, M.; Antonelli, G.; Bustamante Balen, M.; Coron, E.; et al. Advanced Imaging for Detection and Differentiation of Colorectal Neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2019. Endoscopy 2019, 51, 1155–1179. [Google Scholar] [CrossRef]
- Henderson, T.O.; Oeffinger, K.C.; Whitton, J.; Leisenring, W.; Neglia, J.; Meadows, A.; Crotty, C.; Rubin, D.T.; Diller, L.; Inskip, P.; et al. Secondary Gastrointestinal Cancer in Childhood Cancer Survivors: A Cohort Study. Ann. Intern. Med. 2012, 156, 757–766, W-260. [Google Scholar] [CrossRef]
- Nottage, K.; McFarlane, J.; Krasin, M.J.; Li, C.; Srivastava, D.; Robison, L.L.; Hudson, M.M. Secondary Colorectal Carcinoma after Childhood Cancer. J. Clin. Oncol. 2012, 30, 2552–2558. [Google Scholar] [CrossRef]
- Children’s Oncology Group. Available online: http://www.survivorshipguidelines.org/ (accessed on 6 July 2024).
- Hampton, J.S.; Sharp, L.; Craig, D.; Rees, C.J. Colorectal Cancer Screening and Surveillance for Non-Hereditary High-Risk Groups—Is It Time for a Re-Think? Curr. Treat. Options Gastroenterol. 2021, 19, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Torlakovic, E.; Skovlund, E.; Snover, D.C.; Torlakovic, G.; Nesland, J.M. Morphologic Reappraisal of Serrated Colorectal Polyps. Am. J. Surg. Pathol. 2003, 27, 65–81. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Ford, B. Colonoscopy Follow-up: U.S. Multi-Society Task Force on Colorectal Cancer Updates Recommendations. Am. Fam. Physician 2021, 103, 314–316. [Google Scholar] [PubMed]
- O’Brien, M.J.; Winawer, S.J.; Zauber, A.G.; Gottlieb, L.S.; Sternberg, S.S.; Diaz, B.; Dickersin, G.R.; Ewing, S.; Geller, S.; Kasimian, D. The National Polyp Study. Patient and Polyp Characteristics Associated with High-Grade Dysplasia in Colorectal Adenomas. Gastroenterology 1990, 98, 371–379. [Google Scholar]
- Atkin, W.S.; Morson, B.C.; Cuzick, J. Long-Term Risk of Colorectal Cancer after Excision of Rectosigmoid Adenomas. N. Engl. J. Med. 1992, 326, 658–662. [Google Scholar] [CrossRef]
- Lieberman, D.; Moravec, M.; Holub, J.; Michaels, L.; Eisen, G. Polyp Size and Advanced Histology in Patients Undergoing Colonoscopy Screening: Implications for CT Colonography. Gastroenterology 2008, 135, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.A.; Rex, D.K.; Winawer, S.J.; Giardiello, F.M.; Johnson, D.A.; Levin, T.R. Guidelines for Colonoscopy Surveillance after Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012, 143, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Antonelli, G.; Dumonceau, J.-M.; Regula, J.; Bretthauer, M.; Chaussade, S.; Dekker, E.; Ferlitsch, M.; Gimeno-Garcia, A.; Jover, R.; et al. Post-Polypectomy Colonoscopy Surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2020. Endoscopy 2020, 52, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S. Summary and Comparison of Recently Updated Post-Polypectomy Surveillance Guidelines. Intest. Res. 2023, 21, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Mukama, T.; Sundquist, K.; Sundquist, J.; Brenner, H.; Kharazmi, E.; Fallah, M. Longer Interval Between First Colonoscopy with Negative Findings for Colorectal Cancer and Repeat Colonoscopy. JAMA Oncol. 2024, 10, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Bogie, R.; Sanduleanu, S. Optimizing Post-Polypectomy Surveillance: A Practical Guide for the Endoscopist. Dig. Endosc. 2016, 28, 348–359. [Google Scholar] [CrossRef] [PubMed]
- The Influence of Glucose-Lowering Therapies on Cancer Risk in Type 2 Diabetes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19572116/ (accessed on 4 May 2024).
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased Cancer-Related Mortality for Patients with Type 2 Diabetes Who Use Sulfonylureas or Insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Chin, Y.-T.; Yang, Y.-C.S.H.; Lai, H.-Y.; Wang-Peng, J.; Liu, L.F.; Tang, H.-Y.; Davis, P.J. Thyroid Hormone, Cancer, and Apoptosis. Compr. Physiol. 2016, 6, 1221–1237. [Google Scholar] [CrossRef] [PubMed]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and Cancer: The Role of Adipose Tissue and Adipo-Cytokines-Induced Chronic Inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Gillman, M.W.; Harper, D.M.; Kemper, A.R.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 315, 2564–2575. [Google Scholar] [CrossRef]
- Lacroute, J.; Marcantoni, J.; Petitot, S.; Weber, J.; Levy, P.; Dirrenberger, B.; Tchoumak, I.; Baron, M.; Gibert, S.; Marguerite, S.; et al. The Carbon Footprint of Ambulatory Gastrointestinal Endoscopy. Endoscopy 2023, 55, 918–926. [Google Scholar] [CrossRef] [PubMed]
| ACG 2021 | USMSTF 2022 | ACS 2018 | USPSTF 2021 | NCCN 2022 | ACP 2023 | |
|---|---|---|---|---|---|---|
| Age | Begin avg risk at 45 years | Begin avg risk at 45 years | Begin avg risk at 45 years | Begin avg risk at 45 years | Begin avg risk at 45 years | Begin avg risk at 50 years |
| All | 50–75 years | 50–75 years | 50–75 years | 50–75 years | 50–75 years | 50–75 years |
| 75–85 years | Selectively | Selectively | Selectively | Selectively | Selectively | Discontinue at 75 years if life expectancy is 10 years or less |
| 85+ years | Discourage screening | - | Discourage screening | Discourage screening | Discourage screening | - |
| Stool Based | ||||||
| gFOBT | - | - | Annual | Annual | Annual | Every 2 years |
| FIT | Annual | Annual | Annual | Annual | Annual | Every 2 years |
| mt-sDNA | Every 3 years | Every 3 years | Every 1–3 years | Every 1–3 years | Every 3 years | - |
| Endoscopic | ||||||
| Flexible Sigmoidoscopy | Every 5–10 years | Every 5–10 years | Every 5 years or Every 10 years if combined with FIT | Every 5 years | Every 5 years or Every 10 years if combined with FIT | Every 10 years + FIT every 2 years |
| Colonoscopy | Every 10 years | Every 10 years | Every 10 years | Every 10 years | Every 10 years | Every 10 years |
| CT Colonography | Every 5 years | Every 5 years | Every 5 years | Every 5 years | Every 5 years | - |
| Colon Capsule | Every 5 years | If the patient refuses all of the above | - | - | - | - |
| Screening Method | Technique | Advantages | Disadvantages |
|---|---|---|---|
| Colonoscopy | Involves the insertion of a flexible tube with a camera (colonoscope) into the rectum to examine the entire colon for polyps or cancerous growths. | -Direct visualization allows for the detection and removal of precancerous polyps during the procedure. | -Requires bowel preparation, which may be uncomfortable. -Invasive procedure with a small risk of complications, such as bleeding or perforation. |
| Flexible Sigmoidoscopy | Involves the insertion of a thin, flexible tube with a camera (sigmoidoscope) into the rectum and lower part of the colon to examine for polyps or cancerous growths. | -Less invasive than colonoscopy. -Does not require full bowel preparation. | -Limited in scope compared to colonoscopy; only examines the lower part of the colon. -Polyps or cancers in the upper colon may be missed. -Positive findings require follow-up colonoscopy. |
| CT Colonography | Uses CT scans to create detailed images of the colon and rectum, allowing for the detection of polyps or cancerous growths. | -Noninvasive and does not require sedation. -No risk of perforation. -Provides detailed images of the entire colon. | -Requires bowel preparation similar to colonoscopy. -Polyps found may require follow-up colonoscopy for removal. -Radiation exposure from CT scans. |
| Capsule Endoscopy | Capsule endoscopy involves swallowing a small camera that captures images of the colon as it passes through the digestive tract. | -Noninvasive -No need for sedation -Provides comprehensive visualization of the entire GI tract -Better detection of polyps and early cancers | -Requires bowel preparation -Limited availability and accessibility -Risk of capsule retention -Not therapeutic; positive findings require follow-up colonoscopy -Possible technical issues (e.g., battery life, transmission problems) |
| Fecal Immunochemical Test (FIT) | A stool-based test that detects hidden blood in the stool, which can be a sign of colorectal cancer or polyps. | -Noninvasive and simple to perform at home. No dietary or medication restrictions before the test. -No need for bowel preparation. | -Can produce false-positive results due to bleeding from other sources (e.g., hemorrhoids). -Sensitivity may vary, and polyps or early-stage cancer may not always be detected. -A follow-up colonoscopy is required if the test is positive. |
| Stool DNA Test (mt-sDNA) | A stool-based test that combines FIT with analysis of DNA markers associated with colorectal cancer. | -Higher sensitivity for detecting advanced adenomas and colorectal cancer compared to FIT alone. -Noninvasive and can be performed at home without dietary or medication restrictions. | -More expensive than FIT. -False-positive results can occur, leading to unnecessary follow-up testing. -Requires collection of multiple stool samples. |
| gFOBT | gFOBT detects hidden blood in stool samples, suggesting bleeding from polyps or cancer using guaiac-based chemical tests. | -Noninvasive -Easy to perform at home -No need for bowel preparation -Low cost -No sedation needed | -Lower sensitivity and specificity compared to other methods -High false-positive rate -Requires multiple samples -Dietary restrictions prior to the test -Not diagnostic; positive results need follow-up colonoscopy -Can miss polyps and early cancers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maida, M.; Dahiya, D.S.; Shah, Y.R.; Tiwari, A.; Gopakumar, H.; Vohra, I.; Khan, A.; Jaber, F.; Ramai, D.; Facciorusso, A. Screening and Surveillance of Colorectal Cancer: A Review of the Literature. Cancers 2024, 16, 2746. https://doi.org/10.3390/cancers16152746
Maida M, Dahiya DS, Shah YR, Tiwari A, Gopakumar H, Vohra I, Khan A, Jaber F, Ramai D, Facciorusso A. Screening and Surveillance of Colorectal Cancer: A Review of the Literature. Cancers. 2024; 16(15):2746. https://doi.org/10.3390/cancers16152746
Chicago/Turabian StyleMaida, Marcello, Dushyant Singh Dahiya, Yash R. Shah, Angad Tiwari, Harishankar Gopakumar, Ishaan Vohra, Aqsa Khan, Fouad Jaber, Daryl Ramai, and Antonio Facciorusso. 2024. "Screening and Surveillance of Colorectal Cancer: A Review of the Literature" Cancers 16, no. 15: 2746. https://doi.org/10.3390/cancers16152746
APA StyleMaida, M., Dahiya, D. S., Shah, Y. R., Tiwari, A., Gopakumar, H., Vohra, I., Khan, A., Jaber, F., Ramai, D., & Facciorusso, A. (2024). Screening and Surveillance of Colorectal Cancer: A Review of the Literature. Cancers, 16(15), 2746. https://doi.org/10.3390/cancers16152746








