MIRRORS ICG: Perfusion Assessment Using Indocyanine Green (ICG) Peritoneal Angiography during Robotic Interval Cytoreductive Surgery for Advanced Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Objective
2.2. Study Endpoints
2.3. Participants
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Setting
2.5. Statistical Analysis
2.6. Trial Design: Interventional Observational Diagnostic Study
2.7. Trial Registration
3. Results
4. Discussion
4.1. Results in the Context of the Published Literature
4.2. Strengths and Weaknesses
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pruimboom, T.; Schols, R.M.; Van Kuijk, S.M.; Van der Hulst, R.R.; Qiu, S.S. Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction. Cochrane Database Syst. Rev. 2020, 4, CD013280. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic Green. Verdye 5 mg/mL Summary of Product Characteristics. Available online: https://mhraproductsprod.blob.core.windows.net/docs-20200128/587f5400e93d096fc30f5e0e44e23ec99caa8b2b (accessed on 5 July 2024).
- Solass, W.; Horvath, P.; Struller, F.; Konigsrainer, I.; Beckert, S.; Konigsrainer, A.; Weinreich, F.J.; Schenk, M. Functional vascular anatomy of the peritoneum in health and disease. Pleura Peritoneum 2016, 1, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Uwins, C.; Assalaarachchi, H.; Bennett, K.; Read, J.; Tailor, A.; Crawshaw, J.; Chatterjee, J.; Ellis, P.; Skene, S.S.; Michael, A.; et al. MIRRORS: A prospective cohort study assessing the feasibility of robotic interval debulking surgery for advanced-stage ovarian cancer. Int. J. Gynecol. Cancer 2024, 34, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Uwins, C.; Michael, A.; Tailor, A.; Chatterjee, J.; Ellis, P.; Madhuri, T.; Skene, S.; Butler-Manuel, S. Mirrors trial: Minimally invasive robotic surgery, role in optimal debulking ovarian cancer, recovery and survival. Int. J. Gynecol. Cancer 2020, 30, A111–A112. [Google Scholar]
- Uwins, C.; Butler-Manuel, S. MIRRORS: Robotic Surgery for Advanced Ovarian Cancer. On Medicine BMC Biomedcentral: On Medicine BMC Biomedcentral. 2021. Available online: https://blogs.biomedcentral.com/on-medicine/2021/02/19/mirrors-robotic-surgery-ovarian-cancer-isrctn/ (accessed on 17 July 2024).
- Fotopoulou, C.; Hall, M.; Cruickshank, D.; Gabra, H.; Ganesan, R.; Hughes, C.; Kehoe, S.; Ledermann, J.; Morrison, J.; Naik, R.; et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/fallopian tube/primary peritoneal cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 123–139. [Google Scholar] [CrossRef]
- Monaghan, T.F.; Rahman, S.N.; Agudelo, C.W.; Wein, A.J.; Lazar, J.M.; Everaert, K.; Dmochowski, R.R. Foundational Statistical Principles in Medical Research: Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value. Medicina 2021, 57, 503. [Google Scholar] [CrossRef]
- Intuitive Surgical Inc. Firefly Fluorescence Imaging [Apparatus and Software]. Available online: https://manuals.intuitivesurgical.com/c/document_library/get_file?uuid=acee2a7d-bf8b-2c30-1c53-0a706f14f9fe&groupId=73750789 (accessed on 8 May 2021).
- Ito, A.; Ito, Y.; Matsushima, S.; Tsuchida, D.; Ogasawara, M.; Hasegawa, J.; Misawa, K.; Kondo, E.; Kaneda, N.; Nakanishi, H. New whole-body multimodality imaging of gastric cancer peritoneal metastasis combining fluorescence imaging with ICG-labeled antibody and MRI in mice. Gastric Cancer 2014, 17, 497–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liberale, G.; Vankerckhove, S.; Caldon, M.G.; Ahmed, B.; Moreau, M.; Nakadi, I.E.; Larsimont, D.; Donckier, V.; Bourgeois, P.; Group, R.; et al. Fluorescence Imaging After Indocyanine Green Injection for Detection of Peritoneal Metastases in Patients Undergoing Cytoreductive Surgery for Peritoneal Carcinomatosis from Colorectal Cancer: A Pilot Study. Ann. Surg. 2016, 264, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Kurata, M.; Oshiro, Y.; Shimomura, O.; Takahashi, K.; Oda, T.; Ohkohchi, N. Indocyanine green fluorescence-navigated laparoscopic metastasectomy for peritoneal metastasis of hepatocellular carcinoma: A case report. Surg. Case Rep. 2018, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Sato, H.; Yoshida, R.; Yasui, K.; Umeda, Y.; Yoshida, K.; Fuji, T.; Kumano, K.; Takagi, K.; Kagoura, M.; et al. Favorable control of hepatocellular carcinoma with peritoneal dissemination by surgical resection using indocyanine green fluorescence imaging: A case report and review of the literature. J. Med. Case Rep. 2022, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Barabino, G.; Klein, J.P.; Porcheron, J.; Grichine, A.; Coll, J.L.; Cottier, M. Intraoperative Near-Infrared Fluorescence Imaging using indocyanine green in colorectal carcinomatosis surgery: Proof of concept. Eur. J. Surg. Oncol. 2016, 42, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.; Hoogstins, C.E.; Peters, A.A.; de Kroon, C.D.; Trimbos, J.B.; van de Velde, C.J.; Frangioni, J.V.; Vahrmeijer, A.L.; Gaarenstroom, K.N. The Value of Intraoperative Near-Infrared Fluorescence Imaging Based on Enhanced Permeability and Retention of Indocyanine Green: Feasibility and False-Positives in Ovarian Cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef] [PubMed]
- Veys, I.; Pop, F.C.; Vankerckhove, S.; Barbieux, R.; Chintinne, M.; Moreau, M.; Nogaret, J.M.; Larsimont, D.; Donckier, V.; Bourgeois, P.; et al. ICG-fluorescence imaging for detection of peritoneal metastases and residual tumoral scars in locally advanced ovarian cancer: A pilot study. J. Surg. Oncol. 2018, 117, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; Group, A.L.-G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Fengler, J. Near-infrared fluorescence laparoscopy--technical description of PINPOINT(R) a novel and commercially available system. Colorectal Dis. 2015, 17 (Suppl. S3), 3–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]

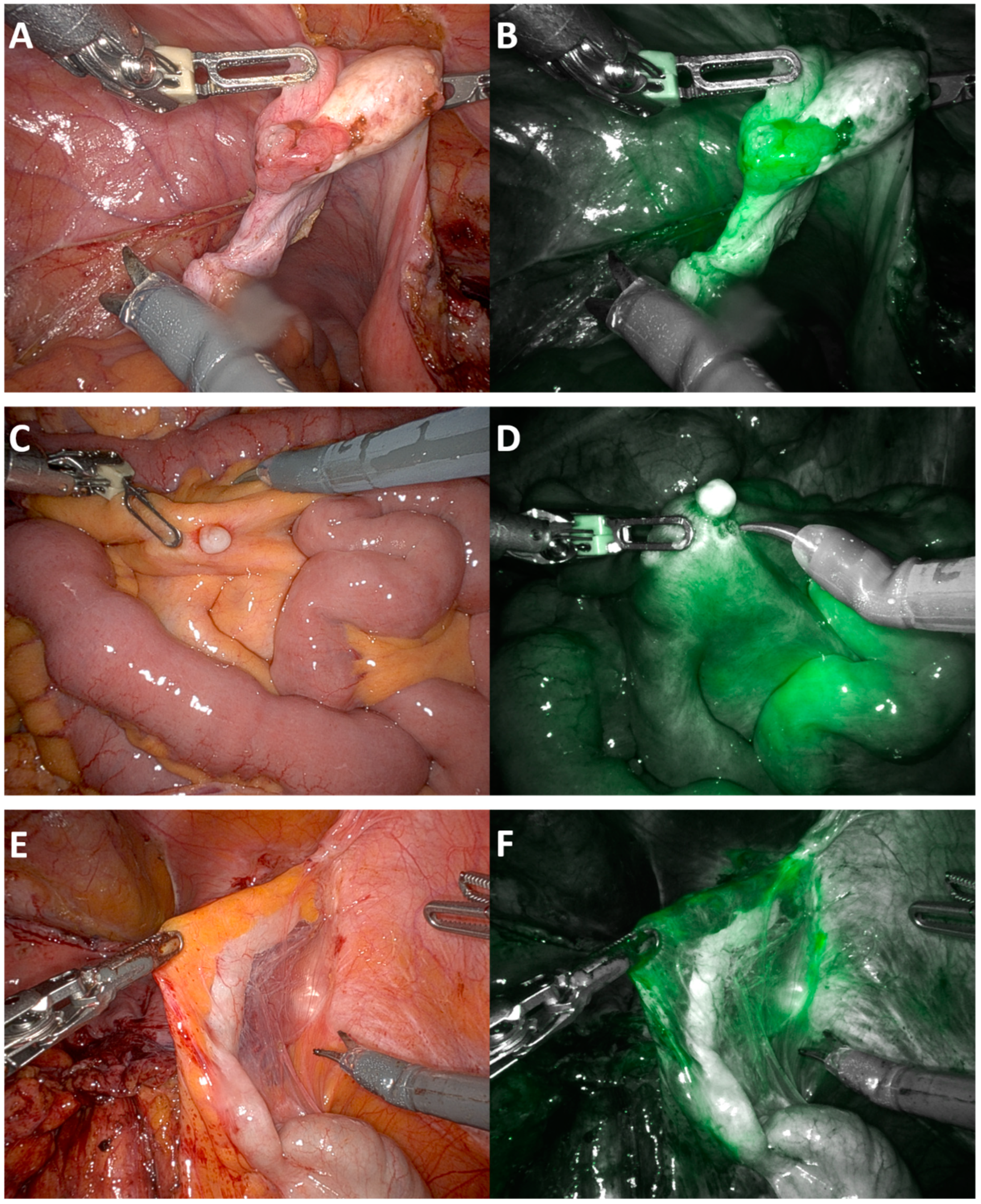
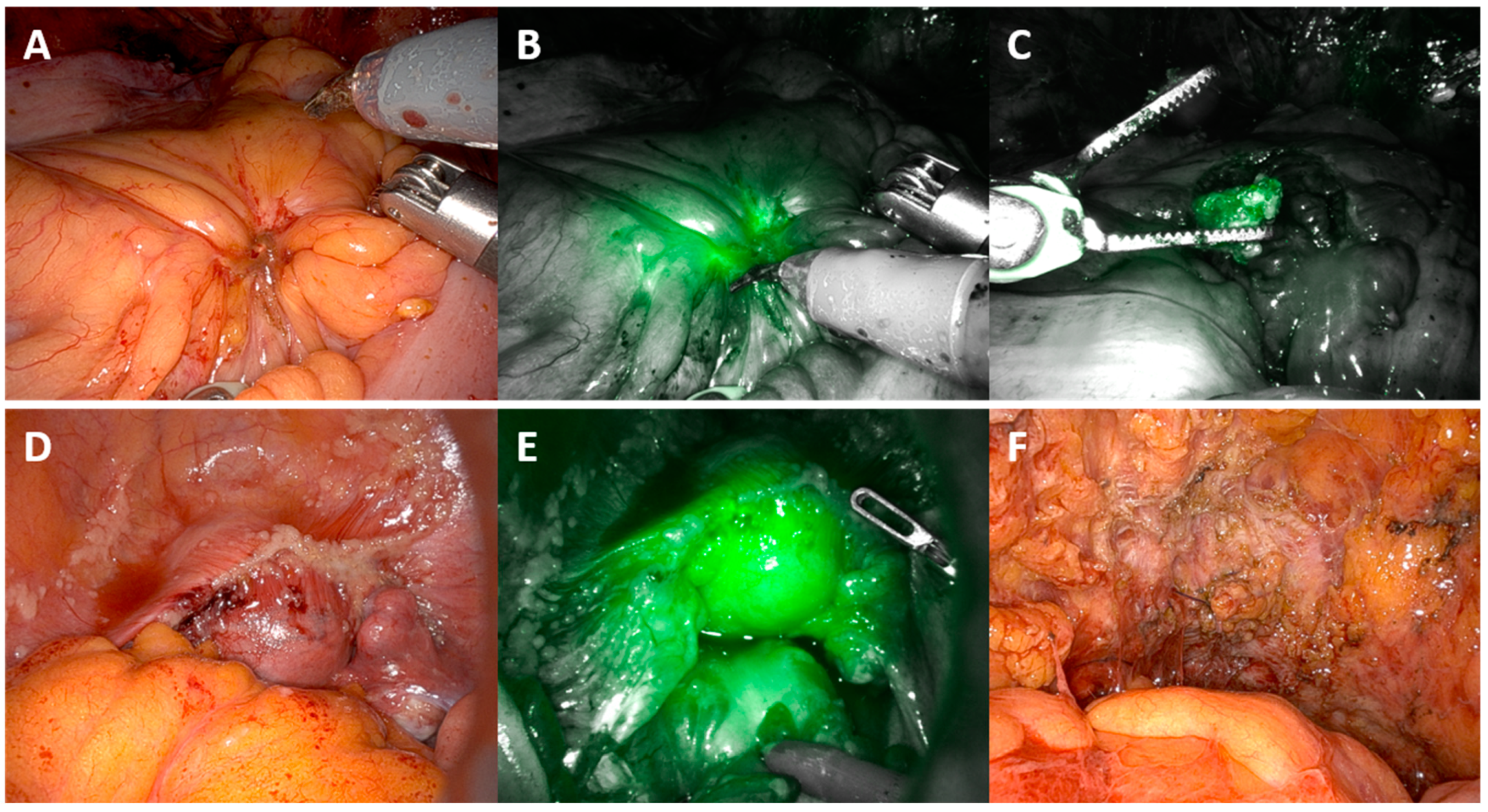
| MIRRORS ICG—Route of Surgery Robotic (n = 20) | |||||||
|---|---|---|---|---|---|---|---|
| Mean | Median (IQR) | Minimum | Maximum | n | n% | ||
| Age (years) | 67.2 | 68.5 (11.5) | 53.0 | 83.0 | 20 | 100.0% | |
| Body Mass Index | 24.7 | 24.0 (5.0) | 15.2 | 38.9 | 20 | 100.0% | |
| ECOG | 0 | 7 | 35.0% | ||||
| 1 | 12 | 60.0% | |||||
| 2 | 1 | 5.0% | |||||
| ASA | 1 | 0 | 0.0% | ||||
| 2 | 6 | 30.0% | |||||
| 3 | 14 | 70.0% | |||||
| Ethnicity | White British | 18 | 90.0% | ||||
| Any other White background | 1 | 5.0% | |||||
| Black Caribbean | 1 | 5.0% | |||||
| Parity (N) | 0 | 5 | 25.0% | ||||
| 1 | 2 | 10.0% | |||||
| 2 | 9 | 45.0% | |||||
| 3 | 2 | 10.0% | |||||
| 4 | 2 | 10.0% | |||||
| Smoking history | Ex-smoker | 6 | 30.0% | ||||
| Never smoked | 12 | 60.0% | |||||
| Smoker | 2 | 10.0% | |||||
| Number of previous abdominal surgeries | 0 | 4 | 20.0% | ||||
| 1 | 8 | 40.0% | |||||
| 2 | 4 | 20.0% | |||||
| 3 | 1 | 5.0% | |||||
| 4 | 2 | 10.0% | |||||
| 5 | 1 | 5.0% | |||||
| Comorbidity | |||||||
| Cardiac condition | 3 | 15.0% | |||||
| Previous VTE | 3 | 15.0% | |||||
| Anaemia | 1 | 5.0% | |||||
| Diabetes | 2 | 10.0% | |||||
| Vascular | 1 | 5.0% | |||||
| Hypertension | 4 | 20.0% | |||||
| Respiratory disease | 5 | 25.0% | |||||
| Dermatology condition | 2 | 10.0% | |||||
| Previous cancer | 4 | 20.0% | |||||
| Musculoskeletal/rheumatology | 5 | 25.0% | |||||
| Mental health | 1 | 5.0% | |||||
| Endocrine/autoimmune | 6 | 30.0% | |||||
| Route of Surgery Robotic | |||||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | n | n% | ||
| Number of cycles of chemotherapy prior to surgery | 4 | 4 | 3 | 6 | 20 | 100.0% | |
| Neoadjuvant chemotherapy | Carboplatin only | 1 | 5.0% | ||||
| Combined Carboplatin and Paclitaxel | 18 | 90.0% | |||||
| Other regimen | 1 | 5.0% | |||||
| BRCA | Negative | 19 | 95.0% | ||||
| BRCA1 | 0 | 0.0% | |||||
| BRCA2 | 0 | 0.0% | |||||
| na | 1 | 5% | |||||
| Tumour type | Adenocarcinoma | 0 | 0.0% | ||||
| Clear cell adenocarcinoma | 0 | 0.0% | |||||
| MMMT | 0 | 0.0% | |||||
| Neuroendocrine | 0 | 0.0% | |||||
| Serous | 20 | 100.0% | |||||
| Tumour site | Endometrial | 1 | 5% | ||||
| Ovary | 9 | 45.0% | |||||
| Peritoneum | 0 | 0.0% | |||||
| Tube | 10 | 50.0% | |||||
| Grade 3 | 20 | 100.0% | |||||
| Stage | 3c | 9 | 45.0% | ||||
| 4a | 2 | 10.0% | |||||
| 4b | 9 | 45.0% | |||||
| RECIST 1.1 | |||||||
| Complete response | 0 | 0.0% | |||||
| Partial response | 18 | 90.0% | |||||
| Stable disease | 2 | 10.0% | |||||
| Chemotherapy Response Score | |||||||
| 0 | 1 | 5.0% | |||||
| 1 | 10 | 50.0% | |||||
| 2 | 5 | 25.0% | |||||
| 3 | 4 | 20.0% | |||||
| Actual (Based on Histology) | ||||
|---|---|---|---|---|
| Total: 102 Biopsies | Positive (79) | Negative (23) | ||
| Observed | ICG Positive (92) | 72 | 20 | PPV 78.3% |
| ICG Negative (10) | 7 | 3 | NPV 30.0% | |
| Sensitivity 91.1% | Specificity 13.0% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwins, C.; Michael, A.; Skene, S.S.; Patel, H.; Ellis, P.; Chatterjee, J.; Tailor, A.; Butler-Manuel, S. MIRRORS ICG: Perfusion Assessment Using Indocyanine Green (ICG) Peritoneal Angiography during Robotic Interval Cytoreductive Surgery for Advanced Ovarian Cancer. Cancers 2024, 16, 2689. https://doi.org/10.3390/cancers16152689
Uwins C, Michael A, Skene SS, Patel H, Ellis P, Chatterjee J, Tailor A, Butler-Manuel S. MIRRORS ICG: Perfusion Assessment Using Indocyanine Green (ICG) Peritoneal Angiography during Robotic Interval Cytoreductive Surgery for Advanced Ovarian Cancer. Cancers. 2024; 16(15):2689. https://doi.org/10.3390/cancers16152689
Chicago/Turabian StyleUwins, Christina, Agnieszka Michael, Simon S. Skene, Hersha Patel, Patricia Ellis, Jayanta Chatterjee, Anil Tailor, and Simon Butler-Manuel. 2024. "MIRRORS ICG: Perfusion Assessment Using Indocyanine Green (ICG) Peritoneal Angiography during Robotic Interval Cytoreductive Surgery for Advanced Ovarian Cancer" Cancers 16, no. 15: 2689. https://doi.org/10.3390/cancers16152689
APA StyleUwins, C., Michael, A., Skene, S. S., Patel, H., Ellis, P., Chatterjee, J., Tailor, A., & Butler-Manuel, S. (2024). MIRRORS ICG: Perfusion Assessment Using Indocyanine Green (ICG) Peritoneal Angiography during Robotic Interval Cytoreductive Surgery for Advanced Ovarian Cancer. Cancers, 16(15), 2689. https://doi.org/10.3390/cancers16152689






