Does Extraesophageal Reflux Support the Development of Lung Adenocarcinoma? Analysis of Pepsin in Bronchoalveolar Lavage in Non-Smoker Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Statistics
- -
- Residuals vs. Fitted Plot: The relationship between predictors and the outcome appears linear, indicating an appropriate fit of the linear model.
- -
- Normal Q-Q Plot: There is no significant deviation from normality in the residuals, as supported by the Shapiro–Wilk normality test (p-value = 0.644).
- -
- Scale-Location Plot: The residuals are randomly spread, suggesting that the assumption of homoscedasticity is likely not violated.
- -
- Residuals vs. Leverage Plot**: There are no influential observations that could unduly affect the model’s results.
3. Results
3.1. Patient Characteristics
3.2. BAL Pepsin Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poelmans, J.; Tack, J. Extraoesophageal manifestations of gastro-oesophageal reflux. Gut 2005, 54, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Gaude, G.S. Pulmonary manifestations of gastroesophageal reflux disease. Ann. Thorac. Med. 2009, 4, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.P.; Parikh, S. Review article: Reflux and its consequences—The laryngeal, pulmonary and oesophageal manifestations. Aliment. Pharmacol. Ther. 2011, 33 (Suppl. S1), 1–71. [Google Scholar] [PubMed]
- Cui, N.; Dai, T. Laryngopharyngeal reflux disease: Updated examination of mechanisms, pathophysiology, treatment, and association with gastroesophageal reflux disease. World J. Gastroenterol. 2024, 30, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.O.; Dunbar, K.B. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T. Pepsinogens, progastricsins, and prochymosins: Structure, function, evolution, and development. Cell. Mol. Life Sci. 2002, 59, 288–306. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.; Dettmar, P.W. Activity/stability of human pepsin: Implications for reflux attributed laryngeal disease. Laryngoscope 2007, 117, 1036–1039. [Google Scholar] [CrossRef]
- Goldberg, H.I.; Dodds, W.J. Role of acid and pepsin in acute experimental esophagitis. Gastroenterology 1969, 56, 223–230. [Google Scholar] [CrossRef]
- Samuels, T.L.; Mandler, E. Mucin gene expression in human laryngeal epithelia: Effect of laryngopharyngeal reflux. Ann. Otol. Rhinol. Laryngol. 2008, 117, 688–695. [Google Scholar] [CrossRef]
- Johnston, N.; Knight, J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope 2004, 114, 2129–2134. [Google Scholar] [CrossRef]
- Johnston, N.; Dettmar, P.W. Effect of pepsin on laryngeal stress protein (Sep70, Sep53, and Hsp70) response: Role in laryngopharyngeal reflux disease. Ann. Otol. Rhinol. Laryngol. 2006, 115, 47–58. [Google Scholar] [CrossRef]
- Johnston, N.; Wells, C.W. Receptor-mediated uptake of pepsin by laryngeal epithelial cells. Ann. Otol. Rhinol. Laryngol. 2007, 116, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Hepworth, E.J. Gastroesophageal reflux disease is a risk factor for laryngeal and pharyngeal cancer. Am. J. Gastroenterol. 2001, 96, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Bacciu, A.; Mercante, G. Effects of gastroesophageal reflux disease in laryngeal carcinoma. Clin. Otolaryngol. Allied Sci. 2004, 29, 545–548. [Google Scholar] [CrossRef]
- Tae, K.; Jin, B.J. The role of laryngopharyngeal reflux as a risk factor in laryngeal cancer: A preliminary report. Clin. Exp. Otorhinolaryngol. 2011, 4, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.M.; Michaud, D.S. Gastric reflux is an independent risk factor for laryngopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1061–1068. [Google Scholar] [CrossRef]
- Parsel, S.M.; Wu, E.L. Gastroesophageal and Laryngopharyngeal Reflux Associated with Laryngeal Malignancy: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1253–1264.e5. [Google Scholar] [CrossRef]
- Johnston, N.; Bulmer, D. Cell Biology of Laryngeal Epithelial Defenses in Health and Disease: Further Studies. Ann. Otol. Rhinol. Laryngol. 2003, 112, 481–491. [Google Scholar] [CrossRef]
- Hsu, C.K.; Lai, C.C. Risk of lung cancer in patients with gastro-esophageal reflux disease: A population-based cohort study. PeerJ 2016, 4, e2753. [Google Scholar] [CrossRef]
- Li, L.; Ren, Q. Causal associations between gastroesophageal reflux disease and lung cancer risk: A Mendelian randomization study. Cancer Med. 2023, 12, 7552–7559. [Google Scholar] [CrossRef] [PubMed]
- Vereczkei, A.; Horvath, O.P. Gastroesophageal reflux disease and non-small cell lung cancer. Results of a pilot study. Dis. Esophagus 2008, 21, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Starr, A. The Association between Gastroesophageal Reflux Disease and Non-Small Cell Lung Cancer: A Retrospective Case-Control Study. Gastroenterol. Res. 2022, 15, 173–179. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.2.2); R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 11 September 2023).
- Argyrou, A.; Legaki, E. Risk factors for gastroesophageal reflux disease and analysis of genetic contributors. World J. Clin. Cases 2018, 6, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Alduais, Y.; Zhang, H. Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Shaykhiev, R. Lung adenocarcinoma subtypes based on expression of human airway basal cell genes. Eur. Respir. J. 2013, 42, 1332–1344. [Google Scholar] [CrossRef]
- Human Pepsin (PP) Elisa Kit. Available online: https://www.elisakit.cc/uploads/file/e01p0699-human-pp-c.pdf (accessed on 22 April 2021).
- Lee, J.S.; Song, J.W. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur. Respir. J. 2012, 39, 352–358. [Google Scholar] [CrossRef]

| Gastroesophageal Reflux | Extraesophageal Reflux | |
|---|---|---|
| Definition | Occurs due to the backflow of gastric contents into the esophagus. If DeMeester criteria are met (pH < 4 or > 7 in more than 50 episodes within 24 h, or reflux lasting longer than 1 h within 24 h according to pH monitoring), it is considered pathological reflux. | Occurs when refluxate rises above the upper esophageal sphincter. |
| Symptoms | Heartburn, regurgitation, dysphagia, chest pain | Chronic cough, postnasal drip syndrome, hoarseness or sore throat, halitosis, dental erosions, globus sensation, chest pain, dyspnoea, sleep disturbance, wheeze |
| Diagnostic tools | Medical history, physical examination, esophagogastroduodenoscopy, 24-h pH-metry, 24-h multichannel intraluminal impedance (MII), esophagogram, esophageal manometry Trial of medication: response to acid-suppressing medications (proton pump inhibitors or PPIs, H2-receptor antagonists) Questionnaires: various standardized questionnaires—such as GERD-Q | Medical history, physical examination, fibrolaryngoscopy, 24-h pH-metry, 24-h multichannel intraluminal impedance (MII), questionnaires: such as RSI |
| Significance of acidity | Yes | Low |
| Group | Lung Adenocarcinoma | Pulmonary Metastases | Lung Sarcoidosis | |
|---|---|---|---|---|
| (Group I) | (Group II) | (Group III) | p-value | |
| Group size (%) | 30/71 (42.25%) | 29/71 (40.85%) | 12/71 (16.9%) | - |
| Age (Median [IQR]) | ||||
| 73 (69, 76) | 68 (62, 76) | 56 (45, 59) | <0.001 a | |
| Sex | ||||
| Male | 15/30 (50%) | 13/29 (44.8%) | 6/12 (50%) | 0.912 b |
| Female | 15/30 (50%) | 16/29 (55.2%) | 6/12 (50%) | |
| Smoking status | ||||
| Never | 15/30 (50%) | 16/29 (55.2%) | 8/12 (66.7%) | 0.618 b |
| Former | 15/30 (50%) | 13/29 (44.8%) | 4/12 (33.3%) | |
| Hemorrhagic BAL | ||||
| No | 23/30 (76.7%) | 24/29 (82.8%) | 11/12 (91.7%) | 0.515 b |
| Yes | 7/30 (23.3%) | 5/29 (17.2%) | 1/12 (8.3%) | |
| Estimate | 95% Confidence Interval for Coefficient | p-Value | ||
|---|---|---|---|---|
| Low | High | |||
| (Intercept) | 474.957 | 322.4074 | 627.5075 | 4.44 × 10−8 |
| Lung adenocarcinoma | −75.623 | −138.457 | −12.7887 | 0.019119 |
| Age | −1.777 | −4.24291 | 0.688678 | 0.154757 |
| Female sex | 16.628 | −42.0543 | 75.3099 | 0.573244 |
| Former smoker | 107.88 | 49.06649 | 166.6941 | 0.000508 |
| Hemorrhagic BAL | 99.924 | 23.23115 | 176.6161 | 0.011489 |
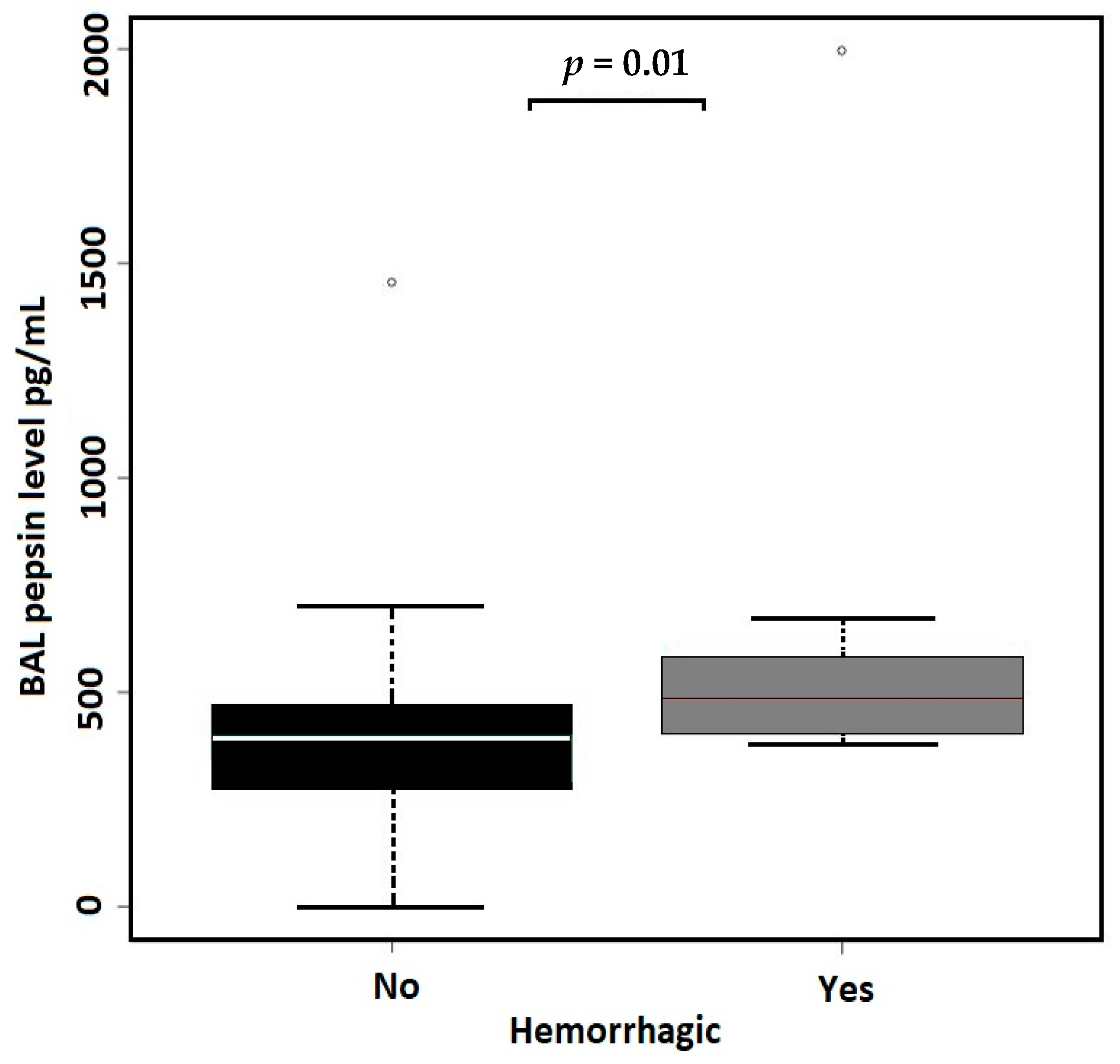
| (A) All Samples, N = 71 | Non-Hemorrhagic BAL | Hemorrhagic BAL | Effect Size b | p-Value | |
| BAL PEPSIN levels | N = 58 | N = 13 | |||
| Median (MAD) [I.Q.] | 395.35 (133.8) [280.2, 472.43] | 483.9 (139.4) [404, 581] | 0.657 | 0.01 a | |
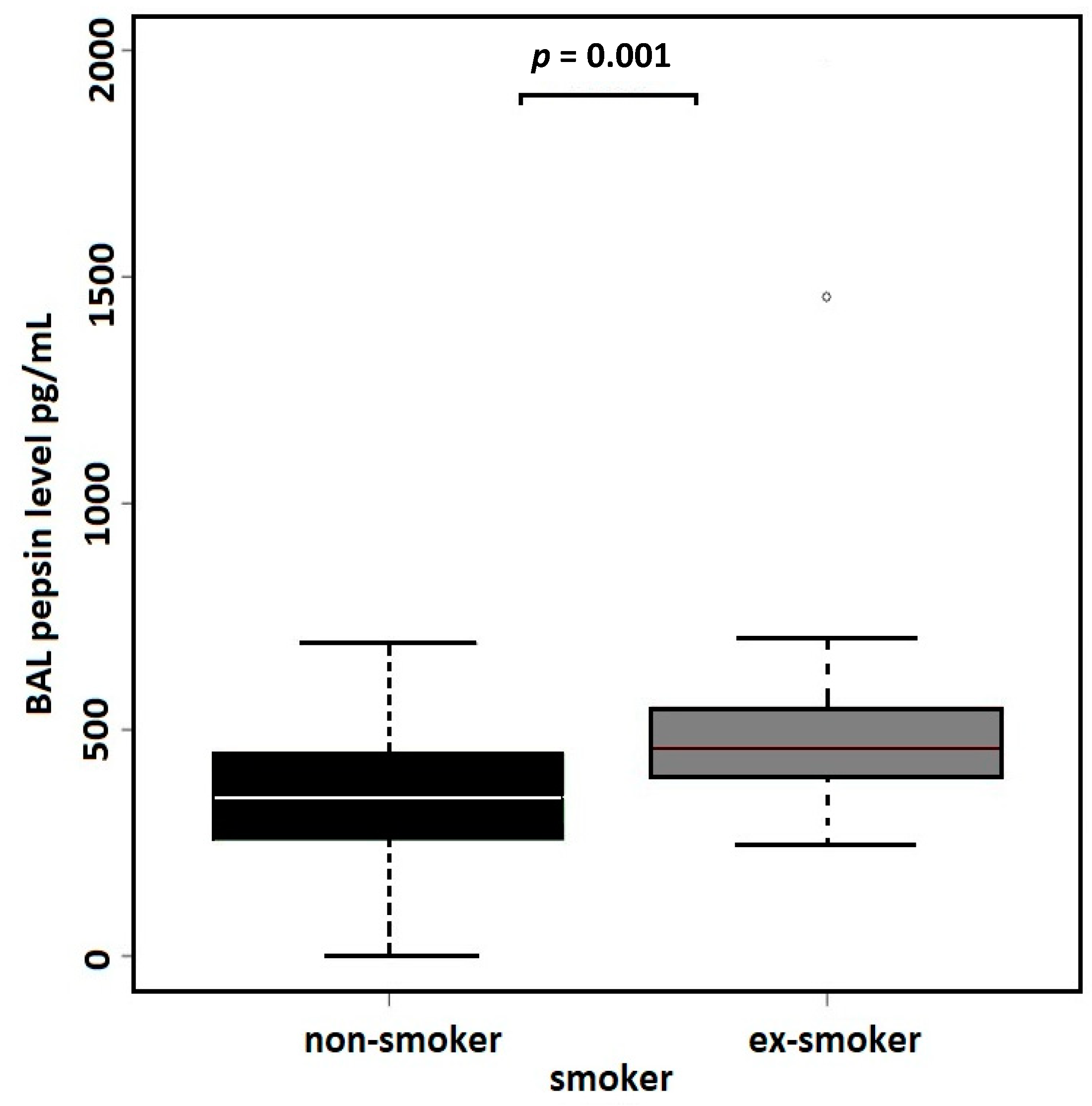
| (B) All Samples, N = 71 | Non-Smokers | Former Smokers | |||
| BAL PEPSIN levels | N = 39 | N = 32 | |||
| Median (MAD) [I.Q.] | 350.9 (143.8) [257.45, 446.6] | 458.4 (98.7) [399.17, 545.95] | 0.856 | 0.001 a | |
| (C) Non-Hemorrhagic Samples, N = 58 | Non-Smokers | Former Smokers | |||
| BAL PEPSIN levels | N = 34 | N = 24 | |||
| Median (MAD) [I.Q.] | 329.5 (145.7) [246.85, 440.42] | 451.5 (82.5) [392.18, 487.3] | 0.986 | 0.002 a | |
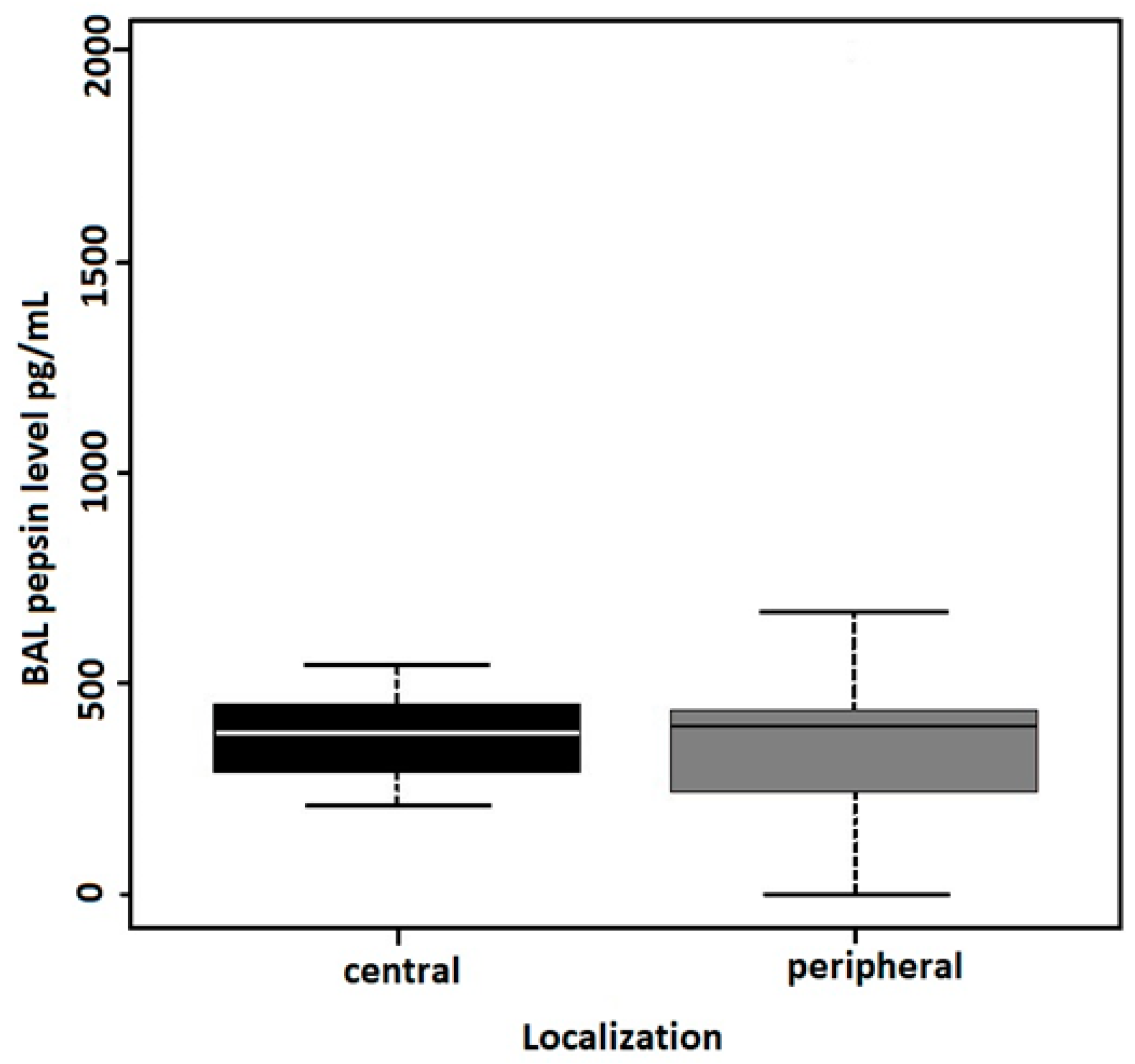
| (D) Lung Adenocarcinoma, N = 30 | Central | Peripheral | |||
| BAL PEPSIN levels | N = 11 | N = 19 | |||
| Median (MAD) [I.Q.] | 382.9 (138.6) [288.45, 450.15] | 398.2 (176.7) [243.8, 434.9] | 0.093 | 0.667 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemanova, P.; Vocka, M.; Vanickova, Z.; Liska, F.; Krizova, L.; Kalab, J.; Votruba, J. Does Extraesophageal Reflux Support the Development of Lung Adenocarcinoma? Analysis of Pepsin in Bronchoalveolar Lavage in Non-Smoker Patients. Cancers 2024, 16, 2687. https://doi.org/10.3390/cancers16152687
Zemanova P, Vocka M, Vanickova Z, Liska F, Krizova L, Kalab J, Votruba J. Does Extraesophageal Reflux Support the Development of Lung Adenocarcinoma? Analysis of Pepsin in Bronchoalveolar Lavage in Non-Smoker Patients. Cancers. 2024; 16(15):2687. https://doi.org/10.3390/cancers16152687
Chicago/Turabian StyleZemanova, Petra, Michal Vocka, Zdislava Vanickova, Frantisek Liska, Ludmila Krizova, Josef Kalab, and Jiri Votruba. 2024. "Does Extraesophageal Reflux Support the Development of Lung Adenocarcinoma? Analysis of Pepsin in Bronchoalveolar Lavage in Non-Smoker Patients" Cancers 16, no. 15: 2687. https://doi.org/10.3390/cancers16152687
APA StyleZemanova, P., Vocka, M., Vanickova, Z., Liska, F., Krizova, L., Kalab, J., & Votruba, J. (2024). Does Extraesophageal Reflux Support the Development of Lung Adenocarcinoma? Analysis of Pepsin in Bronchoalveolar Lavage in Non-Smoker Patients. Cancers, 16(15), 2687. https://doi.org/10.3390/cancers16152687






