The Effect of Immune Checkpoint Inhibitor Therapy on Pre-Existing Gastroparesis and New Onset of Symptoms of Delayed Gastric Emptying
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
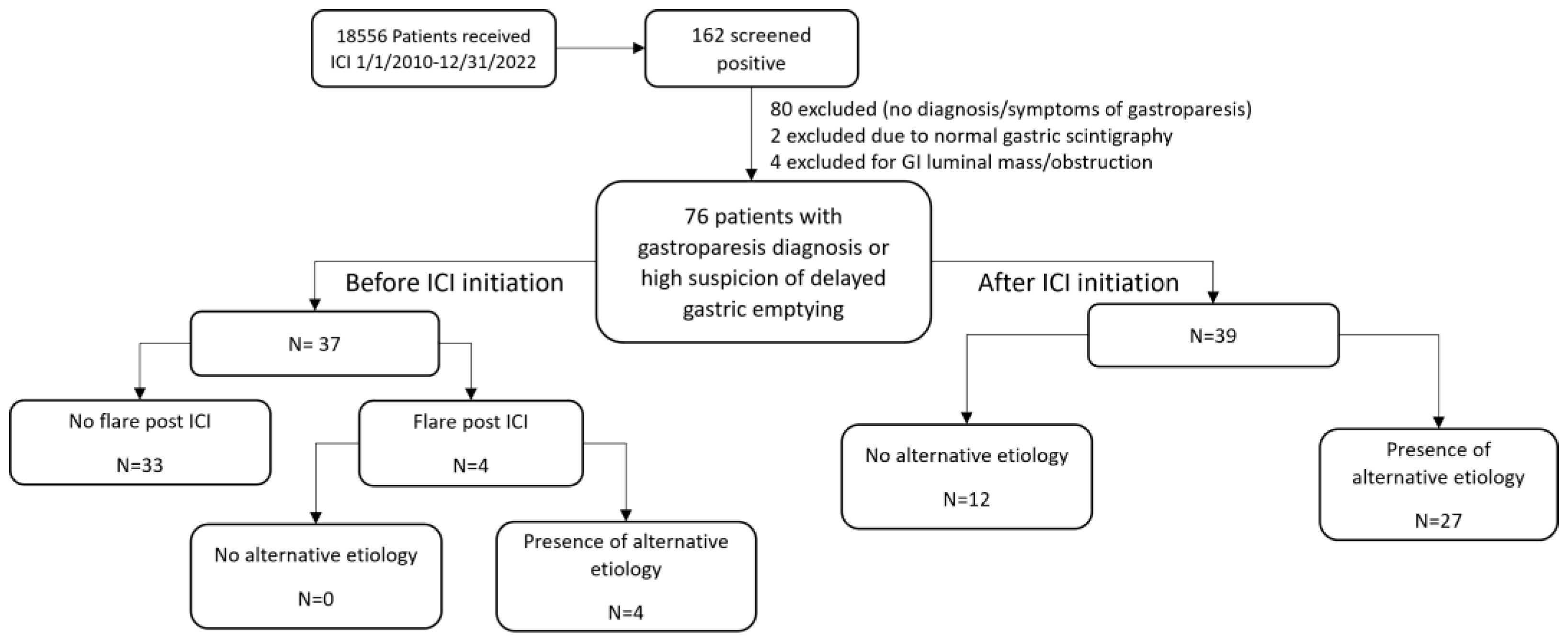
2.1. Patients
2.2. Definitions
2.3. Diagnostic Study
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Subgtoup Analysis of Patients Stratified by the Presence of Alternative Etiologies for Upper GI Symptoms
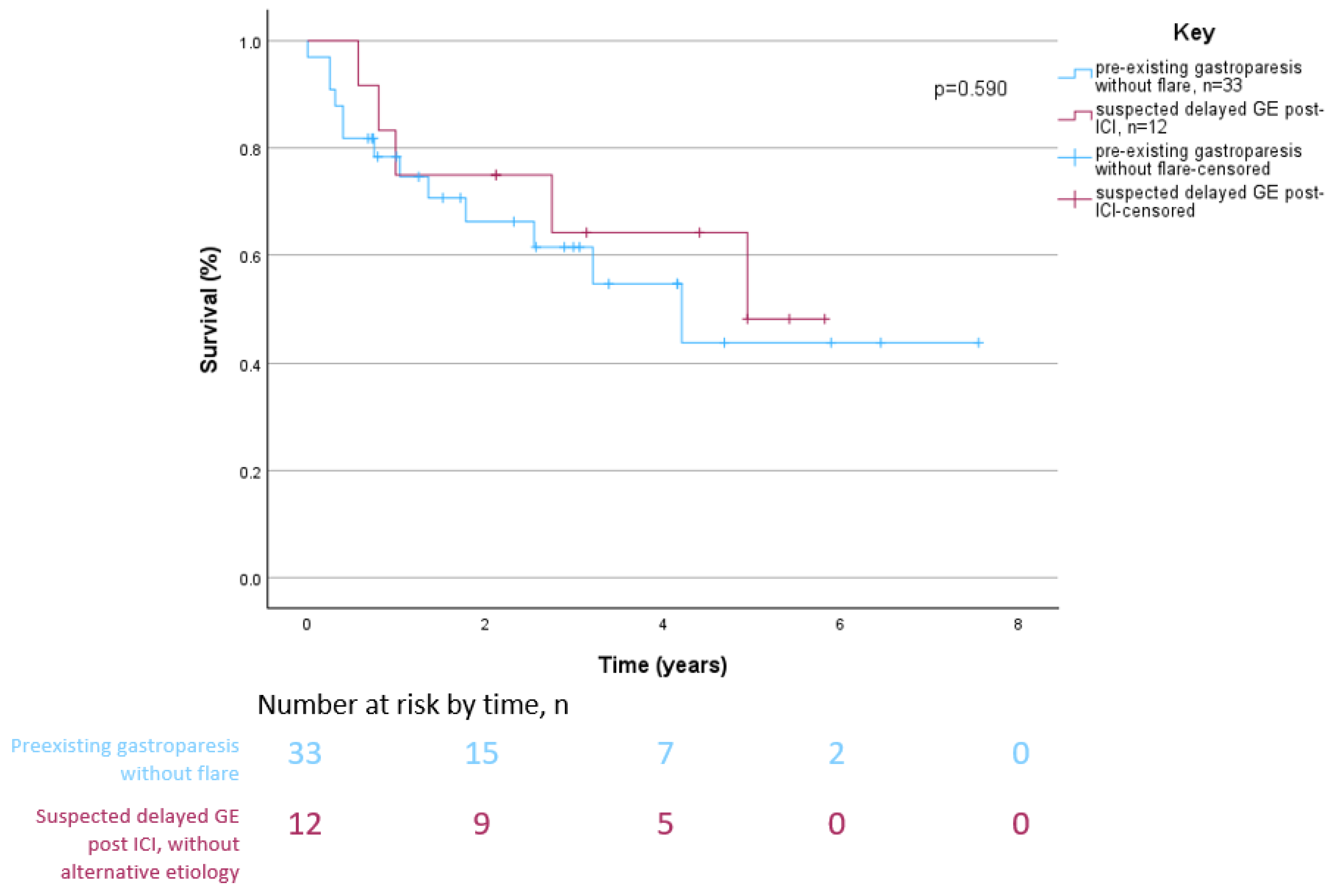
3.3. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Page, D.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Sanders, K.M. Gastroparesis. Gastroenterology 2022, 162, 68–87.e61. [Google Scholar] [CrossRef] [PubMed]
- Hyett, B.; Martinez, F.J.; Gill, B.M.; Mehra, S.; Lembo, A.; Kelly, C.P.; Leffler, D.A. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology 2009, 137, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Malagelada, J.; Brown, M.; Becker, G.; Zinsmeister, A. Relation between antral motility and gastric emptying of solids and liquids in humans. Am. J. Physiol. -Gastrointest. Liver Physiol. 1985, 249, G580–G585. [Google Scholar] [CrossRef] [PubMed]
- Nakane, S.; Mukaino, A.; Ihara, E.; Ogawa, Y. Autoimmune gastrointestinal dysmotility: The interface between clinical immunology and neurogastroenterology. Immunol. Med. 2021, 44, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Farrugia, G.; Lurken, M.S.; Bernard, C.E.; Faussone–Pellegrini, M.S.; Smyrk, T.C.; Parkman, H.P.; Abell, T.L.; Snape, W.J.; Hasler, W.L. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 2011, 140, 1575–1585.e8. [Google Scholar] [CrossRef]
- Cipriani, G.; Gibbons, S.J.; Miller, K.E.; Yang, D.S.; Terhaar, M.L.; Eisenman, S.T.; Ördög, T.; Linden, D.R.; Gajdos, G.B.; Szurszewski, J.H.; et al. Change in Populations of Macrophages Promotes Development of Delayed Gastric Emptying in Mice. Gastroenterology 2018, 154, 2122–2136.e12. [Google Scholar] [CrossRef]
- Choi, K.M.; Kashyap, P.C.; Dutta, N.; Stoltz, G.J.; Ordog, T.; Shea Donohue, T.; Bauer, A.J.; Linden, D.R.; Szurszewski, J.H.; Gibbons, S.J.; et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology 2010, 138, 2399–2409.e1. [Google Scholar] [CrossRef]
- Bhatia, S.; Huber, B.R.; Upton, M.P.; Thompson, J.A. Inflammatory enteric neuropathy with severe constipation after ipilimumab treatment for melanoma: A case report. J. Immunother. 2009, 32, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, J.; Wells, D.; Hiatt, J.B.; Steinbach, G.; Stewart, F.M.; Thomas, H.; Nghiem, P.; Kapur, R.P.; Thompson, J.A.; Bhatia, S. Fatal enteric plexus neuropathy after one dose of ipilimumab plus nivolumab: A case report. J. Immunother. Cancer 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Shroff, S.; Kamiya-Matsuoka, C.; Tummala, S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014, 16, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, J.E.; Steffens, J.D.; Clawson, S.A.; Hill, K.; Dalmau, J. Anti-Hu antibodies in Merkel cell carcinoma. Ann. Neurol. 2002, 52, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A.F. Neurologic complications of immune checkpoint inhibitors. Curr. Opin. Neurol. 2016, 29, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Atieh, J.; Sack, J.; Thomas, R.; Rahma, O.E.; Camilleri, M.; Grover, S. Gastroparesis following immune checkpoint inhibitor therapy: A case series. Dig. Dis. Sci. 2021, 66, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Bernardini, A.; Gigli, G.L.; Valente, M.; Muñiz-Castrillo, S.; Honnorat, J.; Vogrig, A. Neurologic Adverse Events of Immune Checkpoint Inhibitors. A Syst. Rev. 2021, 96, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Schneider, B.; Brahmer, J. NCCN Clinical Practice Guidelines in Oncology: Management of Immunotherapy-Related Toxicities. Available online: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (accessed on 12 January 2024).
- Corinaldesi, R.; Raiti, C.; Stanghellini, V.; Monetti, N.; Rea, E.; Salgemini, R. Comparative effects of oral cisapride and metoclopramide on gastric emptying of solids and symptoms in patients with functional dyspepsia and gastroparesis. Curr. Ther. Res. 1987, 42, 428–435. [Google Scholar]
- Shafi, M.A. Gastrointestinal motility issues in cancer patients. Curr. Gastroenterol. Rep. 2019, 21, 69. [Google Scholar] [CrossRef]
- Amjad, W.; Doycheva, I.; Kamal, F.; Malik, A.; Pandu, A.; Shabbir, M.A.; Mumtaz, M.; Batool, A.; Ukleja, A. Clinical predictors of symptom improvement failure in gastroparesis. Ann. Gastroenterol. 2022, 35, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Annede, P.; Prieux-Klotz, C.; Dubergé, T.; Chargari, C.; Gisserot, O.; de Jaureguiberry, J.P. Radiation induced gastroparesis-case report and literature review. J. Gastrointest. Oncol. 2017, 8, E52–E55. [Google Scholar] [CrossRef]
- Parkman, H.P.; Yates, K.; Hasler, W.L.; Nguyen, L.; Pasricha, P.J.; Snape, W.J.; Farrugia, G.; Koch, K.L.; Calles, J.; Abell, T.L.; et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin. Gastroenterol. Hepatol. 2011, 9, 1056–1064; quiz e1133-1054. [Google Scholar] [CrossRef]
- Ghisoni, E.; Wicky, A.; Bouchaab, H.; Imbimbo, M.; Delyon, J.; Gautron Moura, B.; Gérard, C.L.; Latifyan, S.; Özdemir, B.C.; Caikovski, M.; et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: An overlooked aspect in immunotherapy. Eur. J. Cancer 2021, 149, 153–164. [Google Scholar] [CrossRef]
- Tison, A.; Garaud, S.; Chiche, L.; Cornec, D.; Kostine, M. Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat. Rev. Rheumatol. 2022, 18, 641–656. [Google Scholar] [CrossRef]
- Alexander, S.; Swami, U.; Kaur, A.; Gao, Y.; Fatima, M.; Ginn, M.M.; Stein, J.E.; Grivas, P.; Zakharia, Y.; Singh, N. Safety of immune checkpoint inhibitors in patients with cancer and pre-existing autoimmune disease. Ann. Transl. Med. 2021, 9, 1033. [Google Scholar] [CrossRef]
- Pizuorno Machado, A.; Shatila, M.; Liu, C.; Wang, J.; Altan, M.; Zhang, H.C.; Thomas, A.; Wang, Y. Immune-related adverse events after immune checkpoint inhibitor exposure in adult cancer patients with pre-existing autoimmune diseases. J. Cancer Res. Clin. Oncol. 2023, 149, 6341–6350. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Faleck, D.M.; Ricciuti, B.; Mendelsohn, R.B.; Naqash, A.R.; Cohen, J.V.; Sellers, M.C.; Balaji, A.; Ben-Betzalel, G.; Hajir, I.; et al. Immune Checkpoint Inhibitor Therapy in Patients with Preexisting Inflammatory Bowel Disease. J. Clin. Oncol. 2020, 38, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Chikkamenahalli, L.L.; Jessen, E.; Bernard, C.E.; Ip, W.K.E.; Breen-Lyles, M.; Cipriani, G.; Pullapantula, S.R.; Li, Y.; AlAsfoor, S.; Wilson, L.; et al. Single cell atlas of human gastric muscle immune cells and macrophage-driven changes in idiopathic gastroparesis. iScience 2024, 27, 108991. [Google Scholar] [CrossRef] [PubMed]
- Revicki, D.A.; Rentz, A.M.; Dubois, D.; Kahrilas, P.; Stanghellini, V.; Talley, N.J.; Tack, J. Gastroparesis Cardinal Symptom Index (GCSI): Development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual. Life Res. 2004, 13, 833–844. [Google Scholar] [CrossRef]
- Lacy, B.E.; Tack, J.; Gyawali, C.P. AGA Clinical Practice Update on Management of Medically Refractory Gastroparesis: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 491–500. [Google Scholar] [CrossRef]

| Variable | Patients with Pre-Existing Gastroparesis, n = 37 | Patients with New Onset of Suspected Delayed GE after ICI Therapy, n = 39 | p |
|---|---|---|---|
| Median age at delayed GE onset (IQR), years | 59.4 (45.0–65.7) | 64.5 (55.4–68.5) | 0.034 |
| Male sex | 19 (51) | 26 (67) | 0.243 |
| Cancer type | 0.003 | ||
| Gastrointestinal | 13 (35) | 6 (15) | |
| Biliary/liver | 3 (23) | 1 (17) | |
| Esophagus | 4 (31) | 2 (33) | |
| Gastric/Duodenal | 4 (31) | 1 (17) | |
| Colon/Rectum/Other | 2 (15) | 2 (33) | |
| Lung/head and neck | 8 (22) | 12 (31) | |
| Pharynx/Tonsil | 2 (25) | 4 (33) | |
| Lung | 6 (75) | 8 (67) | |
| Melanoma | 5 (14) | 7 (18) | |
| Gynecological | 5 (14) | 1 (3) | |
| Hematological | 5 (14) | 1 (3) | |
| Genitourinary | 1 (3) | 10 (26) | |
| Endocrine | 0 (0) | 2 (5) | |
| ICI type | 0.328 | ||
| CTLA-4 inhibitor | 5 (14) | 4 (10) | |
| PD-1/PD-L1 inhibitor | 28 (77) | 29 (74) | |
| Combination of both | 4 (11) | 6 (14) | |
| Flare-up of pre-existing gastroparesis after ICI therapy | 4 (11) | -- | -- |
| irAE after ICI therapy | 1 (3) | 13 (33) | 0.001 |
| Hepatitis | 1 (100) | 3 (23) | |
| Colitis | 0 (0) | 1 (8) | |
| Pancreatitis | 0 (0) | 2 (15) | |
| Hypophysitis | 0 (0) | 2 (15) | |
| Vasculitis | 0 (0) | 1 (8) | |
| Pneumonitis | 0 (0) | 4 (31) | |
| All-cause mortality | 15 (41) | 23 (59) | 0.168 |
| Median follow-up duration after ICI initiation (IQR), years | 1.7 (0.7–3.8) | 2.1 (0.8–4.6) | 0.543 |
| Characteristic | Pre-Existing Gastroparesis without Flare-Up after ICI Therapy, n = 33 | Pre-Existing Gastroparesis with Flare-Up after ICI Therapy, n = 4 | New Onset of Suspected Delayed GE or Gastroparesis after ICI Therapy, n = 39 |
|---|---|---|---|
| Alternative etiology for gastroparesis | 26 (79) | 4 (100) | 27 (69) |
| Diabetes | 9 (27) | 1 (25) | 13 (37) |
| Prior bariatric procedures | 4 (12) | 0 (0) | 0 (0) |
| GI cancer | 11 (33) | 2 (50) | 6 (15) |
| Medications | |||
| Opioids | 13 (39) | 1 (25) | 16 (41) |
| PPI | 18 (54) | 1 (25) | 23 (59) |
| H2RA receptor antagonist | 1 (3) | 0 (0) | 1 (3) |
| Anticholinergics | 1 (3) | 0 (0) | 1 (3) |
| GLP-1 agonists | 0 (0) | 0 (0) | 1 (3) |
| Study for delayed GE a | |||
| Gastric scintigraphy | 8 (24) | 0 (0) | 4 (10) |
| UGIS | 0 (0) | 0 (0) | 5 (13) |
| Presenting symptom | |||
| Nausea | 33 (100) | 2 (50) | 35 (90) |
| Vomiting | 31 (94) | 3 (75) | 30 (77) |
| Abdominal pain | 9 (27) | 1 (25) | 11 (28) |
| Early satiety | 8 (24) | 1 (25) | 7 (18) |
| Weight loss | 6 (18) | 0 (0) | 6 (15) |
| Constipation | 5 (15) | 0 (0) | 4 (10) |
| High residuals on NGT | 0 (0) | 0 (0) | 2 (5) |
| No. of hospitalizations for gastroparesis or symptoms related to delayed GE | |||
| 0 | 20 (61) | 3 (75) | 31 (80) |
| 1 | 12 (36) | 1 (25) | 8 (21) |
| 2 | 1 (3) | 0 (0) | 0 (0) |
| 4 | 1 (3) | 0 (0) | 0 (0) |
| Median time from ICI initiation to symptoms of delayed GE (IQR), months | -- | 10.2 (0.7–28.6) | 12.8 (4.4–35.5) |
| Symptomatic treatment for presumed delayed GE, b | |||
| Domperidone | 2 (6) | 0 (0) | 0 (0) |
| Metoclopramide | 28 (85) | 4 (100) | 35 (90) |
| Macrolide | 1 (3) | 0 (0) | 1 (3) |
| Steroids | 1 (3) | 0 (0) | 0 (0) |
| Pyloric botulin | 2 (6) | 0 (0) | 0 (0) |
| Transpyloric stenting | 2 (6) | 0 (0) | 0 (0) |
| Supportive care | |||
| NGT decompression | 2 (6) | 0 (0) | 0 (0) |
| Parenteral nutrition | 0 (0) | 0 (0) | 2 (5) |
| GJ or J tube | 3 (9) | 0 (0) | 7 (18) |
| Clinical response/remission | 9 (27) | 1 (25) | 14 (36) |
| Median duration of clinical symptoms of patients with clinical response/remission (IQR), days | -- | 2 | 108 (14.5–780) |
| Resumption of ICI therapy after symptoms of delayed GE | -- | 0 (0) | 19 (49) |
| Characteristic | No Alternative Etiology, n = 12 | Alternative Etiology, n = 27 | p |
|---|---|---|---|
| Study for delayed GE | |||
| Gastric scintigraphy | 1 (8) | 3 (11) | 1.00 |
| UGIS | 0 (0) | 5 (19) | 0.299 |
| Presenting symptom | |||
| Nausea | 9 (75) | 26 (96) | 0.078 |
| Vomiting | 7 (58) | 23 (85) | 0.102 |
| Abdominal pain | 5 (42) | 6 (22.2) | 0.262 |
| Early satiety | 4 (33) | 3 (11) | 0.172 |
| Weight loss | 2 (17) | 4 (15) | 1.00 |
| Constipation | 2 (17) | 2 (7) | 0.573 |
| High residuals on NGT suctioning | 0 (0) | 2 (7) | 1.00 |
| Median time from ICI initiation to new onset of delayed GE (IQR), months | 14.4 (6.1–26.3) | 10.7 (1.4–39.3) | 0.642 |
| No. of hospitalizations for delayed GE–related symptoms | 0.394 | ||
| 0 | 11 (92) | 20 (74) | |
| 1 | 1 (8) | 7 (26) | |
| Gastroparesis medication | |||
| Metoclopramide | 11 (92) | 24 (89) | 0.640 |
| Macrolide | 0 (0) | 1 (4) | -- |
| Supportive care | 0.417 | ||
| Parenteral nutrition | 1 (8) | 1 (4) | |
| GJ or J tube | 1 (8) | 6 (22) | |
| HbA1c level (in diabetic patients) | 1.00 | ||
| <6.5% | 2 (17) | 4 (15) | |
| ≥6.5% | 3 (25) | 9 (33) | |
| Clinical response/remission achieved | 3 (25) | 11 (41) | 0.477 |
| Median duration of clinical symptoms for patients with clinical response/remission (IQR), days | 290 (147–387) | 74.5 (21.5–690.0) | 1.00 |
| Resumption of ICI therapy after symptoms of delayed GE | 5 (42) | 14 (52) | 0.731 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urias Rivera, A.C.; Pizuorno Machado, A.; Shatila, M.; Triadafilopoulos, G.; McQuade, J.L.; Altan, M.; Zhao, D.; Wang, Y.; Shafi, M.A. The Effect of Immune Checkpoint Inhibitor Therapy on Pre-Existing Gastroparesis and New Onset of Symptoms of Delayed Gastric Emptying. Cancers 2024, 16, 2658. https://doi.org/10.3390/cancers16152658
Urias Rivera AC, Pizuorno Machado A, Shatila M, Triadafilopoulos G, McQuade JL, Altan M, Zhao D, Wang Y, Shafi MA. The Effect of Immune Checkpoint Inhibitor Therapy on Pre-Existing Gastroparesis and New Onset of Symptoms of Delayed Gastric Emptying. Cancers. 2024; 16(15):2658. https://doi.org/10.3390/cancers16152658
Chicago/Turabian StyleUrias Rivera, Andres C., Antonio Pizuorno Machado, Malek Shatila, George Triadafilopoulos, Jennifer L. McQuade, Mehmet Altan, Dan Zhao, Yinghong Wang, and Mehnaz A. Shafi. 2024. "The Effect of Immune Checkpoint Inhibitor Therapy on Pre-Existing Gastroparesis and New Onset of Symptoms of Delayed Gastric Emptying" Cancers 16, no. 15: 2658. https://doi.org/10.3390/cancers16152658
APA StyleUrias Rivera, A. C., Pizuorno Machado, A., Shatila, M., Triadafilopoulos, G., McQuade, J. L., Altan, M., Zhao, D., Wang, Y., & Shafi, M. A. (2024). The Effect of Immune Checkpoint Inhibitor Therapy on Pre-Existing Gastroparesis and New Onset of Symptoms of Delayed Gastric Emptying. Cancers, 16(15), 2658. https://doi.org/10.3390/cancers16152658







