Predicting the Diagnosis of Prostate Cancer with a Novel Blood-Based Biomarker: Comparison of Its Performance with Prostate-Specific Antigen
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
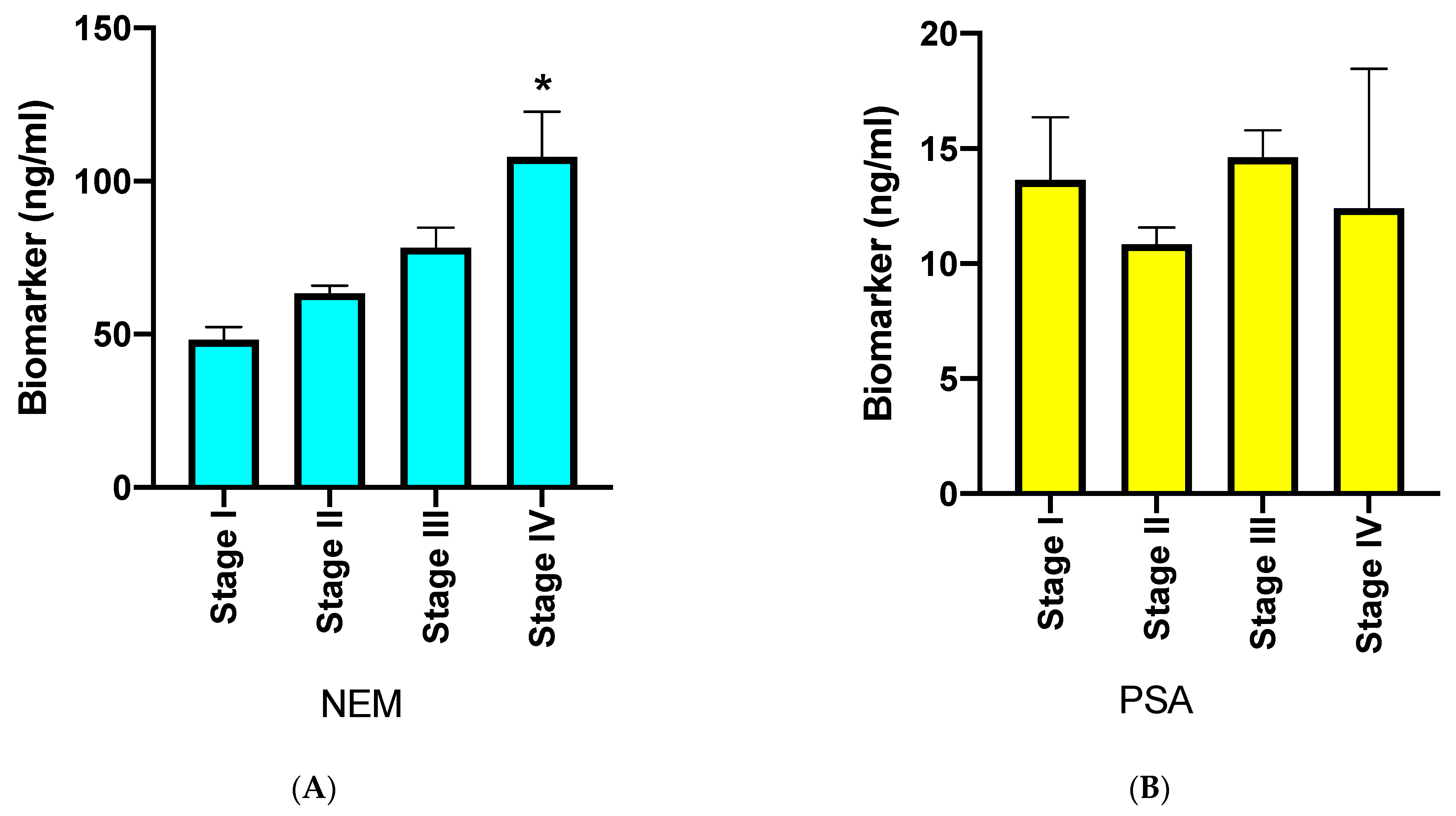
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, Z.; Zheng, Y.; Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Hao, Q.; Song, D.; Wu, Y.; et al. Incidence and disease burden of prostate cancer from 1990 to 2017: Results from the Global Burden of Disease Study 2017. Cancer 2020, 126, 1969–1978. [Google Scholar] [CrossRef]
- Yusim, I.; Krenawi, M.; Mazor, E.; Novack, V.; Mabjeesh, N.J. The use of prostate specific antigen density to predict clinically significant prostate cancer. Sci. Rep. 2020, 10, 20015. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, A.; Yanagisawa, T.; Bekku, K.; Kardoust Parizi, M.; Laukhtina, E.; Klemm, J.; Chiujdea, S.; Mori, K.; Kimura, S.; Fazekas, T.; et al. Comparing the Performance of Digital Rectal Examination and Prostate-specific Antigen as a Screening Test for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2024, 7, 697–704. [Google Scholar] [CrossRef]
- Dragan, J.; Kania, J.; Salagierski, M. Active surveillance in prostate cancer management: Where do we stand now? Arch. Med. Sci. 2021, 17, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Srinivasan, S.; Clements, J.; Batra, J. Beyond the biomarker role: Prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev. 2019, 38, 333–346. [Google Scholar] [CrossRef]
- Balk, S.P.; Ko, Y.J.; Bubley, G.J. Biology of prostate-specific antigen. J. Clin. Oncol. 2003, 21, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chisholm, G.D.; Habib, F.K. The distribution of PSA, cathepsin-D, and pS2 in BPH and cancer of the prostate. Prostate 1992, 21, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lacher, D.A.; Thompson, T.D.; Hughes, J.P.; Saraiya, M. Total, free, and percent free prostate-specific antigen levels among U.S. men, 2001–2004. Adv. Data 2006, 14, 2178–2182. [Google Scholar]
- Vickers, A.; Cronin, A.; Roobol, M.; Savage, C.; Peltola, M.; Pettersson, K.; Scardino, P.T.; Schroder, F.; Lilja, H. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: An independent replication. J. Clin. Oncol. 2010, 28, 2493–2498. [Google Scholar] [CrossRef]
- Adamy, A.; Yee, D.S.; Matsushita, K.; Maschino, A.; Cronin, A.; Vickers, A.; Guillonneau, B.; Scardino, P.T.; Eastham, J.A. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J. Urol. 2011, 185, 477–482. [Google Scholar] [CrossRef]
- David, M.K.; Leslie, S.W. Prostate Specific Antigen. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2024. [Google Scholar]
- Gupta, A.; Roobol, M.J.; Savage, C.J.; Peltola, M.; Pettersson, K.; Scardino, P.T.; Vickers, A.J.; Schroder, F.H.; Lilja, H. A four-kallikrein panel for the prediction of repeat prostate biopsy: Data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands. Br. J. Cancer 2010, 103, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Ditonno, F.; Franco, A.; Manfredi, C.; Veccia, A.; Valerio, M.; Bukavina, L.; Zukowski, L.B.; Vourganti, S.; Stenzl, A.; Andriole, G.L.; et al. Novel non-MRI imaging techniques for primary diagnosis of prostate cancer: Micro-ultrasound, contrast-enhanced ultrasound, elastography, multiparametric ultrasound, and PSMA PET/CT. Prostate Cancer Prostatic Dis. 2024, 27, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Masud, N.; Aldahish, A.; Iczkowski, K.A.; Kale, A.; Shah, G.V. Zinc finger protein-like 1 is a novel neuroendocrine biomarker for prostate cancer. Int. J. Oncol. 2023, 62, 38. [Google Scholar] [CrossRef] [PubMed]
- Alzghoul, S.; Hailat, M.; Zivanovic, S.; Que, L.; Shah, G.V. Measurement of serum prostate cancer markers using a nanopore thin film based optofluidic chip. Biosens. Bioelectron. 2016, 77, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Alzghoul, S.; Hailat, M.; Zivanovic, S.; Shah, G.; Que, L. Detection of neuroendocrine marker in blood samples using an optofluidic chip. In Proceedings of the 18th International Conference on Solid-State Sensors, Anchorage, AK, USA, 21–25 June 2015. [Google Scholar]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Griner, P.F.; Mayewski, R.J.; Mushlin, A.I.; Greenland, P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann. Intern. Med. 1981, 94, 557–592. [Google Scholar] [PubMed]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.P.; Deibl, M.; Steiner, H.; Bektic, J.; Pelzer, A.; Spranger, R.; Klocker, H.; Bartsch, G.; Horninger, W. Longitudinal PSA changes in men with and without prostate cancer: Assessment of prostate cancer risk. Prostate 2005, 64, 240–245. [Google Scholar] [CrossRef]
- Matoso, A.; Epstein, J.I. Defining clinically significant prostate cancer on the basis of pathological findings. Histopathology 2019, 74, 135–145. [Google Scholar] [CrossRef]
- Lazzeri, M.; Haese, A.; Abrate, A.; de la Taille, A.; Redorta, J.P.; McNicholas, T.; Lughezzani, G.; Lista, G.; Larcher, A.; Bini, V.; et al. Clinical performance of serum prostate-specific antigen isoform [-2]proPSA (p2PSA) and its derivatives, %p2PSA and the prostate health index (PHI), in men with a family history of prostate cancer: Results from a multicentre European study, the PROMEtheuS project. BJU Int. 2013, 112, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Punnen, S.; Pavan, N.; Parekh, D.J. Finding the Wolf in Sheep’s Clothing: The 4Kscore Is a Novel Blood Test That Can Accurately Identify the Risk of Aggressive Prostate Cancer. Rev. Urol. 2015, 17, 3–13. [Google Scholar] [PubMed]
- Strom, P.; Nordstrom, T.; Aly, M.; Egevad, L.; Gronberg, H.; Eklund, M. The Stockholm-3 Model for Prostate Cancer Detection: Algorithm Update, Biomarker Contribution, and Reflex Test Potential. Eur. Urol. 2018, 74, 204–210. [Google Scholar] [CrossRef]
- Lazzeri, M.; Abrate, A.; Lughezzani, G.; Gadda, G.M.; Freschi, M.; Mistretta, F.; Lista, G.; Fossati, N.; Larcher, A.; Kinzikeeva, E.; et al. Relationship of chronic histologic prostatic inflammation in biopsy specimens with serum isoform [-2]proPSA (p2PSA), %p2PSA, and prostate health index in men with a total prostate-specific antigen of 4-10 ng/ml and normal digital rectal examination. Urology 2014, 83, 606–612. [Google Scholar] [CrossRef]
- Wang, T.J.; Slawin, K.M.; Rittenhouse, H.G.; Millar, L.S.; Mikolajczyk, S.D. Benign prostatic hyperplasia-associated prostate-specific antigen (BPSA) shows unique immunoreactivity with anti-PSA monoclonal antibodies. Eur. J. Biochem. 2000, 267, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.; Radu, E.; Sajin, M. Neuroendocrine transdifferentiation of prostate carcinoma cells and its prognostic significance. Rom. J. Morphol. Embryol. 2010, 51, 7–12. [Google Scholar]
- Marcu, M.; Radu, E.; Sajin, M. Neuroendocrine differentiation in prostate adenocarcinoma biopsies and its correlation to histological grading. Curr. Health Sci. J. 2010, 36, 37–42. [Google Scholar]
- Sagnak, L.; Topaloglu, H.; Ozok, U.; Ersoy, H. Prognostic significance of neuroendocrine differentiation in prostate adenocarcinoma. Clin. Genitourin. Cancer 2011, 9, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hansson, J.; Abrahamsson, P.A. Neuroendocrine differentiation in prostatic carcinoma. Scand. J. Urol. Nephrol. Suppl. 2003, 212, 28–36. [Google Scholar] [CrossRef]
- Helpap, B.; Kollermann, J.; Oehler, U. Neuroendocrine differentiation in prostatic carcinomas: Histogenesis, biology, clinical relevance, and future therapeutical perspectives. Urol. Int. 1999, 62, 133–138. [Google Scholar] [CrossRef]
- Segawa, N.; Mori, I.; Utsunomiya, H.; Nakamura, M.; Nakamura, Y.; Shan, L.; Kakudo, K.; Katsuoka, Y. Prognostic significance of neuroendocrine differentiation, proliferation activity and androgen receptor expression in prostate cancer. Pathol. Int. 2001, 51, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.H.; Partin, A.W.; Veltri, R.W.; Epstein, J.I. Neuroendocrine differentiation in prostate cancer: Enhanced prediction of progression after radical prostatectomy. Hum. Pathol. 1996, 27, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Beltran, H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front. Oncol. 2014, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, P.A. Neuroendocrine differentiation and hormone-refractory prostate cancer. Prostate Suppl. 1996, 6, 3–8. [Google Scholar] [CrossRef]



| A: The cohort * | |||||
| Source | Normal | BPH | PCa | Other Cancers | Total |
| MCW | 98 | 19 | 150 | 80 | 347 |
| LSUHSC | 0 | 0 | 81 | 0 | 81 |
| Individumed | 0 | 0 | 80 | 0 | 80 |
| Total | 98 | 19 | 311 | 80 | 508 |
| B: Racial distribution | |||||
| Normal | BPH | PCa | Other Cancers | Total | |
| African Americans | 6 | 1 | 54 | 3 | 64 |
| Caucasian | 92 | 18 | 257 | 77 | 444 |
| Total | 98 | 19 | 311 | 80 | 508 |
| C: Clinical profile of the cohort | |||||
| Number of Subjects (n), Source | Average Age (years) | Age Range (years) | Clinical Diagnosis | ||
| 98 (MCW) | 68.66 | 58–86 | Normal | ||
| 19 (MCW) | 71.47 | 59–89 | BPH | ||
| 311 (MCW, LSUHSC, Individumed) | 67.01 | 59–89 | PCa | ||
| 80 (MCW) | 71.3 | 54–93 | Other Cancers | ||
| D: AJCC Prognostic stage distribution of PCa patients | |||||
| Number of Subjects (n) | Average Age (years) | Age Range (years) | Tumor Stage | ||
| 11 (LSUHSC, Individumed) | 60.82 | 48–71 | Stage I | ||
| 89 (LSU, Individumed) | 61.16 | 48–75 | Stage II | ||
| 57 (LSUHSC, Individumed) | 62.26 | 48–75 | Stage III | ||
| 4 (LSUHSC) | 59.67 | 54–62 | Stage IV | ||
| A: Normal Patients | |||||
| Biomarker | FP | FN | TP | TN | Total |
| NEM | 3 | 0 | 0 | 95 | 98 |
| PSA | 8 | 0 | 0 | 90 | 98 |
| B: BPH Patients | |||||
| Biomarker | PCa+ | PCa− | Total | ||
| NEM | 6 | 13 | 19 | ||
| PSA | 14 | 5 | 19 | ||
| C: PCa Patients | |||||
| Biomarker | PCa+ | PCa− | Total | ||
| NEM | 310 | 1 | 311 | ||
| PSA | 269 | 42 | 311 | ||
| D: Biomarker Performance. | |||||
| Biomarker | Accuracy | PPV | NPV | Subjects (n) | |
| NEM (normal + PCa) | 0.933 | 0.982 | 0.993 | 311 + 98 | |
| PSA (normal + PCa) | 0.880 | 0.965 | 0.719 | 311 + 98 | |
| NEM (BPH + PCa) | 0.981 | 0.925 | 0.981 | 311 + 19 | |
| PSA (BPH + PCa) | 0.821 | 0.821 | 0.331 | 311 + 19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanders, J.L.; Iczkowski, K.A.; Shah, G.V. Predicting the Diagnosis of Prostate Cancer with a Novel Blood-Based Biomarker: Comparison of Its Performance with Prostate-Specific Antigen. Cancers 2024, 16, 2619. https://doi.org/10.3390/cancers16152619
Sanders JL, Iczkowski KA, Shah GV. Predicting the Diagnosis of Prostate Cancer with a Novel Blood-Based Biomarker: Comparison of Its Performance with Prostate-Specific Antigen. Cancers. 2024; 16(15):2619. https://doi.org/10.3390/cancers16152619
Chicago/Turabian StyleSanders, Johnmesha L., Kenneth A. Iczkowski, and Girish V. Shah. 2024. "Predicting the Diagnosis of Prostate Cancer with a Novel Blood-Based Biomarker: Comparison of Its Performance with Prostate-Specific Antigen" Cancers 16, no. 15: 2619. https://doi.org/10.3390/cancers16152619
APA StyleSanders, J. L., Iczkowski, K. A., & Shah, G. V. (2024). Predicting the Diagnosis of Prostate Cancer with a Novel Blood-Based Biomarker: Comparison of Its Performance with Prostate-Specific Antigen. Cancers, 16(15), 2619. https://doi.org/10.3390/cancers16152619




