Radium-223 Treatment Produces Prolonged Suppression of Resident Osteoblasts and Decreased Bone Mineral Density in Trabecular Bone in Osteoblast Reporter Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treatment of Mice with Ra-223 and Bioluminescence Measurement
2.3. µCT Analysis and GFP Quantification
2.4. Generation of TRAMP-BMP4 Cell Line
2.5. Mice with TRAMP-BMP4 Tumors
2.6. Statistical Analysis
3. Results
3.1. Ra-223 Treatment Decreases Tail Bone Luciferase Activity in a Col-Luc Osteoblast Reporter Mice
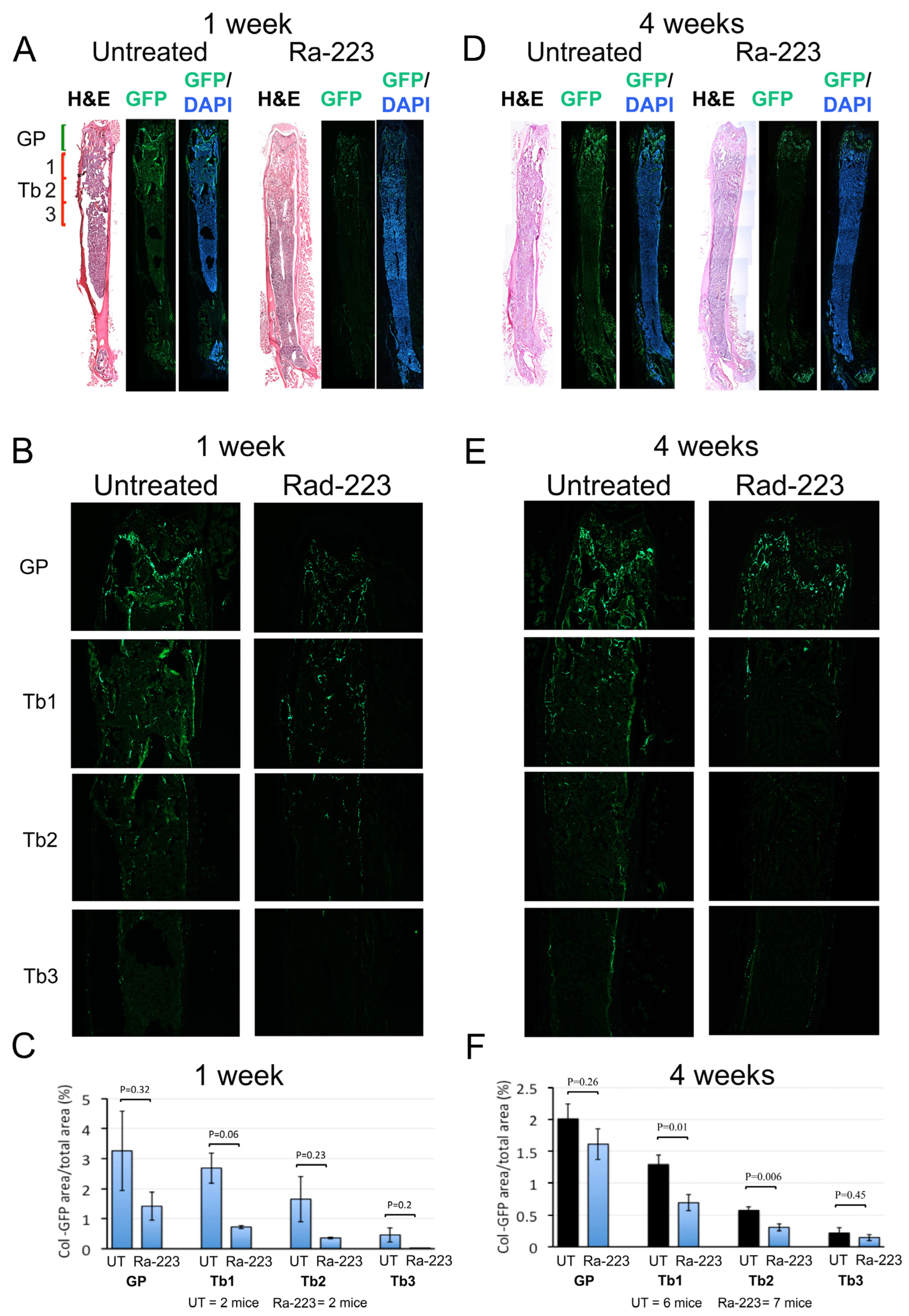
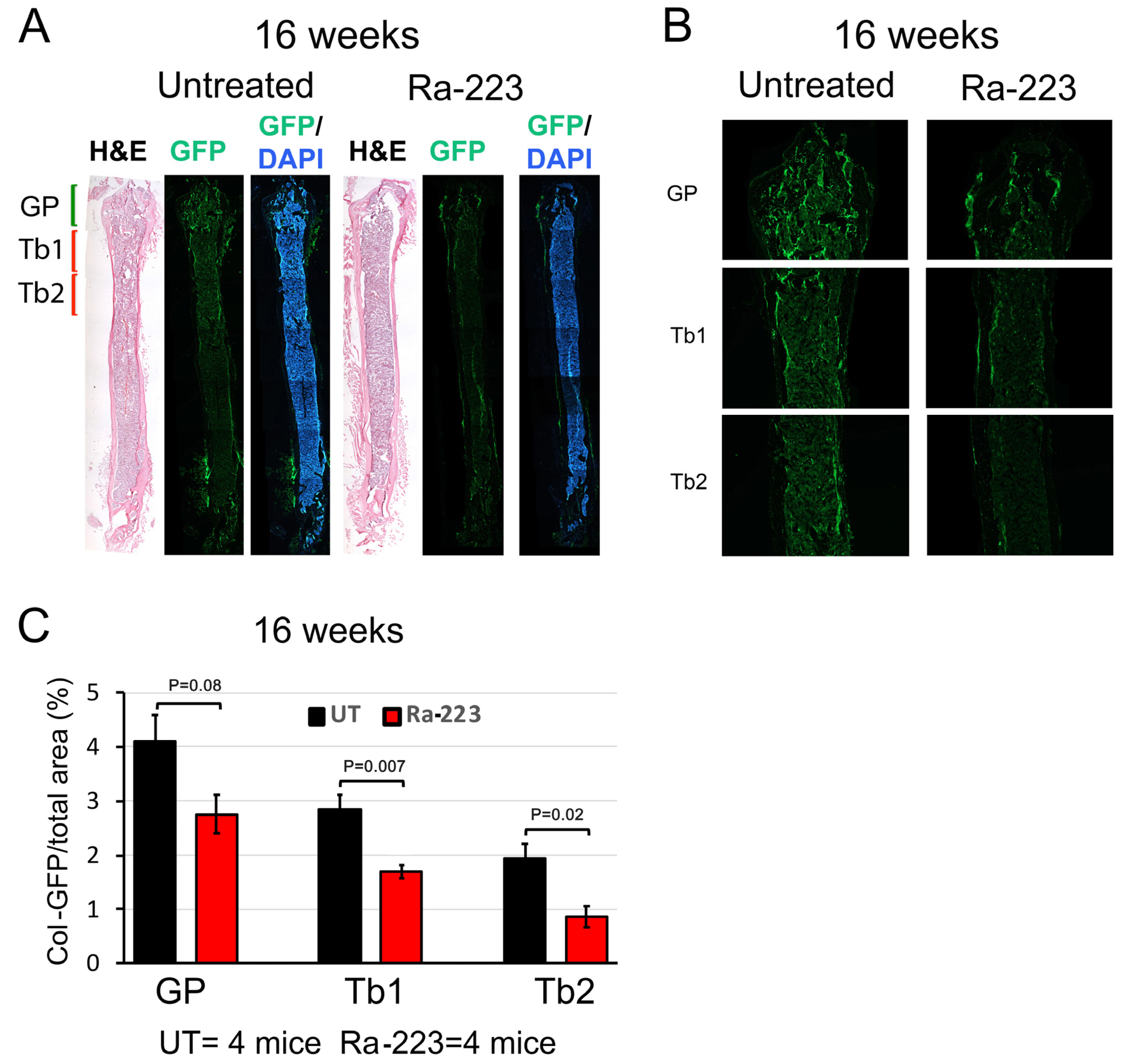
3.2. Ra-223 Decreases GFP-Labeled Osteoblasts in Col-GFP Mice
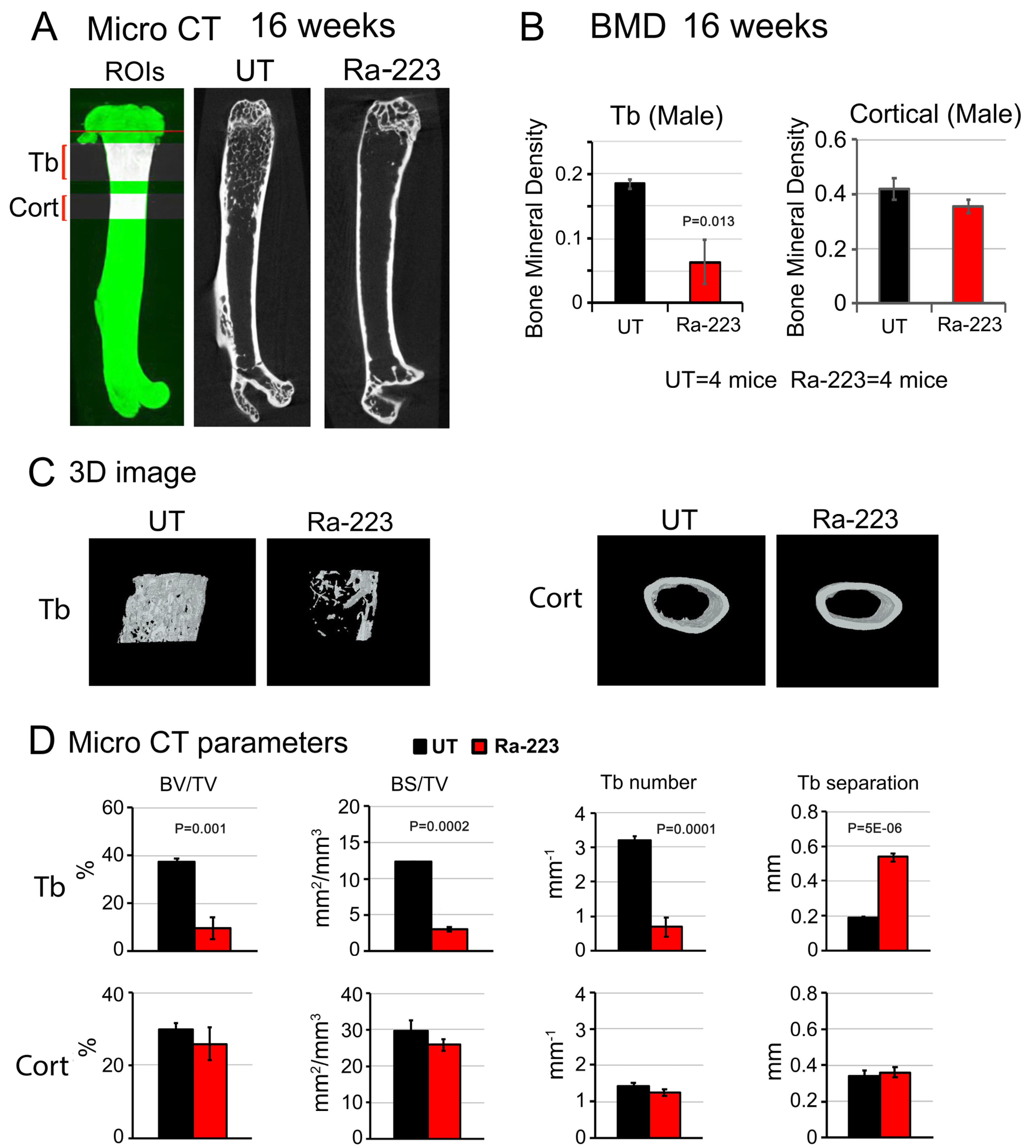
3.3. Ra-223 Decreases Bone Mineral Density of Col-GFP Mice
3.4. Ra-223 Decreases Tumor-Induced Osteoblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bubendorf, L.; Schopfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Cheng, C.J.; Bilen, M.A.; Lu, J.F.; Satcher, R.L.; Yu-Lee, L.Y.; Gallick, G.E.; Maity, S.N.; Lin, S.H. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 2011, 71, 5194–5203. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lin, S.C.; Yu, G.; Zhu, M.; Song, J.H.; Rivera, K.; Pappin, D.J.; Logothetis, C.J.; Panaretakis, T.; Wang, G.; et al. Prostate tumor-induced stromal reprogramming generates Tenascin C that promotes prostate cancer metastasis through YAP/TAZ inhibition. Oncogene 2022, 41, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Lee, Y.C.; Yu, G.; Cheng, C.J.; Zhou, X.; Chu, K.; Murshed, M.; Le, N.T.; Baseler, L.; Abe, J.I.; et al. Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer. Dev. Cell 2017, 41, 467–480 e463. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Hall, C.L.; Escara-Wilke, J.; Mizokami, A.; Keller, J.M.; Keller, E.T. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008, 68, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Keller, J.; Zhang, J.; Lu, Y.; Yao, Z.; Keller, E.T. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005, 65, 8274–8285. [Google Scholar] [CrossRef]
- Fizazi, K.; Yang, J.; Peleg, S.; Sikes, C.R.; Kreimann, E.L.; Daliani, D.; Olive, M.; Raymond, K.A.; Janus, T.J.; Logothetis, C.J.; et al. Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin. Cancer Res. 2003, 9, 2587–2597. [Google Scholar] [PubMed]
- Ribelli, G.; Simonetti, S.; Iuliani, M.; Rossi, E.; Vincenzi, B.; Tonini, G.; Pantano, F.; Santini, D. Osteoblasts Promote Prostate Cancer Cell Proliferation Through Androgen Receptor Independent Mechanisms. Front. Oncol. 2021, 11, 789885. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lin, S.C.; Yu, G.; Cheng, C.J.; Liu, B.; Liu, H.C.; Hawke, D.H.; Parikh, N.U.; Varkaris, A.; Corn, P.; et al. Identification of Bone-Derived Factors Conferring De Novo Therapeutic Resistance in Metastatic Prostate Cancer. Cancer Res. 2015, 75, 4949–4959. [Google Scholar] [CrossRef]
- Bilen, M.A.; Johnson, M.M.; Mathew, P.; Pagliaro, L.C.; Araujo, J.C.; Aparicio, A.; Corn, P.G.; Tannir, N.M.; Wong, F.C.; Fisch, M.J.; et al. Randomized phase 2 study of bone-targeted therapy containing strontium-89 in advanced castrate-sensitive prostate cancer. Cancer 2015, 121, 69–76. [Google Scholar] [CrossRef]
- Tu, S.-M.; Delpassand, E.S.; Jones, D.; Amato, R.J.; Ellerhorst, J.; Logothetis, C.J. Strontium-89 combined with doxorubicin in the treatment of patients with androgen-independent prostate cancer. Urol. Oncol. 1996, 2, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Reid, R.H.; Hoskin, P.J.; Quick, D.P.; Ell, P.J.; Coleman, R.E.; Kotler, J.A.; Freeman, L.M.; Olivier, P. Quadramet 424Sm10/11 Study Group. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 2004, 63, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Serafini, A.N.; Houston, S.J.; Resche, I.; Quick, D.P.; Grund, F.M.; Ell, P.J.; Bertrand, A.; Ahmann, F.R.; Orihuela, E.; Reid, R.H.; et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: A double-blind placebo-controlled clinical trial. J. Clin. Oncol. 1998, 16, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P. Samarium for osteoblastic bone metastases and osteosarcoma. Expert. Opin. Pharmacother. 2006, 7, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, O.S.; Larsen, R.H. Targeting of osseous sites with alpha-emitting 223Ra: Comparison with the beta-emitter 89Sr in mice. J. Nucl. Med. 2003, 44, 252–259. [Google Scholar] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O‘Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hijab, A.; Curcean, S.; Tunariu, N.; Tovey, H.; Alonzi, R.; Staffurth, J.; Blackledge, M.; Padhani, A.; Tree, A.; Stidwill, H.; et al. Fracture Risk in Men with Metastatic Prostate Cancer Treated With Radium-223. Clin. Genitourin. Cancer 2021, 19, e299–e305. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- Paindelli, C.; Casarin, S.; Wang, F.; Diaz-Gomez, L.; Zhang, J.; Mikos, A.G.; Logothetis, C.J.; Friedl, P.; Dondossola, E. Enhancing (223)Ra Treatment Efficacy by Anti-beta1 Integrin Targeting. J. Nucl. Med. 2022, 63, 1039–1045. [Google Scholar] [CrossRef]
- Vardaki, I.; Corn, P.; Gentile, E.; Song, J.H.; Madan, N.; Hoang, A.; Parikh, N.; Guerra, L.; Lee, Y.C.; Lin, S.C.; et al. Radium-223 Treatment Increases Immune Checkpoint Expression in Extracellular Vesicles from the Metastatic Prostate Cancer Bone Microenvironment. Clin. Cancer Res. 2021, 27, 3253–3264. [Google Scholar] [CrossRef]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Huang, C.F.; Murshed, M.; Chu, K.; Araujo, J.C.; Ye, X.; deCrombrugghe, B.; Yu-Lee, L.Y.; Gallick, G.E.; Lin, S.H. Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene 2010, 29, 3196–3207. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.A.; Gingrich, J.R.; Kwon, E.D.; Madias, C.; Greenberg, N.M. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997, 57, 3325–3330. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Rossert, J.; Eberspaecher, H.; de Crombrugghe, B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J. Cell Biol. 1995, 129, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.C. Spread of prostatic cancer to bone. Urology 1983, 21, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Charhon, S.A.; Chapuy, M.C.; Delvin, E.E.; Valentin-Opran, A.; Edouard, C.M.; Meunier, P.J. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer 1983, 51, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, C.; Lin, S.-H. Osteoblasts in prostate cancer metastasis to bone. Nat. Rev. Cancer 2005, 5, 21–28. [Google Scholar] [CrossRef]
- Li, Z.; Mathew, P.; Yang, J.; Starbuck, M.-W.; Zurita, A.J.; Liu, J.; Sikes, C.; Multani, A.S.; Efstathiou, E.; Lopez, A.; et al. Androgen receptor–negative human prostate cancer cells induce osteogenesis through FGF9-mediated mechanisms. J. Clin. Investig. 2008, 118, 2697–2710. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Vessella, R.L.; Morrissey, C.; Brown, L.G.; Coleman, I.M.; Higano, C.S.; Mostaghel, E.A.; Zhang, X.; True, L.D.; Lam, H.M.; et al. LuCaP Prostate Cancer Patient-Derived Xenografts Reflect the Molecular Heterogeneity of Advanced Disease and Serve as Models for Evaluating Cancer Therapeutics. Prostate 2017, 77, 654–671. [Google Scholar] [CrossRef]
- Greenberg, N.M.; DeMayo, F.; Finegold, M.J.; Medina, D.; Tilley, W.D.; Aspinall, J.O.; Cunha, G.R.; Donjacour, A.A.; Matusik, R.J.; Rosen, J.M. Prostate cancer in a transgenic mouse. Proc. Natl. Acad. Sci. USA 1995, 92, 3439–3443. [Google Scholar] [CrossRef]
- Yu, G.; Corn, P.G.; Shen, P.; Song, J.H.; Lee, Y.C.; Lin, S.C.; Pan, J.; Agarwal, S.K.; Panaretakis, T.; Pacifici, M.; et al. Retinoic Acid Receptor Activation Reduces Metastatic Prostate Cancer Bone Lesions by Blocking the Endothelial-to-Osteoblast Transition. Cancer Res. 2022, 82, 3158–3171. [Google Scholar] [CrossRef]
- Al-Bari, A.A.; Al Mamun, A. Current advances in regulation of bone homeostasis. FASEB Bioadv. 2020, 2, 668–679. [Google Scholar] [CrossRef]
- Corral, D.A.; Amling, M.; Priemel, M.; Loyer, E.; Fuchs, S.; Ducy, P.; Baron, R.; Karsenty, G. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13835–13840. [Google Scholar] [CrossRef]
- Suominen, M.I.; Rissanen, J.P.; Kakonen, R.; Fagerlund, K.M.; Alhoniemi, E.; Mumberg, D.; Ziegelbauer, K.; Halleen, J.M.; Kakonen, S.M.; Scholz, A. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J. Natl. Cancer Inst. 2013, 105, 908–916. [Google Scholar] [CrossRef]
- Abou, D.S.; Ulmert, D.; Doucet, M.; Hobbs, R.F.; Riddle, R.C.; Thorek, D.L. Whole-Body and Microenvironmental Localization of Radium-223 in Naive and Mouse Models of Prostate Cancer Metastasis. J. Natl. Cancer Inst. 2016, 108, djv380. [Google Scholar] [CrossRef]
- Pan, T.; Lin, S.C.; Lee, Y.C.; Yu, G.; Song, J.H.; Pan, J.; Titus, M.; Satcher, R.L.; Panaretakis, T.; Logothetis, C.; et al. Statins reduce castration-induced bone marrow adiposity and prostate cancer progression in bone. Oncogene 2021, 40, 4592–4603. [Google Scholar] [CrossRef]
- Trieu, J.; Chang, M.; Rojas, V.; Varada, N.; Cao, Y.; Anderson, M.; Vogelzang, N.J. Lower Fracture Rates in Patients Treated with Radium-223, Abiraterone or Enzalutamide, When Given Concurrently with Bone Health Agents: A Real-World Analysis. Clin. Genitourin. Cancer 2022, 20, 399–403. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-C.; Yu, G.; Corn, P.G.; Damasco, J.; Lee, Y.-C.; Song, J.H.; Navone, N.M.; Logothetis, C.J.; Melancon, M.P.; Panaretakis, T.; et al. Radium-223 Treatment Produces Prolonged Suppression of Resident Osteoblasts and Decreased Bone Mineral Density in Trabecular Bone in Osteoblast Reporter Mice. Cancers 2024, 16, 2603. https://doi.org/10.3390/cancers16142603
Lin S-C, Yu G, Corn PG, Damasco J, Lee Y-C, Song JH, Navone NM, Logothetis CJ, Melancon MP, Panaretakis T, et al. Radium-223 Treatment Produces Prolonged Suppression of Resident Osteoblasts and Decreased Bone Mineral Density in Trabecular Bone in Osteoblast Reporter Mice. Cancers. 2024; 16(14):2603. https://doi.org/10.3390/cancers16142603
Chicago/Turabian StyleLin, Song-Chang, Guoyu Yu, Paul G. Corn, Jossana Damasco, Yu-Chen Lee, Jian H. Song, Nora M. Navone, Christopher J. Logothetis, Marites P. Melancon, Theocharis Panaretakis, and et al. 2024. "Radium-223 Treatment Produces Prolonged Suppression of Resident Osteoblasts and Decreased Bone Mineral Density in Trabecular Bone in Osteoblast Reporter Mice" Cancers 16, no. 14: 2603. https://doi.org/10.3390/cancers16142603
APA StyleLin, S.-C., Yu, G., Corn, P. G., Damasco, J., Lee, Y.-C., Song, J. H., Navone, N. M., Logothetis, C. J., Melancon, M. P., Panaretakis, T., & Lin, S.-H. (2024). Radium-223 Treatment Produces Prolonged Suppression of Resident Osteoblasts and Decreased Bone Mineral Density in Trabecular Bone in Osteoblast Reporter Mice. Cancers, 16(14), 2603. https://doi.org/10.3390/cancers16142603







