Imaging and Metabolic Diagnostic Methods in the Stage Assessment of Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Transrectal Ultrasound (TRUS)
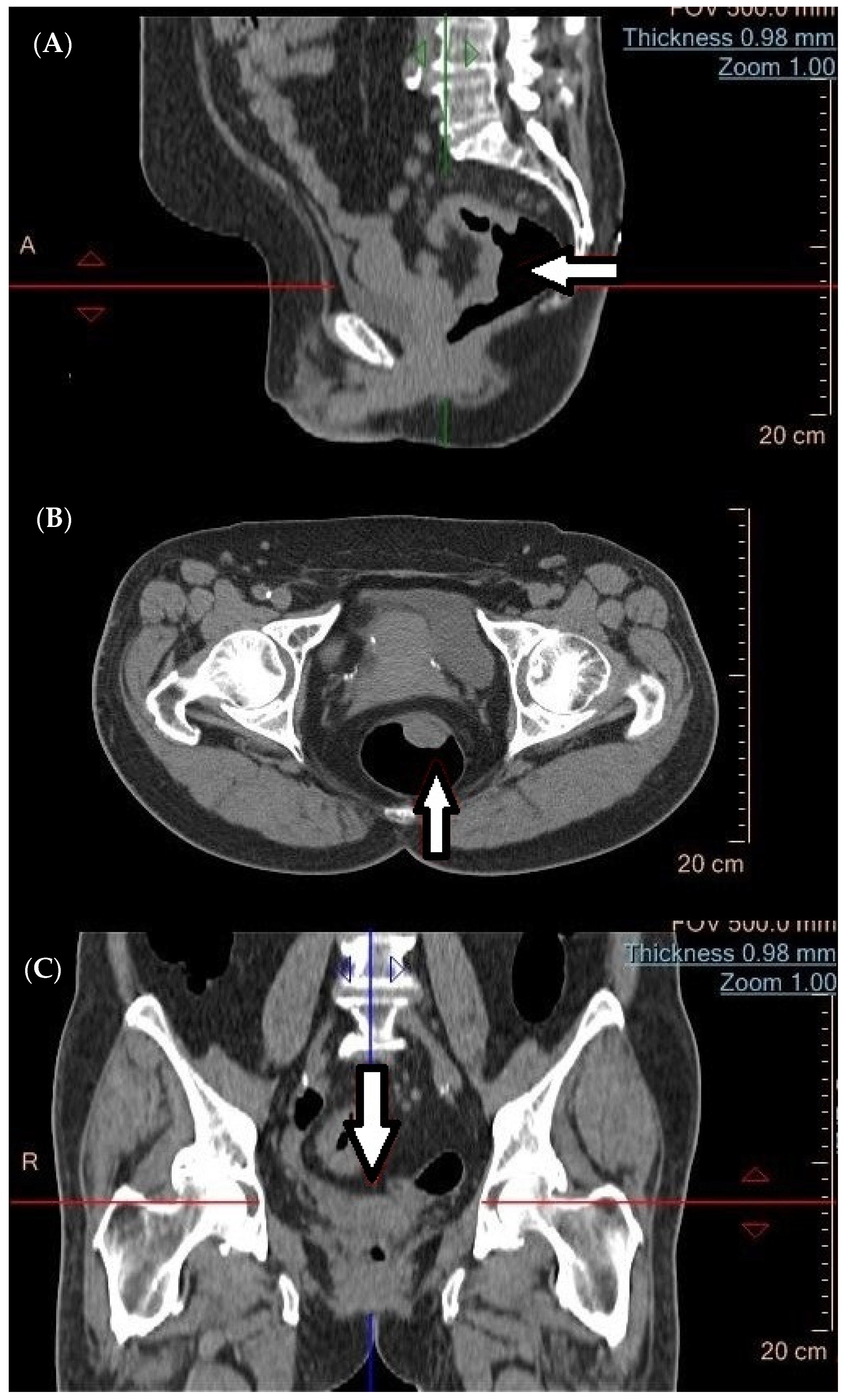
4. Computed Tomography (CT) in the Diagnosis of Metastatic Tumors
5. Virtual Colonoscopy (CTC)
6. Magnetic Resonance Imaging (MRI)
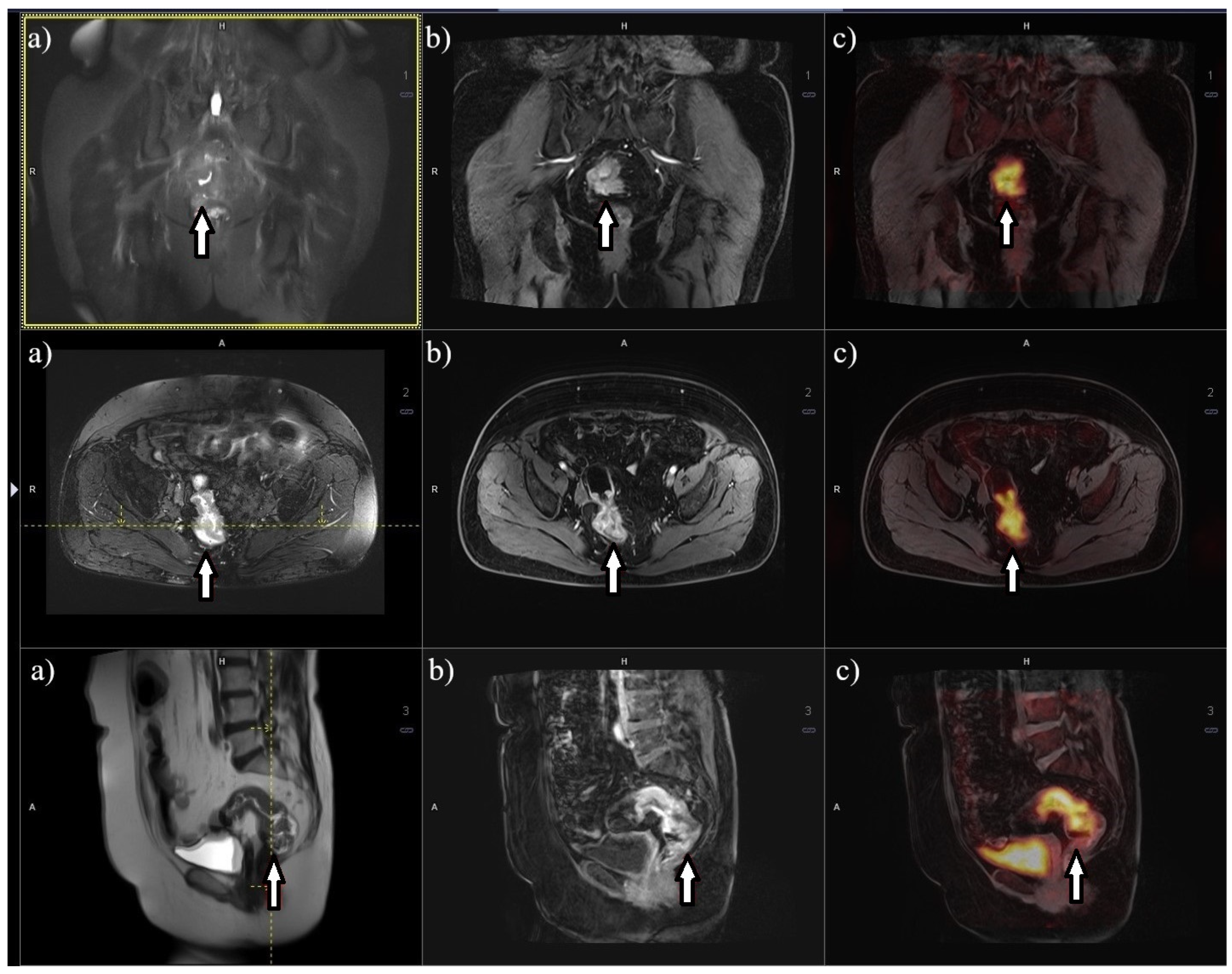
7. Metabolic Imaging Using Positron Emission Tomography/Computed Tomography (PET/CT)
7.1. 2-Deoxy-2-[fluorine-18]fluoro-D-glucose Tracer (18F-FDG)
7.2. 18F-FLT (Fluorothymidine) PET/CT
7.3. 68Ga-DOTATATE (Gallium-68 DOTATATE) and 68Ga-DOTANOC PET-CT
7.4. Future Perspectives of PET/CT
8. Metabolic Imaging 18F-FDG PET/MRI
9. Discussion
10. Improving the Future of Imaging Diagnostic Procedure Performance
11. Limitation of the Study
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giesen, L.J.X.; Olthof, P.B.; Elferink, M.A.G.; van Westreenen, H.L.; Beets, G.L.; Verhoef, C.; Dekker, J.W.T. Changes in rectal cancer treatment after the introduction of a national screening program; Increasing use of less invasive strategies within a national cohort. Eur. J. Surg. Oncol. 2022, 48, 1117–1122. [Google Scholar] [CrossRef]
- Lang, D.; Ciombor, K.K. Diagnosis and Management of Rectal Cancer in Patients Younger Than 50 Years: Rising Global Incidence and Unique Challenges. J. Natl. Compr. Cancer Netw. 2022, 20, 1169–1175. [Google Scholar] [CrossRef]
- Didkowska, J.; Barańska, K.; Miklewska, M.J.; Wojciechowska, U. Cancer incidence and mortality in Poland in 2023, Nowotwory. J. Oncol. 2024, 74, 75–93. [Google Scholar] [CrossRef]
- Fazeli, M.S.; Keramati, M.R. Rectal cancer: A review. Med. J. Islam. Repub. Iran 2015, 29, 171. [Google Scholar]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int. J. Cancer 2008, 123, 867–873. [Google Scholar] [CrossRef]
- Hupkens, B.J.P.; Martens, M.H.; Stoot, J.H.; Berbee, M.; Melenhorst, J.; Beets-Tan, R.G.; Beets, G.L.; Breukink, S.O. Quality of life in rectal cancer patients after chemoradiation: Watch-and-wait policy versus standard resection—A matched-controlled study. Dis. Colon Rectum. 2017, 60, 1032–1040. [Google Scholar] [CrossRef]
- Han, C.; Tang, X.; Yang, M.; Zhang, K.; Liu, J.; Lin, R.; Ding, Z. How Useful Is Endoscopic Ultrasound in Differentiating T3/T4a T Stage of Colorectal Cancer: A Prospective Study. Front. Oncol. 2022, 11, 5524. [Google Scholar] [CrossRef]
- Deptała, A.; Wojtukiewicz, M.Z. Rak Jelita Grubego; Termedia: Poznań, Poland, 2018. [Google Scholar]
- Boot, J.; Gomez-Munoz, F.; Beets-Tan, R.G.H. Imaging of rectal cancer. Radiologe 2019, 59, 46–50. [Google Scholar] [CrossRef]
- Horvat, N.; Rocha, C.C.T.; Oliveira, B.C.; Petkovska, I.; Gollub, M.J. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019, 39, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Curvo-Semedo, L.; Cancer, R. Rectal Cancer: Staging, Magn. Reson. Imaging Clin. N. Am. 2020, 28, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Okocha, M.; Lotfollahzadeh, S.; Microsurgery, R.C. StatPearls. 2022. Available online: http://www.ncbi.nlm.nih.gov/pubmed/35710032 (accessed on 4 June 2023).
- Bujko, K.; Wyrwicz, L.; Rutkowski, A.; Malinowska, M.; Pietrzak, L.; Kryński, J.; Michalski, W.; Oledzki, J.; Kuśnierz, J.; Zajac, L.; et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann. Oncol. 2016, 27, 834–842. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Tepper, J.E. Current options for the management of rectal cancer. Curr. Treat. Options Oncol. 2007, 8, 331–338. [Google Scholar] [CrossRef]
- Wender, R.; Brooks, D.; Sharpe, K.; Doroshenk, M. The National Colorectal Cancer Roundtable: Past Performance, Current and Future Goals. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 499–509. [Google Scholar] [CrossRef]
- Smith, R.A.; Fedewa, S.; Siegel, R. Early colorectal cancer detection-Current and evolving challenges in evidence, guidelines, policy, and practices. Adv. Cancer Res. 2021, 151, 69–107. [Google Scholar] [CrossRef]
- Meyenberger, C.; Boni, R.A.H.; Bertschinger, P.; Zala, G.F.; Klotz, H.P.; Krestin, G.P. Endoscopic ultrasound and endorectal magnetic resonance imaging: A prospective, comparative study for preoperative staging and follow-up of rectal cancer. Endoscopy 1995, 27, 469–479. [Google Scholar] [CrossRef]
- Taylor, F.G.M.; Quirke, P.; Heald, R.J.; Moran, B.J.; Blomqvist, L.; Swift, I.R.; Sebag-Montefiore, D.; Tekkis, P.; Brown, G. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J. Clin. Oncol. 2014, 32, 34–43. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- The EQUATOR Network|Enhancing the QUAlity and Transparency of Health Research, (n.d.). Available online: https://www.equator-network.org/ (accessed on 15 October 2020).
- Hasak, S.; Kushnir, V. Rectal Endoscopic Ultrasound in Clinical Practice. Curr. Gastroenterol. Rep. 2019, 21, 18. [Google Scholar] [CrossRef]
- Kocaman, O.; Baysal, B.; Şentürk, H.; Ince, A.T.; Müslümanoʇlu, M.; Kocakoç, E.; Arici, S.; Uysal, O.; Yildiz, K.; Türkdoʇan, K.; et al. Staging of rectal carcinoma: MDCT, MRI or EUS. Single center experience. Turk. J. Gastroenterol. 2014, 25, 669–673. [Google Scholar] [CrossRef]
- Marone, P.; de Bellis, M.; D’Angelo, V.; Delrio, P.; Passananti, V.; Di Girolamo, E.; Rossi, G.B.; Rega, D.; Tracey, M.C.; Tempesta, A.M. Role of endoscopic ultrasonography in the loco-regional staging of patients with rectal cancer. World J. Gastrointest. Endosc. 2015, 7, 688. [Google Scholar] [CrossRef]
- Marusch, F.; Ptok, H.; Sahm, M.; Schmidt, U.; Ridwelski, K.; Gastinger, I.; Lippert, H. Endorectal ultrasound in rectal carcinoma—Do the literature results really correspond to the realities of routine clinical care? Endoscopy 2011, 43, 425–431. [Google Scholar] [CrossRef]
- Cote, A.; Graur, F.; Lebovici, A.; Mois, E.; Hajjar, N.A.L.; Mare, C.; Badea, R.; Iancu, C. The accuracy of endorectal ultrasonography in rectal cancer staging. Clujul Med. 2015, 88, 348. [Google Scholar] [CrossRef]
- Shapiro, R.; Ali, U.A.; Lavery, I.C.; Kiran, R.P. Endorectal ultrasound does not reliably identify patients with uT3 rectal cancer who can avoid neoadjuvant chemoradiotherapy. Int. J. Color. Dis. 2013, 28, 993–1000. [Google Scholar] [CrossRef]
- Marusch, F.; Lippert, H.; Koch, A.; Schmidt, U.; Zippel, R.; Kuhn, R.; Wolff, S.; Pross, M.; Wierth, A.; Gastinger, I. Routine use of transrectal ultrasound in rectal carcinoma: Results of a prospective multicenter study. Endoscopy 2002, 34, 385–390. [Google Scholar] [CrossRef]
- Entee, P.D.M.; Shokuhi, P.; Rogers, A.C.; Mehigan, B.J.; McCormick, P.H.; Gillham, C.M.; Kennedy, M.J.; Gallagher, D.J.; Ryan, C.E.; Muldoon, C.B.; et al. Extramural venous invasion (EMVI) in colorectal cancer is associated with increased cancer recurrence and cancer-related death. Eur. J. Surg. Oncol. 2022, 48, 1638–1642. [Google Scholar] [CrossRef]
- Stipa, F.; Zernecke, A.; Moore, H.G.; Minsky, B.D.; Wong, W.D.; Weiser, M.; Paty, P.B.; Shia, J.; Guillem, J.G. Residual mesorectal lymph node involvement following neoadjuvant combined-modality therapy: Rationale for radical resection? Ann. Surg. Oncol. 2004, 11, 187–191. [Google Scholar] [CrossRef]
- Mroczkowski, P.; Dziki, Ł.; Vosikova, T.; Otto, R.; Merecz-Sadowska, A.; Zajdel, R.; Zajdel, K.; Lippert, H.; Jannasch, O. Rectal Cancer: Are 12 Lymph Nodes the Limit? Cancers 2023, 15, 3447. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Ma, X.; Li, T.; Yan, Y.; Xue, C.; Hui, B.; Liu, R.; Ma, H.; Ren, J. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int. J. Biol. Sci. 2016, 12, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Hav, M.; Libbrecht, L.; Ferdinande, L.; Geboes, K.; Pattyn, P.; Cuvelier, C.A. Pathologic Assessment of Rectal Carcinoma after Neoadjuvant Radio(chemo)therapy: Prognostic Implications. BioMed Res. Int. 2015, 2015, 574540. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Q.; Li, J.; Song, Z.; Cheng, Y. Short-Term Clinical and Oncological Outcome of Prolonging Operation Interval After Neoadjuvant Chemoradiotherapy for Locally Advanced Middle and Low Rectal Cancer. Cancer Manag. Res. 2020, 12, 2315. [Google Scholar] [CrossRef]
- Puli, S.R.; Bechtold, M.L.; Reddy, J.B.K.; Choudhary, A.; Antillon, M.R.; Brugge, W.R. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann. Surg. Oncol. 2009, 16, 254–265. [Google Scholar] [CrossRef]
- Sun, F.; Chen, T.; Han, J.; Ye, P.; Hu, J. Staging accuracy of endoscopic ultrasound for esophageal cancer after neoadjuvant chemotherapy: A meta-analysis and systematic review. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2015, 28, 757–771. [Google Scholar] [CrossRef]
- Ren, Y.; Ye, J.; Wang, Y.; Xiong, W.; Xu, J.; He, Y.; Cai, S.; Tan, M.; Yuan, Y. The Optimal Application of Transrectal Ultrasound in Staging of Rectal Cancer Following Neoadjuvant Therapy: A Pragmatic Study for Accuracy Investigation. J. Cancer 2018, 9, 784. [Google Scholar] [CrossRef]
- Costa, J.M.; Gonçalves, B.; Gomes, M.M.; Fernandes, D.; Gonçalves, R.; Soares, J.B. Accuracy of endoscopic ultrasound in gastric adenocarcinoma patient selection for neoadjuvant therapy. United Eur. Gastroenterol. J. 2019, 7, 278. [Google Scholar] [CrossRef]
- de Nucci, G.; Gabbani, T.; Impellizzeri, G.; Deiana, S.; Biancheri, P.; Ottaviani, L.; Frazzoni, L.; Mandelli, E.D.; Soriani, P.; Vecchi, M.; et al. Linear EUS Accuracy in Preoperative Staging of Gastric Cancer: A Retrospective Multicenter Study. Diagnostics 2023, 13, 1842. [Google Scholar] [CrossRef]
- Gonzalo-Marin, J.; Vila, J.J.; Perez-Miranda, M. Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. World J. Gastrointest. Oncol. 2014, 6, 360. [Google Scholar] [CrossRef]
- Ghoneem, E.; Shabana, A.S.A.; El Sherbini, M.; Zuhdy, M.; Eldamshety, O.; Gouda, M.; El Shamy, A.; Saleh, G.A.; Saleh, A.A.G. Endoluminal ultrasound versus magnetic resonance imaging in assessment of rectal cancer after neoadjuvant therapy. BMC Gastroenterol. 2022, 22, 542. [Google Scholar] [CrossRef]
- Okafor, P.N.; Swanson, K.; Shah, N.; Talwalkar, J.A. Endoscopic ultrasound for rectal cancer staging: A population-based study of utilization, impact on treatment patterns, and survival. J. Gastroenterol. Hepatol. 2018, 33, 1469–1476. [Google Scholar] [CrossRef]
- Tan, C.H.; Iyer, R. Use of computed tomography in the management of colorectal cancer. World J. Radiol. 2010, 2, 151. [Google Scholar] [CrossRef]
- Klang, E.; Eifer, M.; Kopylov, U.; Belsky, V.; Raskin, S.; Konen, E.; Amitai, M.M. Pitfalls in diagnosing colon cancer on abdominal CT. Clin. Radiol. 2017, 72, 858–863. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Alabousi, A. Incidental findings on staging CT for rectal cancer: Frequency, clinical significance and outcomes. Clin. Imaging 2023, 93, 14–22. [Google Scholar] [CrossRef]
- Colvin, H.; Lukram, A.; Sohail, I.; Chung, K.T.; Jehangir, E.; Berry, J.; Babu, H.; Hinson, F. The performance of routine computed tomography for the detection of colorectal cancer. Ann. R. Coll. Surg. Engl. 2013, 95, 473–476. [Google Scholar] [CrossRef]
- Sanon, S.; Bos, P.D. In Vivo Imaging to Measure Spontaneous Lung Metastasis of Orthotopically-injected Breast Tumor Cells. J. Vis. Exp. 2022, 2022, e64002. [Google Scholar] [CrossRef]
- Freitas, P.S.; Janicas, C.; Veiga, J.; Matos, A.P.; Herédia, V.; Ramalho, M. Imaging evaluation of the liver in oncology patients: A comparison of techniques. World J. Hepatol. 2021, 13, 1936. [Google Scholar] [CrossRef]
- Jensen, C.T.; Wagner-Bartak, N.A.; Vu, L.N.; Liu, X.; Raval, B.; Martinez, D.; Wei, W.; Cheng, Y.; Samei, E.; Gupta, S. Detection of Colorectal Hepatic Metastases Is Superior at Standard Radiation Dose CT versus Reduced Dose CT. Radiology 2019, 290, 400–409. [Google Scholar] [CrossRef]
- Ozaki, K.; Higuchi, S.; Kimura, H.; Gabata, T. Liver Metastases: Correlation between Imaging Features and Pathomolecular Environments. Radiographics 2022, 42, 1994–2013. [Google Scholar] [CrossRef]
- Chandarana, M.; Arya, S.; de Menezes, J.L.; Engineer, R.; Ostwal, V.; Patil, P.; Kumar, S.; Dusane, R.; D’souza, A.; Saklani, A. Can CRM Status on MRI Predict Survival in Rectal Cancers: Experience from the Indian Subcontinent. Indian J. Surg. Oncol. 2019, 10, 364. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Z.; Zhang, B.; Wu, Y.; Li, G.; Wang, Z. Comparison of 68Ga-DOTANOC and 18F-FDG PET-CT Scans in the Evaluation of Primary Tumors and Lymph Node Metastasis in Patients with Rectal Neuroendocrine Tumors. Front. Endocrinol. 2021, 12, 727327. [Google Scholar] [CrossRef]
- White, T.J.; Avery, G.R.; Kennan, N.; Syed, A.M.; Hartley, J.E.; Monson, J.R.T. Virtual colonoscopy vs conventional colonoscopy in patients at high risk of colorectal cancer—A prospective trial of 150 patients. Color. Dis. 2009, 11, 138–145. [Google Scholar] [CrossRef]
- Gollub, M.J. Virtual colonoscopy. Lancet 2002, 360, 964. [Google Scholar] [CrossRef]
- Ganeshan, D.; Elsayes, K.M.; Vining, D. Virtual colonoscopy: Utility, impact and overview. World J. Radiol. 2013, 5, 61. [Google Scholar] [CrossRef]
- Rossi, M.; Mallardo, V.; Rosati, I.; Prisco, M.R.; Califano, T.; Della Vecchia, N.; Romano, G.; Di Lanno, I.; Genovese, E.A. Virtual colonoscopy and PET/CT for diagnosis and staging of colorectal cancer. Recent. Prog. Med. 2013, 104, 345–349. [Google Scholar] [CrossRef]
- Heiken, J.P.; Peterson, C.M.; Menias, C.O. Virtual colonoscopy for colorectal cancer screening: Current status: Wednesday 5 October 2005, 14:00–16:00. Cancer Imaging 2005, 5, S133. [Google Scholar] [CrossRef]
- Plumb, A.A.; Halligan, S.; Pendsé, D.A.; Taylor, S.A.; Mallett, S. Sensitivity and specificity of CT colonography for the detection of colonic neoplasia after positive faecal occult blood testing: Systematic review and meta-analysis. Eur. Radiol. 2014, 24, 1049–1058. [Google Scholar] [CrossRef]
- Atkin, W.; Dadswell, E.; Wooldrage, K.; Kralj-Hans, I.; Von Wagner, C.; Edwards, R.; Yao, G.; Kay, C.; Burling, D.; Faiz, O.; et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): A multicentre randomised trial. Lancet 2013, 381, 1194–1202. [Google Scholar] [CrossRef]
- Cai, W.; Lee, J.G.; Zhang, D.; Kim, S.H.; Zalis, M.; Yoshida, H. Electronic cleansing in fecal-tagging dual-energy CT colonography based on material decomposition and virtual colon tagging. IEEE Trans. Biomed. Eng. 2015, 62, 754–765. [Google Scholar] [CrossRef]
- Torkzad, M.R.; Påhlman, L.; Glimelius, B. Magnetic resonance imaging (MRI) in rectal cancer: A comprehensive review. Insights Imaging 2010, 1, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Saklani, A.P.; Bae, S.U.; Clayton, A.; Kim, N.K. Magnetic resonance imaging in rectal cancer: A surgeon’s perspective. World J. Gastroenterol. 2014, 20, 2030. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, H.S.; Jung, I.; Shin, S.J.; Beom, S.H.; Chang, J.S.; Koom, W.S.; Kim, T.I.; Hur, H.; Min, B.S.; et al. Upfront radical surgery with total mesorectal excision followed by adjuvant FOLFOX chemotherapy for locally advanced rectal cancer (TME-FOLFOX): An open-label, multicenter, phase II randomized controlled trial. Trials 2020, 21, 320. [Google Scholar] [CrossRef]
- Taylor, F.G.M.; Quirke, P.; Heald, R.J.; Moran, B.; Blomqvist, L.; Swift, I.; Sebag-Montefiore, D.J.; Tekkis, P.; Brown, G. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: A prospective, multicenter, European study. Ann. Surg. 2011, 253, 711–719. [Google Scholar] [CrossRef]
- Patel, U.B.; Taylor, F.; Blomqvist, L.; George, C.; Evans, H.; Tekkis, P.; Quirke, P.; Sebag-Montefiore, D.; Moran, B.; Heald, R.; et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J. Clin. Oncol. 2011, 29, 3753–3760. [Google Scholar] [CrossRef]
- Battersby, N.J.; How, P.; Moran, B.; Stelzner, S.; West, N.P.; Branagan, G.; Strassburg, J.; Quirke, P.; Tekkis, P.; Pedersen, B.G.; et al. Prospective Validation of a Low Rectal Cancer Magnetic Resonance Imaging Staging System and Development of a Local Recurrence Risk Stratification Model: The MERCURY II Study. Ann. Surg. 2016, 263, 751–760. [Google Scholar] [CrossRef]
- Patra, A.; Baheti, A.D.; Ankathi, S.K.; Desouza, A.; Engineer, R.; Ostwal, V.; Ramaswamy, A.; Saklani, A. Can Post-Treatment MRI Features Predict Pathological Circumferential Resection Margin (pCRM) Involvement in Low Rectal Tumors. Indian J. Surg. Oncol. 2020, 11, 720–725. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, D.; Cai, S.; Li, Q.; Li, X. Circumferential resection margin as a prognostic factor after rectal cancer surgery: A large population-based retrospective study. Cancer Med. 2018, 7, 3673. [Google Scholar] [CrossRef]
- Khani, H.M.; Smedh, K.; Kraaz, W. Is the circumferential resection margin a predictor of local recurrence after preoperative radiotherapy and optimal surgery for rectal carcinoma? Color. Dis. 2007, 9, 706–712. [Google Scholar] [CrossRef]
- Yuval, J.B.; Patil, S.; Gangai, N.; Omer, D.M.; Akselrod, D.G.; Fung, A.; Harmath, C.B.; Kampalath, R.; Krehbiel, K.; Lee, S.; et al. MRI assessment of rectal cancer response to neoadjuvant therapy: A multireader study. Eur. Radiol. 2023, 33, 5761–5768. [Google Scholar] [CrossRef]
- Srisajjakul, S.; Prapaisilp, P.; Bangchokdee, S. Pitfalls in MRI of rectal cancer: What radiologists need to know and avoid. Clin. Imaging 2018, 50, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Zhang, Y.; Wei, M.; Yang, X.; Wang, Z. Magnetic Resonance Imaging Evaluation of the Accuracy of Various Lymph Node Staging Criteria in Rectal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 709070. [Google Scholar] [CrossRef] [PubMed]
- Maino, C.; Vernuccio, F.; Cannella, R.; Cortese, F.; Franco, P.N.; Gaetani, C.; Giannini, V.; Inchingolo, R.; Ippolito, D.; Defeudis, A.; et al. Liver metastases: The role of magnetic resonance imaging. World J. Gastroenterol. 2023, 29, 5180. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, Y.; Cheng, L.; Fu, L.; Yan, H.; Ru, H.; Mo, X.; Yan, L.; Su, Z. Multi-omics analyses of glucose metabolic reprogramming in colorectal cancer. Front. Immunol. 2023, 14, 1179699. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Miles, K.; Taylor, S.A.; Ganeshan, B.; Rodriquez, M.; Fraioli, F.; Wan, S.; Afaq, A.; Shortman, R.; Walls, D.; et al. FDG-PET/CT in colorectal cancer: Potential for vascular-metabolic imaging to provide markers of prognosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 371. [Google Scholar] [CrossRef] [PubMed]
- Brush, J.; Boyd, K.; Chappell, F.; Crawford, F.; Dozier, M.; Fenwick, E.; Glanville, J.; Mcintosh, H.; Renehan, A.; Weller, D.; et al. The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: A systematic review and economic evaluation. Health Technol. Assess. 2011, 15, 1–192. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.X.; Xie, M.Z.; Liang, X.Q.; Ye, M.L.; Li, J.L.; Hu, B.L. Clinical Significance and Prognostic Value of the Maximum Standardized Uptake Value of 18F-Flurodeoxyglucose Positron Emission Tomography-Computed Tomography in Colorectal Cancer. Front. Oncol. 2021, 11, 741612. [Google Scholar] [CrossRef] [PubMed]
- Bijlstra, O.D.; Boreel, M.M.E.; van Mossel, S.; Burgmans, M.C.; Kapiteijn, E.H.W.; Oprea-Lager, D.E.; Rietbergen, D.D.D.; van Velden, F.H.P.; Vahrmeijer, A.L.; Swijnenburg, R.J.; et al. The Value of 18F-FDG-PET-CT Imaging in Treatment Evaluation of Colorectal Liver Metastases: A Systematic Review. Diagnostics 2022, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Kim, J.S.; Kim, H.J.; Ha, H.K.; Yu, C.S.; Kim, J.C. Diagnostic value of FDG-PET/CT for lymph node metastasis of colorectal cancer. World J. Surg. 2012, 36, 1898–1905. [Google Scholar] [CrossRef]
- Staib, L.; Schirrmeister, H.; Reske, S.N.; Beger, H.G. Is 18F-fluorodeoxyglucose positron emission tomography in recurrent colorectal cancer a contribution to surgical decision making? Am. J. Surg. 2000, 180, 1–5. [Google Scholar] [CrossRef]
- Rodríguez-Fraile, M.; Cózar-Santiago, M.P.; Sabaté-Llobera, A.; Caresia-Aróztegui, A.P.; Bolton, R.C.D.; Orcajo-Rincon, J.; de Arcocha-Torres, M.; García-Velloso, M.J.; García-Talavera, P. FDG PET/CT in colorectal cancer. Rev. Esp. Med. Nucl. Imagen Mol. 2020, 39, 57–66. [Google Scholar] [CrossRef]
- McKinley, E.T.; Watchmaker, J.M.; Chakravarthy, A.B.; Meyerhardt, J.A.; Engelman, J.A.; Walker, R.C.; Washington, M.K.; Coffey, R.J.; Manning, H.C. [18F]-FLT PET to predict early response to neoadjuvant therapy in KRAS wild-type rectal cancer: A pilot study. Ann. Nucl. Med. 2015, 29, 535. [Google Scholar] [CrossRef]
- LeBlanc, R.A.; Oza, U.D.; Hayden, R.; Fanous, H. Use of 68Ga DOTATATE, a new molecular imaging agent, for neuroendocrine tumors. Bayl. Univ. Med. Cent. Proc. 2020, 33, 51. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Ferdani, R.; Anderson, C.J.; Lewis, J.S. Copper-64 Radiopharmaceuticals for Oncologic Imaging. PET Clin. 2009, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Balogova, S.; Talbot, J.N.; Nataf, V.; Michaud, L.; Huchet, V.; Kerrou, K.; Montravers, F. 18F-Fluorodihydroxyphenylalanine vs other radiopharmaceuticals for imaging neuroendocrine tumours according to their type. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 943. [Google Scholar] [CrossRef] [PubMed]
- Musafargani, S.; Ghosh, K.K.; Mishra, S.; Mahalakshmi, P.; Padmanabhan, P.; Gulyás, B. PET/MRI: A frontier in era of complementary hybrid imaging. Eur. J. Hybrid Imaging 2018, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Spick, C.; Herrmann, K.; Czernin, J. 18F-FDG PET/CT and PET/MRI Perform Equally Well in Cancer: Evidence from Studies on More Than 2300 Patients. J. Nucl. Med. 2016, 57, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Pichler, B.J.; Wehrl, H.F.; Kolb, A.; Judenhofer, M.S. PET/MRI: The Next Generation of Multi-Modality Imaging? Semin. Nucl. Med. 2008, 38, 199. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.; Choi, M. Clinical Utility of Positron Emission Tomography Magnetic Resonance Imaging (PET-MRI) in Gastrointestinal Cancers. Diagnostics 2016, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Chen, Y.; Shao, X.; Guo, L.; Xu, X. Lymph nodes primary staging of colorectal cancer in 18F-FDG PET/MRI: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 162. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Chen, W.H.; Luo, S.L.; Luo, Y.X.; Liu, Z.H.; Huang, M.J.; Wang, J.P. Transanal total mesorectal excision for rectal cancer: A preliminary report. Surg. Endosc. 2016, 30, 2552–2562. [Google Scholar] [CrossRef]
- Dayde, D.; Tanaka, I.; Jain, R.; Tai, M.C.; Taguchi, A. Predictive and Prognostic Molecular Biomarkers for Response to Neoadjuvant Chemoradiation in Rectal Cancer. Int. J. Mol. Sci. 2017, 18, 573. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Gollub, M.J.; Brown, G. The importance of MRI for rectal cancer evaluation. Surg. Oncol. 2022, 43, 101739. [Google Scholar] [CrossRef]
- Broer, S.L.; Broekmans, F.J.M.; Laven, J.S.E.; Fauser, B.C.J.M. Anti-Müllerian hormone: Ovarian reserve testing and its potential clinical implications. Hum. Reprod. Update 2014, 20, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Brent, R.J. Cost-Benefit Analysis versus Cost-Effectiveness Analysis from a Societal Perspective in Healthcare. Int. J. Environ. Res. Public Health 2023, 20, 4637. [Google Scholar] [CrossRef] [PubMed]
- Gigli, A.; Francisci, S.; Capodaglio, G.; Pierannunzio, D.; Mallone, S.; Tavilla, A.; Lopez, T.; Zorzi, M.; Stracci, F.; Busco, S.; et al. The Economic Impact of Rectal Cancer: A Population-Based Study in Italy. Int. J. Environ. Res. Public Health 2021, 18, 474. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.B.J.; Wille-Jørgensen, P. National and international guidelines for rectal cancer. Color. Dis. 2014, 16, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Van Weel, C.; Van Weel-Baumgarten, E.; Mold, J. The importance of longitudinal studies in family medicine: Experiences of two practice-based research networks. J. Am. Board Fam. Med. 2006, 19, 69–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaupp, R.; Dinius, J.; Drazic, I.; Körner, M. Long-term effects of an e-learning course on patient safety: A controlled longitudinal study with medical students. PLoS ONE 2019, 14, e0210947. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Cindea-Drimus, R.; Auernhammer, C.; Schmidt, G.; Bartenstein, P.; Hacker, M. 68Ga-DOTATATE PET/CT in the diagnosis of recurrent neuroendocrine tumors. J. Nucl. Med. 2012, 53, 419. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Causa Andrieu, P.; Nincevic, J.; Gomes de Farias, L.D.P.; Khasawneh, H.; Arita, Y.; Stanietzky, N.; Fernandes, M.C.; De Castria, T.B.; Horvat, N. Advances in MRI-Based Assessment of Rectal Cancer Post-Neoadjuvant Therapy: A Comprehensive Review. J. Clin. Med. 2023, 13, 172. [Google Scholar] [CrossRef]
- Partovi, S.; Kohan, A.; Rubbert, C.; Vercher-Conejero, J.L.; Gaeta, C.; Yuh, R.; Zipp, L.; Herrmann, K.A.; Robbin, M.R.; Lee, Z.; et al. Clinical oncologic applications of PET/MRI: A new horizon. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 202. [Google Scholar]
- Rutegård, M.K.; Båtsman, M.; Axelsson, J.; Brynolfsson, P.; Brännström, F.; Rutegård, J.; Ljuslinder, I.; Blomqvist, L.; Palmqvist, R.; Rutegård, M.; et al. PET/MRI and PET/CT hybrid imaging of rectal cancer–description and initial observations from the RECTOPET (REctal Cancer trial on PET/MRI/CT) study. Cancer Imaging 2019, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Crimì, F.; Valeggia, S.; Baffoni, L.; Stramare, R.; Lacognata, C.; Spolverato, G.; Albertoni, L.; Spimpolo, A.; Evangelista, L.; Zucchetta, P.; et al. [18F]FDG PET/MRI in rectal cancer. Ann. Nucl. Med. 2021, 35, 281. [Google Scholar] [CrossRef]
- Bailey, J.J.; Jordan, E.J.; Burke, C.; Ohliger, M.A.; Wang, Z.J.; Van Loon, K.; Varma, M.G.; Hope, T.A. Does Extended PET Acquisition in PET/MRI Rectal Cancer Staging Improve Results? AJR Am. J. Roentgenol. 2018, 211, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Edwards, L.; Singh, R. The Role of Artificial Intelligence in Colorectal Cancer Screening: Lesion Detection and Lesion Characterization. Cancers 2023, 15, 5126. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, Y.; Lv, W.; Liu, L.; Zhou, Q.; Yang, H.; Ren, J.; Liu, G.; Wang, X.; Zhang, X.; et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: A multicentre diagnostic study. Lancet Digit. Health 2021, 3, e250–e259. [Google Scholar] [CrossRef]
- Thomas, J.; Ravichandran, R.; Nag, A.; Gupta, L.; Singh, M.; Panjiyar, B.K. Advancing Colorectal Cancer Screening: A Comprehensive Systematic Review of Artificial Intelligence (AI)-Assisted Versus Routine Colonoscopy. Cureus 2023, 15, e45278. [Google Scholar] [CrossRef]
- Koh, D.M.; Papanikolaou, N.; Bick, U.; Illing, R.; Kahn, C.E.; Kalpathi-Cramer, J.; Matos, C.; Martí-Bonmatí, L.; Miles, A.; Mun, S.K.; et al. Artificial intelligence and machine learning in cancer imaging. Commun. Med. 2022, 2, 133. [Google Scholar] [CrossRef] [PubMed]
- Trivizakis, E.; Papadakis, G.Z.; Souglakos, I.; Papanikolaou, N.; Koumakis, L.; Spandidos, D.A.; Tsatsakis, A.; Karantanas, A.H.; Marias, K. Artificial intelligence radiogenomics for advancing precision and effectiveness in oncologic care (Review). Int. J. Oncol. 2020, 57, 43–53. [Google Scholar] [CrossRef]
- Bedrikovetski, S.; Dudi-Venkata, N.N.; Kroon, H.M.; Seow, W.; Vather, R.; Carneiro, G.; Moore, J.W.; Sammour, T. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 1058. [Google Scholar] [CrossRef]
- Yu, C.; Helwig, E.J. The role of AI technology in prediction, diagnosis and treatment of colorectal cancer. Artif. Intell. Rev. 2022, 55, 323. [Google Scholar] [CrossRef]
- Rao, H.B.; Sastry, N.B.; Venu, R.P.; Pattanayak, P. The role of artificial intelligence based systems for cost optimization in colorectal cancer prevention programs. Front. Artif. Intell. 2022, 5, 955399. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.C. Artificial intelligence in medical imaging. Magn. Reson. Imaging 2020, 68, A1–A4. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.; Hawk, K.E.; Rohren, E.; Vial, A.; Klein, R. Machine Learning and Deep Learning in Medical Imaging: Intelligent Imaging. J. Med. Imaging Radiat. Sci. 2019, 50, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.P.; Chen, Y.; Fishman, E.K. Evolution of imaging in rectal cancer: Multimodality imaging with MDCT, MRI, and PET. J. Gastrointest. Oncol. 2015, 6, 172–184. [Google Scholar] [CrossRef]
- Laurent, C.; Nobili, S.; Rullier, A.; Vendrely, V.; Saric, J.; Rullier, E. Efforts to Improve Local Control in Rectal Cancer Compromise Survival by the Potential Morbidity of Optimal Mesorectal Excision. J. Am. Coll. Surg. 2006, 203, 684–691. [Google Scholar] [CrossRef]
- Thomas, R.; Chalkidou, K. Cost–Effectiveness Analysis. 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK436886/ (accessed on 1 July 2024).

| Interstitial Space Status in MRI | MF Status in MRI | Distance of Invasion from the Levator Ani Muscle | Recommended Surgical Treatment |
|---|---|---|---|
| Safe: tumor above the inter-sphincteric space | Tumor > 1 mm from MF | >10 mm | Inter-sphincteric resection ± anastomosis |
| Safe: tumor limited to the submucosal layer, with the preserved full thickness of the muscularis propria | Tumor > 1 mm from MF | ≤10 mm | Local excision |
| Safe: tumor involving a portion of the muscularis propria | Tumor > 1 mm from MF | ≤10 mm | Inter-sphincteric resection ± anastomosis |
Endangered: any of the following is sufficient:
| Tumor > 1 mm from MF | ≤10 mm | Abdominopelvic excision of the rectum—extralevatory abdominoperineal excision (ELAPE) |
| Safe or Endangered | Infiltration beyond the MF into adjacent organs: prostate bladder vagina sacrum pelvic fascia | Prolapse of the small pelvis |
| Diagnostic Method | T Stage Accuracy | N Stage Accuracy | Strengths | Limitations | Literature |
|---|---|---|---|---|---|
| EUS | 63–96% | 63–85% | Cost-effective and widely available Detailed anatomical assessment No contrast agents required High accuracy for T staging Effective for specific tumor locations | Variable accuracy in N staging Inter-operator variability Limited accuracy for high rectal tumors Tendency to overestimate or underestimate stages | [17,26,36] |
| CT | 38.7–86% | 60.70% | Effective for detecting distant metastases Rapid and precise whole-body scanning | Suboptimal for T and N staging Limited anatomical resolution | [50,57,101] |
| MR | 76–100% | 73–78% | High sensitivity and specificity Accurate assessment of unfavorable factors Better prognostic value Enhanced diagnostic accuracy Reduced risk of non-radical surgery Effective in liver metastasis detection | Steep learning curve Long acquisition time Protocol dependency Challenges in lymph node assessment Technical limitations | [94,102,103] |
| PET/CT | N/A | 74.20% | Comprehensive imaging modality Versatility with various tracers Metabolic activity insight Evaluation of lymph nodes and metastases Detection of synchronous neoplastic diseases Potential for early treatment Response prediction | Limited specific evidence for rectal cancer Variable sensitivity and specificity Mixed results in treatment Planning impact Technical limitations Tracers’ variable efficacy Requirement for a multidisciplinary approach | [82,103,104] |
| PET/MRI | 92–100% | 42–92% | Comprehensive diagnostic examination High tissue resolution Diffusion-weighted imaging No ionizing radiation Improved visualization of extracolonic lesions Impact on treatment strategy Metabolic activity insight Evaluation of lymph nodes and metastases | Cost Longer imaging acquisition Contraindications Learning curve and interpretation Limited availability | [103,105,106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksim, R.; Buczyńska, A.; Sidorkiewicz, I.; Krętowski, A.J.; Sierko, E. Imaging and Metabolic Diagnostic Methods in the Stage Assessment of Rectal Cancer. Cancers 2024, 16, 2553. https://doi.org/10.3390/cancers16142553
Maksim R, Buczyńska A, Sidorkiewicz I, Krętowski AJ, Sierko E. Imaging and Metabolic Diagnostic Methods in the Stage Assessment of Rectal Cancer. Cancers. 2024; 16(14):2553. https://doi.org/10.3390/cancers16142553
Chicago/Turabian StyleMaksim, Rafał, Angelika Buczyńska, Iwona Sidorkiewicz, Adam Jacek Krętowski, and Ewa Sierko. 2024. "Imaging and Metabolic Diagnostic Methods in the Stage Assessment of Rectal Cancer" Cancers 16, no. 14: 2553. https://doi.org/10.3390/cancers16142553
APA StyleMaksim, R., Buczyńska, A., Sidorkiewicz, I., Krętowski, A. J., & Sierko, E. (2024). Imaging and Metabolic Diagnostic Methods in the Stage Assessment of Rectal Cancer. Cancers, 16(14), 2553. https://doi.org/10.3390/cancers16142553






