Review on the Increasing Role for PSMA-Based Radioligand Therapy in Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
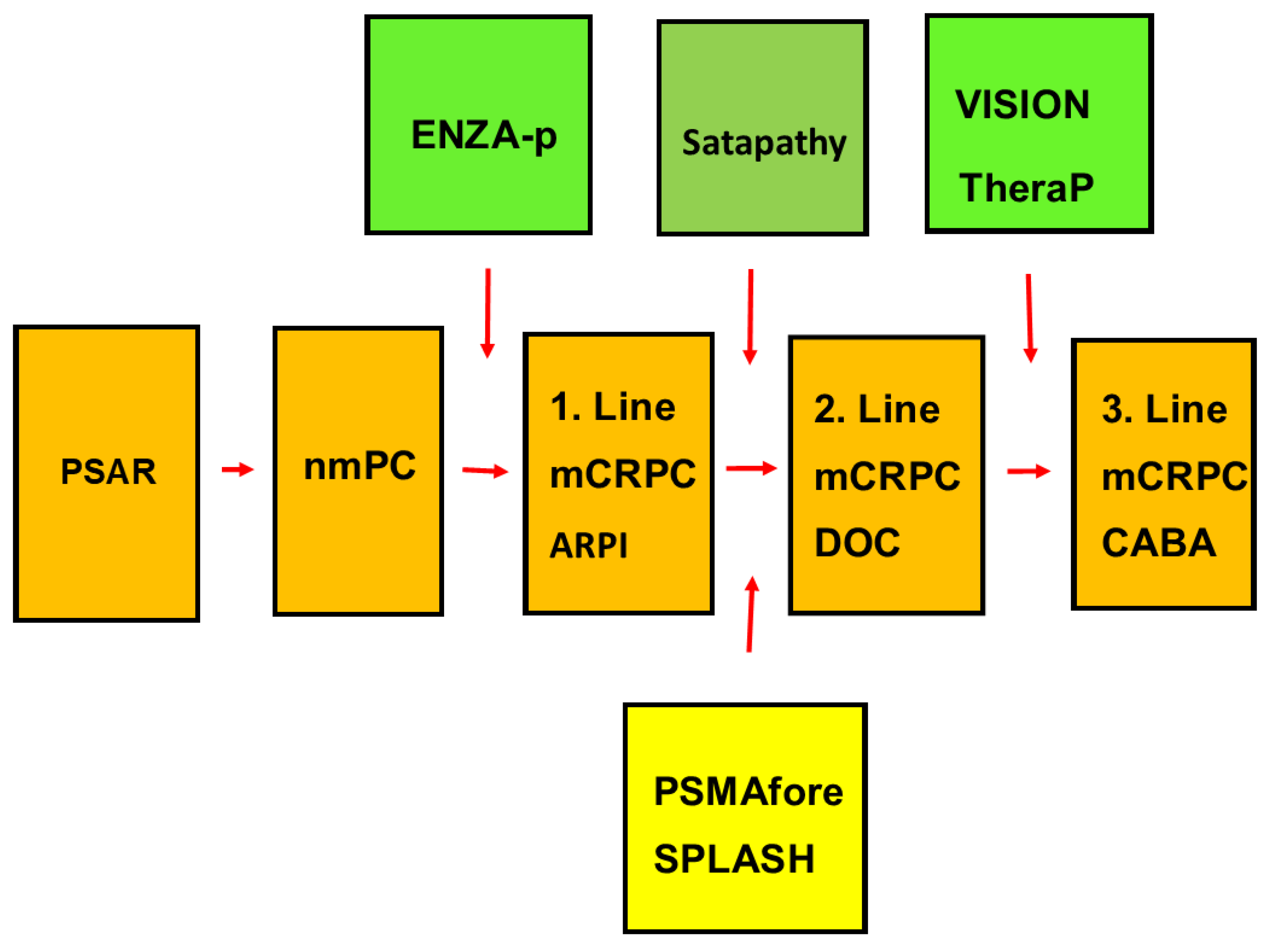
2. Material and Methods
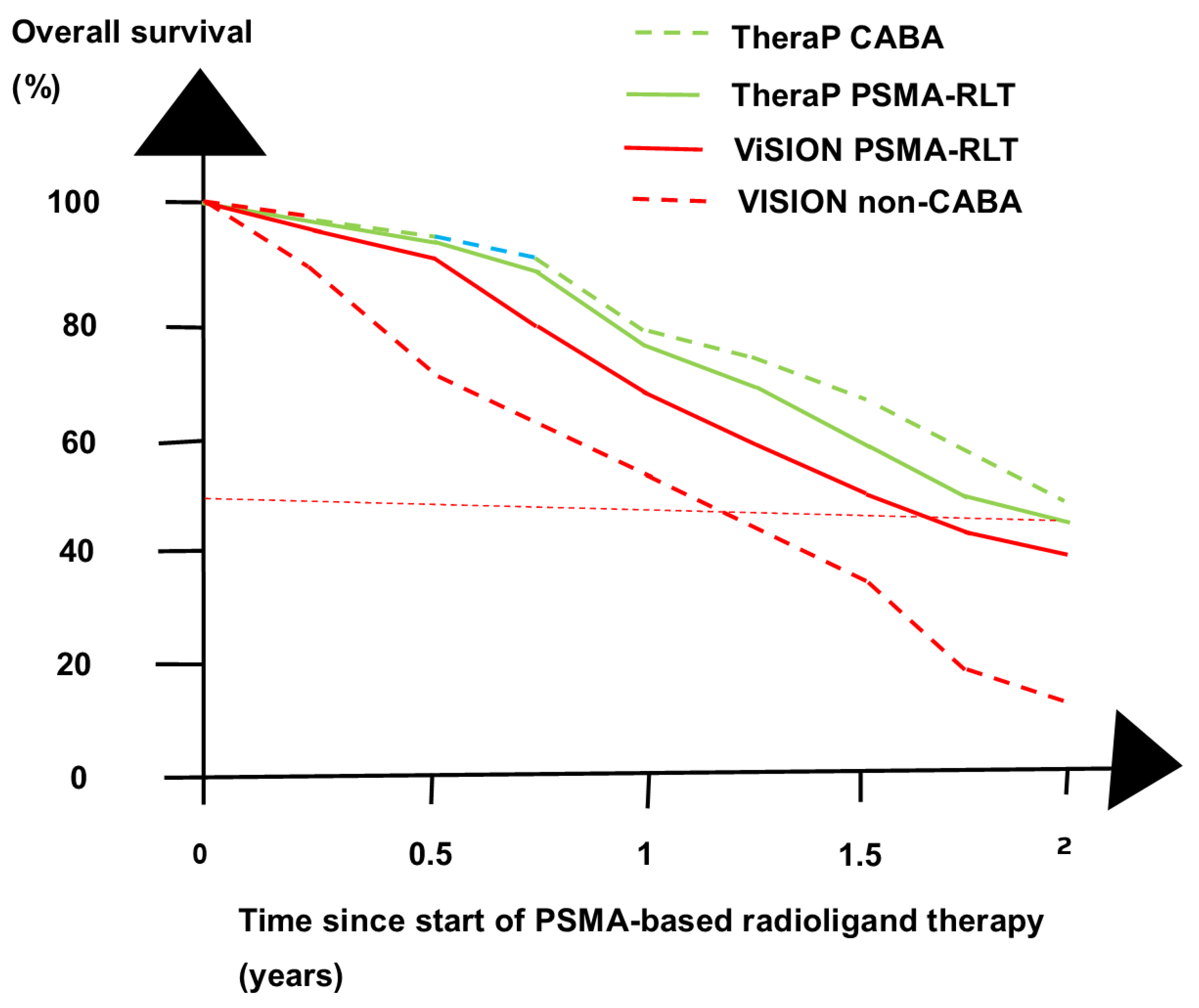
3. Recent RCTs
4. Discussion
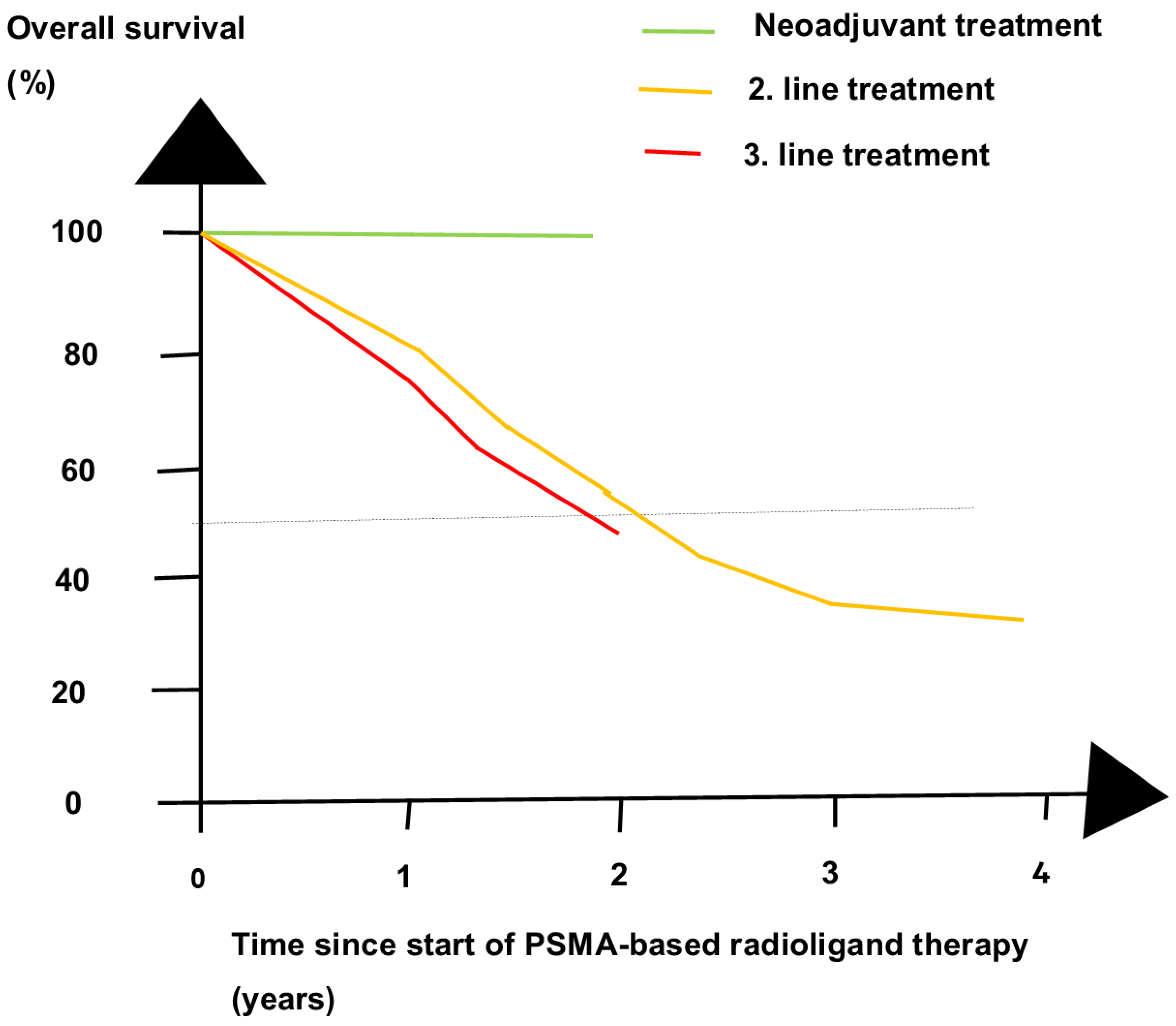
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| ABI | abiraterone |
| Act | Actinium |
| ADT | androgen deprivation therapy |
| ARPI | androgen receptor pathway inhibitor |
| BCR | Biochemical recurrence |
| CABA | cabazitaxel |
| CT | computed tomography |
| DOC | docetaxel |
| ENZA | enzalutamide |
| ENZA-P | randomized controlled phase 2 trial of the combination of enzalutamide and PSMA-based radioligand therapy |
| ESMO | European Society of Medical Oncology |
| LNM | lymph node metastases |
| Lu | lutetium |
| mCRPC | metastatic castration-resistant prostate cancer |
| mHSPC | metastatic hormone-sensitive prostate cancer |
| nmCRPC | nonmetastatic castration-resistant prostate cancer |
| OS | overall survival |
| PCa | prostate cancer |
| PCWG3 | Prostate Cancer Clinical Trials Working Group 3 |
| PET | positron emission tomography |
| PSA | prostate-specific antigen |
| PSAR | prostate-specific antigen relapse |
| PSMA | prostate-specific membrane antigen |
| Ra | Radium |
| RCT | randomized controlled trial |
| RLT | radioligand therapy |
| rPFS | radiological progression-free survival |
| Tb | Terbium |
References
- Montgomery, B.; Tretiakova, M.S.; Joshua, A.M.; Gleave, M.E.; Fleshner, N.; Bubley, G.J.; Mostaghel, E.A.; Chi, K.N.; Lin, D.W.; Sanda, M.; et al. Neoadjuvant Enzalutamide Prior to Prostatectomy. Clin. Cancer Res. 2017, 23, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Ye, H.; Xie, W.; Lis, R.; Calagua, C.; Zhang, Z.; Trinh, Q.D.; Chang, S.L.; Harshman, L.C.; Ross, A.E.; et al. Evaluation of Intense Androgen Deprivation Before Prostatectomy: A Randomized Phase II Trial of Enzalutamide and Leuprolide with or Without Abiraterone. J. Clin. Oncol. 2019, 37, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Murphy, L.; Clarke, N.W.; Cross, W.; Jones, R.J.; Parker, C.C.; Gillessen, S.; Cook, A.; Brawley, C.; Amos, C.L.; et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022, 399, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann. Oncol. 2019, 30, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Pillai, S.; Semira, M.C.; Wong, S.; Shapiro, J.; Weickhardt, A.; Azad, A.; Kwan, E.M.; Spain, L.; Gunjur, A.; et al. Real-world first-line systemic therapy patterns in metastatic castration-resistant prostate cancer. BJUI Compass 2022, 3, 205–213. [Google Scholar] [CrossRef] [PubMed]
- George, D.J.; Sartor, O.; Miller, K.; Saad, F.; Tombal, B.; Kalinovsky, J.; Jiao, X.; Tangirala, K.; Sternberg, C.N.; Higano, C.S. Treatment Patterns and Outcomes in Patients with Metastatic Castration-resistant Prostate Cancer in a Real-world Clinical Practice Setting in the United States. Clin. Genitourin. Cancer 2020, 18, 284–294. [Google Scholar] [CrossRef] [PubMed]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wulfing, C.; Kramer, G.; Eymard, J.C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Freedland, S.J.; Davis, M.; Epstein, A.J.; Arondekar, B.; Ivanova, J.I. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2024, 27, 327–333. [Google Scholar] [CrossRef]
- de Wit, R.; Freedland, S.J.; Oudard, S.; Marinov, G.; Capart, P.; Combest, A.J.; Peterson, R.; Ozatilgan, A.; Morgans, A.K. Real-world evidence of patients with metastatic castration-resistant prostate cancer treated with cabazitaxel: Comparison with the randomized clinical study CARD. Prostate Cancer Prostatic Dis. 2023, 26, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; de Almeida Luz, M.; De Giorgi, U.; Gleave, M.; Gotto, G.T.; Pieczonka, C.M.; Haas, G.P.; Kim, C.S.; Ramirez-Backhaus, M.; Rannikko, A.; et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N. Engl. J. Med. 2023, 389, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Buteau, J.P.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): Secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 2024, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- von Eyben, F.E.; Bauman, G.; von Eyben, R.; Rahbar, K.; Soydal, C.; Haug, A.R.; Virgolini, I.; Kulkarni, H.; Baum, R.; Paganelli, G. Optimizing PSMA radioligand therapy for patients with metastatic castration-resistant prostate cancer. A systematic review and meta-analysis. Int. J. Mol. Sci. 2020, 21, 9054. [Google Scholar] [CrossRef]
- Parida, G.K.; Panda, R.A.; Bishnoi, K.; Agrawal, K. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med. Princ. Pract. 2023, 32, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.M.; Lawal, I.O.; Bal, C.; Bruchertseifer, F.; Ballal, S.; Cardaci, G.; Davis, C.; Eiber, M.; Hekimsoy, T.; Knoesen, O.; et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): A multicentre, retrospective study. Lancet Oncol. 2024, 25, 175–183. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholoma, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: Pilot experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef]
- Banda, A.; Privé, B.M.; Allach, Y.; Uijen, M.J.M.; Peters, S.M.B.; Loeff, C.C.; Gotthardt, M.; Muselaers, C.H.J.; Witjes, J.A.; van Oort, I.M.; et al. PSMA-RLT in Patients with Metastatic Hormone-Sensitive Prostate Cancer: A Retrospective Study. Cancers 2023, 15, 297. [Google Scholar] [CrossRef]
- Langbein, T.; Kulkarni, H.R.; Schuchardt, C.; Mueller, D.; Volk, G.F.; Baum, R.P. Salivary Gland Toxicity of PSMA-Targeted Radioligand Therapy with 177Lu-PSMA and Combined 225Ac- and 177Lu-Labeled PSMA Ligands (TANDEM-PRLT) in Advanced Prostate Cancer: A Single-Center Systematic Investigation. Diagnostics 2022, 12, 1926. [Google Scholar] [CrossRef]
- Emmett, L.; Subramaniam, S.; Crumbaker, M.; Nguyen, A.; Joshua, A.M.; Weickhardt, A.; Lee, S.T.; Ng, S.; Francis, R.J.; Goh, J.C.; et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 563–571. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Tobias, A. Meta-analysis of p values. Stata Tech. Bull. 1999, 49, 15–17. [Google Scholar]
- Suman, S.; Parghane, R.V.; Joshi, A.; Prabhash, K.; Talole, S.; Basu, S. Combined 177Lu-PSMA-617 PRLT and abiraterone acetate versus 177Lu-PSMA-617 PRLT monotherapy in metastatic castration-resistant prostate cancer. An observational study comparing the response and durability. Prostate 2021, 81, 1225–1234. [Google Scholar] [CrossRef]
- Parker, D.; Zambelli, J.; Lara, M.K.; Wolf, T.H.; McDonald, A.; Lee, E.; Abou-Elkacem, L.; Gordon, E.J.; Baum, R.P. Case Report: Long-term complete response to PSMA-targeted radioligand therapy and abiraterone in a metastatic prostate cancer patient. Front. Oncol. 2023, 13, 1192792. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R.; Sood, A.; Das, C.K.; Mavuduru, R.S.; Goyal, S.; Shukla, J.; Singh, S.K. 177Lu-PSMA-617 versus docetaxel in chemotherapy-naive metastatic castration-resistant prostate cancer: A randomized, controlled, phase 2 non-inferiority trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1754–1764. [Google Scholar] [CrossRef]
- Sartor, O.C.; Castellano Gauna, D.E.; Herman, K.; de Bono, J.S.; Shore, N.D.; Chi, K.N.N.; Crosby, M.; Piulats Rodriguez, J.M.; Flechon, A.; Wei, X.X.; et al. LBA13 phase 3 trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore. Ann. Oncol. 2023, 34, S1324–S1325. [Google Scholar] [CrossRef]
- Sartor, O.; Fizazi, K.; Herrmann, K.; Morris, M.J. Design Considerations in the PSMAfore Trial. J. Nucl. Med. 2024, 65, 226–227. [Google Scholar] [CrossRef]
- Hansen, A.R.; Probst, S.; Tulane, R.F.; Osman, M.M.; Delpassand, E.S.; Nordquist, L.T.; Viglianti, B.L.; Michalski, J.M.; Beauregard, J.-M.; Oz, O.K.; et al. 1400P Efficacy and Safety of 177Lu PNT2002 Prostate-Specific Membrane Antigen (PSMA) Therapy in Metatatic Castration-Resistant Prostate Cancer (mCRPC) Initial Results from SPLASH. Ann. Oncol. 2022, 33, S1185. [Google Scholar] [CrossRef]
- Satapathy, S.; Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. [177Lu]Lu-PSMA-617 as first-line systemic therapy in patients with metastatic castration-resistant prostate cancer: A real-world study. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2495–2503. [Google Scholar] [CrossRef]
- Oprea-Lager, D.E.; MacLennan, S.; Bjartell, A.; Briganti, A.; Burger, I.A.; de Jong, I.; De Santis, M.; Eberlein, U.; Emmett, L.; Fizazi, K.; et al. European Association of Nuclear Medicine Focus 5: Consensus on Molecular Imaging and Theranostics in Prostate Cancer. Eur. Urol. 2024, 85, 49–60. [Google Scholar] [CrossRef]
- Mattana, F.; Muraglia, L.; Barone, A.; Colandrea, M.; Saker Diffalah, Y.; Provera, S.; Cascio, A.S.; Omodeo Salè, E.; Ceci, F. Prostate-Specific Membrane Antigen-Targeted Therapy in Prostate Cancer: History, Combination Therapies, Trials, and Future Perspective. Cancers 2024, 16, 1643. [Google Scholar] [CrossRef]
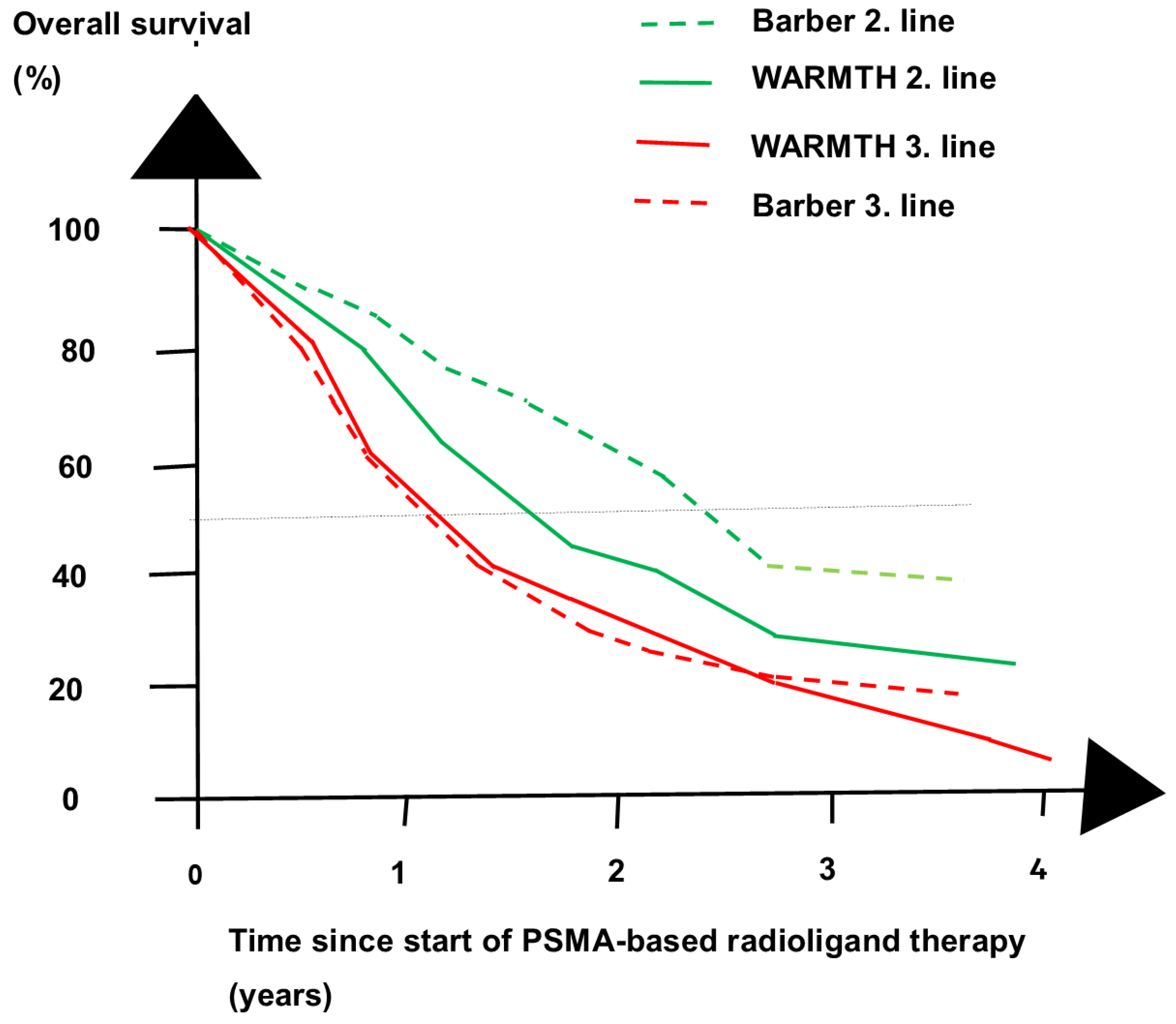
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical outcomes of 177Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bogemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef]
- Satapathy, S.; Sahoo, R.K.; Bal, C. [177Lu]Lu-PSMA-radioligand therapy efficacy outcomes in taxane-naive versus taxane-treated patients with metastatic castration-resistant prostate cancer: A systematic review and metaanalysis. J. Nucl. Med. 2023, 64, 1266–1271. [Google Scholar] [CrossRef]
- Mader, N.; Schoeler, C.; Pezeshkpour, N.; Klimek, K.; Groener, D.; Happel, C.; Tselis, N.; Mandel, P.; Grunwald, F.; Sabet, A. Intermittent radioligand therapy with (177)Lu-PSMA-617 for oligometastatic castration-resistant prostate cancer. Cancers 2023, 15, 4605. [Google Scholar] [CrossRef]
- Edler von Eyben, F.; Singh, A.; Zhang, J.; Nipsch, K.; Meyrick, D.; Lenzo, N.; Kairemo, K.; Joensuu, T.; Virgolini, I.; Soydal, C.; et al. 177Lu-PSMA radioligand therapy of predominant lymph node metastatic prostate cancer. Oncotarget 2019, 10, 2451–2461. [Google Scholar] [CrossRef][Green Version]
- von Eyben, F.E.; Kulkarni, H.R.; Baum, R.P. Metastatic extent predicts survival as patients with metastatic castration-resistant prostate cancer are treated with (177)Lu-PSMA radioligand therapy. Theranostics 2020, 10, 4900–4902. [Google Scholar] [CrossRef]
- Yaxley, W.J.; McBean, R.; Wong, D.; Grimes, D.; Vasey, P.; Frydenberg, M.; Yaxley, J.W. Should Lutetium-prostate specific membrane antigen radioligand therapy for metastatic prostate cancer be used earlier in men with lymph node only metastatic prostate cancer? Investig. Clin. Urol. 2021, 62, 650–657. [Google Scholar] [CrossRef]
- Kostos, L.; Buteau, J.P.; Hofman, M.S.; Azad, A.A. Determinants of outcome following PSMA-based radioligand therapy and mechanisms of resistance in patients with metastatic castration-resistant prostate cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231179309. [Google Scholar] [CrossRef]
- Stuparu, A.D.; Capri, J.R.; Meyer, C.A.L.; Le, T.M.; Evans-Axelsson, S.L.; Current, K.; Lennox, M.; Mona, C.E.; Fendler, W.P.; Calais, J.; et al. Mechanisms of Resistance to Prostate-Specific Membrane Antigen-Targeted Radioligand Therapy in a Mouse Model of Prostate Cancer. J. Nucl. Med. 2021, 62, 989–995. [Google Scholar] [CrossRef]
- De Giorgi, U.; Sansovini, M.; Severi, S.; Nicolini, S.; Monti, M.; Gurioli, G.; Foca, F.; Casadei, C.; Conteduca, V.; Celli, M.; et al. Circulating androgen receptor gene amplification and resistance to 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: Results of a Phase 2 trial. Br. J. Cancer 2021, 125, 1226–1232. [Google Scholar] [CrossRef]
- Chaiswing, L.; Weiss, H.L.; Jayswal, R.D.; Clair, D.K.S.; Kyprianou, N. Profiles of Radioresistance Mechanisms in Prostate Cancer. Crit. Rev. Oncog. 2018, 23, 39–67. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Schulman, C.C.; Debruyne, F.M.; Forster, G.; Selvaggi, F.P.; Zlotta, A.R.; Witjes, W.P. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur. Urol. 2000, 38, 706–713. [Google Scholar] [CrossRef]
- Carles, J.; Gallardo, E.; Domenech, M.; Font, A.; Bellmunt, J.; Figols, M.; Mellado, B.; Saez, M.I.; Suarez, C.; Mendez, M.J.; et al. Phase 2 Randomized Study of Radiation Therapy and 3-Year Androgen Deprivation with or Without Concurrent Weekly Docetaxel in High-Risk Localized Prostate Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 344–352. [Google Scholar] [CrossRef]
- Perera, M.B.; Beech, B.B.; De Jesus Escano, M.; Gmelich, C.; Yip, W.; Boorjan, S.A.; Eastham, J.A. Neoadjuvant Systemic Therapy Prior to Radical Prostatectomy for Clinically Localized High-Risk Prostate Cancer. Front. Oncol. 2022, 2, 864646. [Google Scholar] [CrossRef]
- Eapen, R.S.; Buteau, J.P.; Jackson, P.; Mitchell, C.; Oon, S.F.; Alghazo, O.; McIntosh, L.; Dhiantravan, N.; Scalzo, M.J.; O’Brien, J.; et al. Administering [177Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-risk Localised Prostate Cancer (LuTectomy): A Single-centre, Single-arm, Phase 1/2 Study. Eur. Urol. 2024, 85, 217–226. [Google Scholar] [CrossRef]
- Golan, S.; Frumer, M.; Zohar, Y.; Rosenbaum, E.; Yakimov, M.; Kedar, D.; Margel, D.; Baniel, J.; Steinmetz, A.P.; Groshar, D.; et al. Neoadjuvant 177Lu-PSMA-I&T Radionuclide Treatment in Patients with High-risk Prostate Cancer Before Radical Prostatectomy: A Single-arm Phase 1 Trial. Eur. Urol. Oncol. 2023, 6, 151–159. [Google Scholar] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Catalano, M.; Santi, R.; Santoni, M.; Galli, I.C.; Amorosi, A.; Polom, W.; De Giorgi, U.; Nesi, G. Neoadjuvant Treatment in Muscle-Invasive Bladder Cancer: From the Beginning to the Latest Developments. Front. Oncol. 2022, 12, 912699. [Google Scholar] [CrossRef] [PubMed]
- Horesh, N.; Freund, M.R.; Garoufalia, Z.; Gefen, R.; Nagarajan, A.; Suarez, E.; Emile, S.H.; Wexner, S.D. Total Neoadjuvant Therapy Is a Predictor for Complete Pathological Response in Patients Undergoing Surgery for Rectal Cancer. J. Gastrointest. Surg. 2022, 26, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; van der Meulen, N.P.; Schibli, R. Opportunities and potential challenges of using terbium-161 for targeted radionuclide therapy in clinics. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3181–3184. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Singh, A.; Kulkarni, H.R.; Bernhardt, P.; Ryden, T.; Schuchardt, C.; Gracheva, N.; Grundler, P.V.; Koster, U.; Muller, D.; et al. First-in-Humans Application of 161Tb: A Feasibility Study Using 161Tb-DOTATOC. J. Nucl. Med. 2021, 62, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Schuler, A.; Burgard, C.; Blickle, A.; Maus, S.; Petrescu, C.; Petto, S.; Bartholoma, M.; Stemler, T.; Ezziddin, S.; Rosar, F. [161Tb]Tb-PSMA-617 radioligand therapy in patients with mCRPC: Preliminary dosimetry results and intra-individual head-to-head comparison to [177Lu]Lu-PSMA-617. Theranostics 2024, 14, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Al-Ibraheem, A.; Scott, A.M. 161Tb-PSMA Unleashed: A Promising New Player in the Theranostics of Prostate Cancer. Nucl. Med. Mol. Imaging 2023, 57, 168–171. [Google Scholar] [CrossRef]
- Buteau, J.P.; Kostos, L.K.; Alipour, R.; Jackson, P.; McIntosh, L.; Emmerson, B.; Haskali, M.B.; Yeung, T.; Xie, S.; Medhurst, E.; et al. VIOLET: A phase I/II trial evaluation of radioligand treatment in men with metastatic castration-resistant prostatecancer with [161Tb]Tb-PSMA I&T. J. Clin. Oncol. 2023, 41 (Suppl), TPS281. [Google Scholar]
- Sandhu, S.; Josha, A.M.; Emmett, L.; Crumbaker, M.; Bressel, M.; Huynh, R.; Banks, P.D.; Wallace, R.; Hamid, A.; Inderjeeth, A.J.; et al. LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPPC). J. Clin. Oncol. 2023, 41 (Suppl), 5005. [Google Scholar] [CrossRef]

| Phase of Treatment | Author/NCT Number | Reference | Name of Trial | Type of RLT | No of Patients |
|---|---|---|---|---|---|
| First-line treatment | Emmett 2024 | [23] | ENZA-p | PSMA-617 | 162 |
| Second-line treatment | Satapathy 2023 | [28] | NR | 40 | |
| NCT04689838 | [29,30] | PSMAfore | PSMA-617 | 467 | |
| Hansen 2022 | [31] | SPLASH | PSMA I&T | 412 | |
| Overall total | 1081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Eyben, F.E.; Virgolini, I.; Baum, R. Review on the Increasing Role for PSMA-Based Radioligand Therapy in Prostate Cancer. Cancers 2024, 16, 2520. https://doi.org/10.3390/cancers16142520
von Eyben FE, Virgolini I, Baum R. Review on the Increasing Role for PSMA-Based Radioligand Therapy in Prostate Cancer. Cancers. 2024; 16(14):2520. https://doi.org/10.3390/cancers16142520
Chicago/Turabian Stylevon Eyben, Finn Edler, Irene Virgolini, and Richard Baum. 2024. "Review on the Increasing Role for PSMA-Based Radioligand Therapy in Prostate Cancer" Cancers 16, no. 14: 2520. https://doi.org/10.3390/cancers16142520
APA Stylevon Eyben, F. E., Virgolini, I., & Baum, R. (2024). Review on the Increasing Role for PSMA-Based Radioligand Therapy in Prostate Cancer. Cancers, 16(14), 2520. https://doi.org/10.3390/cancers16142520







