B7H4 Role in Solid Cancers: A Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. B7H4 in Immunity and Autoimmunological Diseases
3. B7H4 Expression in Solid Cancers
3.1. Breast Cancer
3.2. Gastrointestinal Cancers
3.2.1. Oesophageal Cancer
3.2.2. Gastric Cancer
3.2.3. Pancreatic Cancer
3.2.4. Cholangiocarcinoma and Gallbladder Cancer
3.2.5. Hepatocellular Carcinoma
3.2.6. Colorectal Cancer
3.3. Urinary
3.3.1. Renal Cell Carcinoma
3.3.2. Urothelial Cancer
3.3.3. Prostate Cancer
3.4. Gynecological Cancers
3.4.1. Cervical Cancer
3.4.2. Ovarian Cancer
3.4.3. Endometrial Cancer
3.5. Head and Neck Cancers
3.6. Lung Cancer
3.7. Central Nervous System Malignancies
4. B7H4 Targeting Immunotherapies
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Zang, X.; Loke, P.; Kim, J.; Murphy, K.; Waitz, R.; Allison, J.P. B7x: A Widely Expressed B7 Family Member That Inhibits T Cell Activation. Proc. Natl. Acad. Sci. USA 2003, 100, 10388–10392. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-H.; Zhu, G.; Sica, G.L.; Strome, S.E.; Cheville, J.C.; Lau, J.S.; Zhu, Y.; Flies, D.B.; Tamada, K.; Chen, L. Genomic Organization and Expression Analysis of B7-H4, an Immune Inhibitory Molecule of the B7 Family. J. Immunol. 2003, 171, 4650–4654. [Google Scholar] [CrossRef] [PubMed]
- Sica, G.L.; Choi, I.H.; Zhu, G.; Tamada, K.; Wang, S.D.; Tamura, H.; Chapoval, A.I.; Flies, D.B.; Bajorath, J.; Chen, L. B7-H4, a Molecule of the B7 Family, Negatively Regulates T Cell Immunity. Immunity 2003, 18, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.V.R.; Richards, S.; Mai, X.M.; Dong, C. B7S1, a Novel B7 Family Member That Negatively Regulates T Cell Activation. Immunity 2003, 18, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jin, K.; Zeng, H.; Shao, F.; Chang, Y.; Wang, Y.; Xu, L.; Wang, Z.; Cui, X.; Zhu, Y.; et al. B7-H4 Correlates with Clinical Outcome and Immunotherapeutic Benefit in Muscle-Invasive Bladder Cancer. Eur. J. Cancer 2022, 171, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, M.; Kula, A.; Mielcarska, S.; Kiczmer, P.; Skiba, H.; Krygier, M.; Chrabańska, M.; Piecuch, J.; Szrot, M.; Robotycka, J.; et al. B7H4 Expression Is More Frequent in MSS Status Colorectal Cancer and Is Negatively Associated with Tumour Infiltrating Lymphocytes. Cells 2023, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- John, P.; Wei, Y.; Liu, W.; Du, M.; Guan, F.; Zang, X. The B7x Immune Checkpoint Pathway: From Discovery to Clinical Trial. Trends Pharmacol. Sci. 2019, 40, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Podojil, J.R.; Chiang, M.-Y.; Ifergan, I.; Copeland, R.; Liu, L.N.; Maloveste, S.; Langermann, S.; Liebenson, D.; Balabanov, R.; Chi, H.; et al. B7-H4 Modulates Regulatory CD4+ T Cell Induction and Function via Ligation of a Semaphorin 3a/Plexin A4/Neuropilin-1 Complex. J. Immunol. 2018, 201, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Augustine, M.M.; Azuma, T.; Luo, L.; Yao, S.; Anand, S.; Rietz, A.C.; Huang, J.; Xu, H.; Flies, A.S.; et al. B7-H4-Deficient Mice Display Augmented Neutrophil-Mediated Innate Immunity. Blood 2009, 113, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Abadi, Y.M.; Jeon, H.; Ohaegbulam, K.C.; Scandiuzzi, L.; Ghosh, K.; Hofmeyer, K.A.; Lee, J.S.; Ray, A.; Gravekamp, C.; Zang, X. Host B7x Promotes Pulmonary Metastasis of Breast Cancer. J. Immunol. 2013, 190, 3806–3814. [Google Scholar] [CrossRef] [PubMed]
- Radichev, I.A.; Maneva-Radicheva, L.V.; Amatya, C.; Parker, C.; Ellefson, J.; Wasserfall, C.; Atkinson, M.; Burn, P.; Savinov, A.Y. Nardilysin-Dependent Proteolysis of Cell-Associated VTCN1 (B7-H4) Marks Type 1 Diabetes Development. Diabetes 2014, 63, 3470–3482. [Google Scholar] [CrossRef] [PubMed]
- Albers, H.M.; Reinards, T.H.C.M.; Brinkman, D.M.C.; Kamphuis, S.S.M.; van Rossum, M.A.J.; Hoppenreijs, E.P.A.H.; Girschick, H.J.; Wouters, C.; Saurenmann, R.K.; Bakker, E.; et al. Genetic Variation in VTCN1 (B7-H4) Is Associated with Course of Disease in Juvenile Idiopathic Arthritis. Ann. Rheum. Dis. 2014, 73, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Zhu, G.; Xu, H.; Rietz, A.C.; Drake, C.G.; Matteson, E.L.; Chen, L. Potential Role of Decoy B7-H4 in the Pathogenesis of Rheumatoid Arthritis: A Mouse Model Informed by Clinical Data. PLoS Med. 2009, 6, e1000166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Scandiuzzi, L.; Ray, A.; Wei, J.; Hofmeyer, K.A.; Abadi, Y.M.; Loke, P.; Lin, J.; Yuan, J.; Serreze, D.V.; et al. B7x in the Periphery Abrogates Pancreas-Specific Damage Mediated by Self-Reactive CD8 T Cells. J. Immunol. 2012, 189, 4165–4174. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, Y.; Li, Y.; Jiang, Y.; Lu, H.; Zang, W.; Zhao, X.; Liu, L.; Chen, Y.; Tan, H.; et al. Co-Inhibitory Molecule B7 Superfamily Member 1 Expressed by Tumor-Infiltrating Myeloid Cells Induces Dysfunction of Anti-Tumor CD8+ T Cells. Immunity 2018, 48, 773–786.e5. [Google Scholar] [CrossRef] [PubMed]
- Ohaegbulam, K.C.; Liu, W.; Jeon, H.; Almo, S.C.; Zang, X. Tumor-Expressed Immune Checkpoint B7x Promotes Cancer Progression and Antigen-Specific CD8 T Cell Exhaustion and Suppressive Innate Immune Cells. Oncotarget 2017, 8, 82740–82753. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Zou, L.; Rodriguez, P.; Zhu, G.; Wei, S.; Mottram, P.; Brumlik, M.; Cheng, P.; Curiel, T.; Myers, L.; et al. B7-H4 Expression Identifies a Novel Suppressive Macrophage Population in Human Ovarian Carcinoma. J. Exp. Med. 2006, 203, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ye, H.; Qi, Z.; Mo, L.; Yue, Q.; Baral, A.; Hoon, D.S.B.; Vera, J.C.; Heiss, J.D.; Chen, C.C.; et al. B7-H4(B7x)-Mediated Cross-Talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin. Cancer Res. 2016, 22, 2778–2790. [Google Scholar] [CrossRef]
- Kryczek, I.; Wei, S.; Zhu, G.; Myers, L.; Mottram, P.; Cheng, P.; Chen, L.; Coukos, G.; Zou, W. Relationship between B7-H4, Regulatory T Cells, and Patient Outcome in Human Ovarian Carcinoma. Cancer Res. 2007, 67, 8900–8905. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Yang, Z.; Jin, J.; Ni, W.; Qi, W.; Xuan, Y. B7H4 Is Associated with Stemness and Cancer Progression in Esophageal Squamous Cell Carcinoma. Hum. Pathol. 2018, 80, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K. Hypoxia Induces Core-to-Edge Transition of Progressive Tumoral Cells: A Critical Review on Differential yet Corroborative Roles for HIF-1α and HIF-2α. Life Sci. 2020, 242, 117145. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K. Enriched Cancer Stem Cells, Dense Stroma, and Cold Immunity: Interrelated Events in Pancreatic Cancer. J. Biochem. Mol. Toxicol. 2021, 35, e22708. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Vigdorovich, V.; Garrett-Thomson, S.C.; Janakiram, M.; Ramagopal, U.A.; Abadi, Y.M.; Lee, J.S.; Scandiuzzi, L.; Ohaegbulam, K.C.; Chinai, J.M.; et al. Structure and Cancer Immunotherapy of the B7 Family Member B7x. Cell Rep. 2014, 9, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Borczuk, A.; Janakiram, M.; Ren, X.; Lin, J.; Assal, A.; Halmos, B.; Perez-Soler, R.; Zang, X. Wide Expression and Significance of Alternative Immune Checkpoint Molecules, B7x and HHLA2, in PD-L1-Negative Human Lung Cancers. Clin. Cancer Res. 2018, 24, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.I.; Park, M.H.; Lee, J.S. Associations of B7-H3 and B7-H4 Expression in Ductal Carcinoma In Situ of the Breast with Clinicopathologic Features and T-Cell Infiltration. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Altan, M.; Pelekanou, V.; Schalper, K.A.; Toki, M.; Gaule, P.; Syrigos, K.; Herbst, R.S.; Rimm, D.L. B7-H3 Expression in NSCLC and Its Association with B7-H4, PD-L1 and Tumor-Infiltrating Lymphocytes. Clin. Cancer Res. 2017, 23, 5202–5209. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhou, Z.; Li, H.; Xue, Y.; Lu, X.; Bahar, I.; Kepp, O.; Hung, M.-C.; Kroemer, G.; Wan, Y. Pharmacologic Suppression of B7-H4 Glycosylation Restores Antitumor Immunity in Immune-Cold Breast Cancers. Cancer Discov. 2020, 10, 1872–1893. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Zhang, Y.; Xie, L.; Jiang, H.; Zeng, H.; Chen, C.; Liu, L.; He, X.; Hao, X.; Fang, X.; et al. B7-H4 Enhances the Differentiation of Murine Leukemia-Initiating Cells via the PTEN/AKT/RCOR2/RUNX1 Pathways. Leukemia 2017, 31, 2260–2264. [Google Scholar] [CrossRef]
- Wartewig, T.; Kurgyis, Z.; Keppler, S.; Pechloff, K.; Hameister, E.; Öllinger, R.; Maresch, R.; Buch, T.; Steiger, K.; Winter, C.; et al. PD-1 Is a Haploinsufficient Suppressor of T Cell Lymphomagenesis. Nature 2017, 552, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.I.; Park, M.H.; Kweon, S.-S.; Lee, J.S. B7-H3 and B7-H4 Expression in Breast Cancer and Their Association with Clinicopathological Variables and T Cell Infiltration. Pathobiology 2020, 87, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, C.; Ren, G. Clinical Significance of the B7-H4 as a Novel Prognostic Marker in Breast Cancer. Gene 2017, 623, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Altan, M.; Kidwell, K.M.; Pelekanou, V.; Carvajal-Hausdorf, D.E.; Schalper, K.A.; Toki, M.I.; Thomas, D.G.; Sabel, M.S.; Hayes, D.F.; Rimm, D.L. Association of B7-H4, PD-L1, and Tumor Infiltrating Lymphocytes with Outcomes in Breast Cancer. NPJ Breast Cancer 2018, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, C.; Liu, X.-B.; Wang, L.; Kang, F.-B. B7-H4 Overexpression Contributes to Poor Prognosis and Drug-Resistance in Triple-Negative Breast Cancer. Cancer Cell Int. 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, J.; Ruan, M.; Xiao, Y.; Lan, H.; Wu, Q.; Yu, C.-W.; Zhang, Q. The Loss of B7-H4 Expression in Breast Cancer Cells Escaping from T Cell Cytotoxicity Contributes to Epithelial-to-Mesenchymal Transition. Breast Cancer Res. 2023, 25, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, J.; Wu, H.; Zhou, S.; Tan, Y.; Tan, M.; Shan, B.; Lu, B.; Zhang, X. B7-H4 Expression Associates with Cancer Progression and Predicts Patient’s Survival in Human Esophageal Squamous Cell Carcinoma. Cancer Immunol. Immunother. 2011, 60, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, Q.; Wang, Z.; Shi, L.; Wu, C.; Jiang, J. Assessment of Combined Expression of B7-H3 and B7-H4 as Prognostic Marker in Esophageal Cancer Patients. Oncotarget 2016, 7, 77237–77243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, N.-N.; Wang, S.; Man, H.-W.; Li, P.-F.; Shan, B.-E. Roles of Coinhibitory Molecules B7-H3 and B7-H4 in Esophageal Squamous Cell Carcinoma. Tumour Biol. 2016, 37, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, W.; Man, H.; Li, P.; Shan, B. Increased B7-H4 Expression during Esophageal Squamous Cell Carcinogenesis Is Associated with IL-6/STAT3 Signaling Pathway Activation in Mice. Oncol. Lett. 2017, 13, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Wang, W.; Zhao, L.; Shan, B. B7-H4 Facilitates Proliferation of Esophageal Squamous Cell Carcinoma Cells through Promoting Interleukin-6/Signal Transducer and Activator of Transcription 3 Pathway Activation. Cancer Sci. 2016, 107, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, Z.; Zhang, Y.; Quan, Q.; Huang, L.; Xu, Y.; Cao, L.; Zhang, X. Association of Increased B7 Protein Expression by Infiltrating Immune Cells with Progression of Gastric Carcinogenesis. Medicine 2019, 98, e14663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, Y.; Wu, C.; Shen, Y.; Wei, W.; Chen, L.; Zheng, X.; Sun, J.; Lu, B.; Zhang, X. Tumor Expression of B7-H4 Predicts Poor Survival of Patients Suffering from Gastric Cancer. Cancer Immunol. Immunother. 2010, 59, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ji, M.; Wu, J.; Zhou, Q.; Li, X.; Li, Z.; Zheng, X.; Xu, B.; Zhao, W.; Wu, C.; et al. Serum B7-H4 Expression Is a Significant Prognostic Indicator for Patients with Gastric Cancer. World J. Surg. Oncol. 2014, 12, 188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsunaga, T.; Saito, H.; Ikeguchi, M. Increased B7-H1 and B7-H4 Expressions on Circulating Monocytes and Tumor-Associated Macrophages Are Involved in Immune Evasion in Patients with Gastric Cancer. Yonago Acta Med. 2011, 54, 1–10. [Google Scholar] [PubMed]
- Shan, Z.; Yan, Z.; Peng, L.; Cheng, P.; Teng, Y.; Mao, F.; Fan, K.; Zhuang, Y.; Zhao, Y. Granulocyte-Macrophage Colony-Stimulating Factor-Activated Neutrophils Express B7-H4 That Correlates with Gastric Cancer Progression and Poor Patient Survival. J. Immunol. Res. 2021, 2021, 6613247. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, H.; Lu, C.; Li, Q.; Xu, B.; Jiang, J.; Wu, C. Expression of Costimulatory Molecules B7-H1, B7-H4 and Foxp3+ Tregs in Gastric Cancer and Its Clinical Significance. Int. J. Clin. Oncol. 2015, 20, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Maskey, N.; Li, K.; Hu, M.; Xu, Z.; Peng, C.; Yu, F.; Cao, H.; Chen, J.; Li, Y.; Yang, G. Impact of Neoadjuvant Chemotherapy on Lymphocytes and Co-Inhibitory B7-H4 Molecule in Gastric Cancer: Low B7-H4 Expression Associates with Favorable Prognosis. Tumour Biol. 2014, 35, 11837–11843. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, Y.; Li, C.; Yang, L. Silencing of B7-H4 Suppresses the Tumorigenicity of the MGC-803 Human Gastric Cancer Cell Line and Promotes Cell Apoptosis via the Mitochondrial Signaling Pathway. Int. J. Oncol. 2018, 52, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Tsiaousidou, A.; Lambropoulou, M.; Chatzitheoklitos, E.; Tripsianis, G.; Tsompanidou, C.; Simopoulos, C.; Tsaroucha, A.K. B7H4, HSP27 and DJ-1 Molecular Markers as Prognostic Factors in Pancreatic Cancer. Pancreatology 2013, 13, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, X.; Tao, M.; Chen, K.; Chen, C.; Xu, G.; Li, W.; Yuan, S.; Mao, Y. B7-H3 and B7-H4 Are Independent Predictors of a Poor Prognosis in Patients with Pancreatic Cancer. Oncol. Lett. 2016, 11, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Sang, Y.; Wang, F.X.C.; Hong, B.; Wang, Q.; Zhou, X.; Weng, T.; Wu, Z.; Zheng, M.; Zhang, H.; et al. Prognostic Significance of B7-H4 Expression in Matched Primary Pancreatic Cancer and Liver Metastases. Oncotarget 2016, 7, 72242–72249. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Mo, S.; Ma, H.; Lu, Z.; Yu, S.; Chen, J. Prognostic Value of Programmed Death Ligand-1 in Discriminating Patients With Lymph Node–Negative, P53–Wild-Type, or Low-BRCA1/2-Expression Pancreatic Ductal Adenocarcinoma. Arch. Pathol. Lab. Med. 2022, 147, 465–473. [Google Scholar] [CrossRef]
- Loch, F.N.; Kamphues, C.; Beyer, K.; Schineis, C.; Rayya, W.; Lauscher, J.C.; Horst, D.; Dragomir, M.P.; Schallenberg, S. The Immune Checkpoint Landscape in Tumor Cells of Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 2160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, J.; Liu, Y.; Zheng, X.; Feng, J.; Chen, X.; Jiang, T.; Li, Y.; Chen, L. Prognostic Values of B7-H3, B7-H4, and HHLA2 Expression in Human Pancreatic Cancer Tissues Based on mIHC and Spatial Distribution Analysis. Pathol. Res. Pract. 2022, 234, 153911. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, Z.; Gao, H.; Xiong, F.; Cao, C.; Yu, J.; Shi, W.; Zhan, Q.; Yang, C. Clinical Significance and Correlation of PD-L1, B7-H3, B7-H4, and TILs in Pancreatic Cancer. BMC Cancer 2022, 22, 584. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Wang, L.; Cao, H.; Xu, Y.; Zhan, Q. Co-Deficiency of B7-H3 and B7-H4 Identifies High CD8 + T Cell Infiltration and Better Prognosis in Pancreatic Cancer. BMC Cancer 2022, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-S.; Li, H.; Li, T.-J.; Li, S.; Xia, H.-Y.; Long, J.; Wu, C.-T.; Wang, W.-Q.; Zhang, W.-H.; Gao, H.-L.; et al. Neutrophil Extracellular Traps and Macrophage Extracellular Traps Predict Postoperative Recurrence in Resectable Nonfunctional Pancreatic Neuroendocrine Tumors. Front. Immunol. 2021, 12, 577517. [Google Scholar] [CrossRef] [PubMed]
- Kaltenmeier, C.; Yazdani, H.O.; Morder, K.; Geller, D.A.; Simmons, R.L.; Tohme, S. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2021, 12, 785222. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, H.; Mo, S.; Yu, S.; Lu, Z.; Chen, J. Intratumoral Neutrophil Extracellular Traps Are Associated with Unfavorable Clinical Outcomes and Immunogenic Context in Pancreatic Ductal Adenocarcinoma. Front. Immunol. 2022, 13, 1027459. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Hong, B.; Shen, L.; Wu, Z.; Yao, H.; Zhang, L. B7-H4 Enhances Oncogenicity and Inhibits Apoptosis in Pancreatic Cancer Cells. Cell Tissue Res. 2013, 353, 139–151. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Li, Z.; Jiang, P.; Deng, X.; Tian, F.; Li, X.; Wang, S. Aberrant Expression of B7-H4 Correlates with Poor Prognosis and Suppresses Tumor-Infiltration of CD8+ T Lymphocytes in Human Cholangiocarcinoma. Oncol. Rep. 2016, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Cai, J.-B.; Zhang, L.; Zhang, P.-F.; Shen, Y.-H.; Yang, X.; Lu, J.-C.; Gao, D.-M.; Kang, Q.; Liu, L.-X.; et al. Upregulation of B7-H4 Promotes Tumor Progression of Intrahepatic Cholangiocarcinoma. Cell Death Dis. 2017, 8, 3205. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zeng, L.; Hu, Y.; Chen, S.; Tian, M.; Hu, Q. Detection of Early-Stage Extrahepatic Cholangiocarcinoma in Patients with Biliary Strictures by Soluble B7-H4 in the Bile. Am. J. Cancer Res. 2018, 8, 699–707. [Google Scholar] [PubMed]
- Lv, C.; Han, S.; Wu, B.; Liang, Z.; Li, Y.; Zhang, Y.; Lang, Q.; Zhong, C.; Fu, L.; Yu, Y.; et al. Novel Immune Scoring Dynamic Nomograms Based on B7-H3, B7-H4, and HHLA2: Potential Prediction in Survival and Immunotherapeutic Efficacy for Gallbladder Cancer. Front. Immunol. 2022, 13, 984172. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Zang, X.-X.; Huang, H.; Zhang, H.; Wang, C.; Kong, Y.-L.; Zhang, H.-Y. The Expression of B7-H3 and B7-H4 in Human Gallbladder Carcinoma and Their Clinical Implications. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4466–4473. [Google Scholar]
- Kang, F.-B.; Wang, L.; Sun, D.-X.; Li, H.-J.; Li, D.; Wang, Y.; Kang, J.-W. B7-H4 Overexpression Is Essential for Early Hepatocellular Carcinoma Progression and Recurrence. Oncotarget 2017, 8, 80878–80888. [Google Scholar] [CrossRef][Green Version]
- Liao, X.; Zhang, D. HHLA2 Immune Checkpoint Is a Novel Prognostic Predictor in Hepatocellular Carcinoma. Am. J. Clin. Pathol. 2022, 158, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.A.; Wu, Z.X.; Zhang, X.; Zeng, Z.Y.; Li, D.L. Circulating B7-H4 in Serum Predicts Prognosis in Patients with Hepatocellular Carcinoma. Genet. Mol. Res. 2015, 14, 13041–13048. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, Y. Diagnostic Value of Serum B7-H4 for Hepatocellular Carcinoma. J. Surg. Res. 2015, 197, 301–306. [Google Scholar] [CrossRef]
- Yuan, L.; Dong, L.; Yu, G.; Fan, W.; Zhang, L.; Wang, P.; Hu, X.; Zhao, M. Aberrant Expression of B7-H4 May Contribute to the Development of Hepatocellular Carcinoma. Mol. Med. Rep. 2016, 14, 5015–5024. [Google Scholar] [CrossRef][Green Version]
- Zhang, P.; Chen, Z.; Chen, L.; Zang, H.; Zhu, B.; Shao, W.; Niu, W. Clinical Significance of Changes in AFP, HTATIP2/TIP30, B7-H4 and Inflammatory Cytokines after Transcatheter Arterial Chemoembolization. J. BUON 2020, 25, 1206–1211. [Google Scholar] [PubMed]
- Dong, L.; Xie, L.; Li, M.; Dai, H.; Wang, X.; Wang, P.; Zhang, Q.; Liu, W.; Hu, X.; Zhao, M. Downregulation of B7-H4 Suppresses Tumor Progression of Hepatocellular Carcinoma. Sci. Rep. 2019, 9, 14854. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.-T.; Liao, R.; Lei, D.-L.; Hu, G.-L.; Luo, F. Inhibition of B7-H4 Promotes Hepatocellular Carcinoma Cell Apoptosis and Autophagy through the PI3K Signaling Pathway. Int. Immunopharmacol. 2020, 88, 106889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lu, Y.; Zhao, Z.; Shi, T.; Wu, H.; Chen, W.; Zhang, L.; Zhang, X. B7-H4 Expression Is Upregulated by PKCδ Activation and Contributes to PKCδ-Induced Cell Motility in Colorectal Cancer. Cancer Cell Int. 2022, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhao, Z.-X.; Cheng, P.; Huang, F.; Guan, X.; Zhang, M.-G.; Chen, H.-P.; Liu, Z.; Jiang, Z.; Zheng, Z.-X.; et al. B7-H3 Immune Checkpoint Expression Is a Poor Prognostic Factor in Colorectal Carcinoma. Mod. Pathol. 2020, 33, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, Q.; Gao, Z.; Xu, X.; Lu, Q.; Wu, Y. Clinical Value of Detecting IQGAP3, B7-H4 and Cyclooxygenase-2 in the Diagnosis and Prognostic Evaluation of Colorectal Cancer. Cancer Cell Int. 2019, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Liu, G.; Zhou, N.; Xu, D.; Feng, N.; Lei, Y.; Ma, L.; Tang, M.; Tong, G.; Tang, N.; et al. Interferon-γ in the Tumor Microenvironment Promotes the Expression of B7H4 in Colorectal Cancer Cells, Thereby Inhibiting Cytotoxic T Cells. Sci. Rep. 2024, 14, 6053. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Shi, L.; Yang, J.; Wang, H.; Yang, H.; Wang, Q. B7 Family Member H4 Induces Epithelial-Mesenchymal Transition and Promotes the Proliferation, Migration and Invasion of Colorectal Cancer Cells. Bioengineered 2022, 13, 107–118. [Google Scholar] [CrossRef]
- Ding, S.; Lv, X.; Liu, Z.; Zhan, S.; Xu, Y.; Zhang, X.; Liu, C.; Cao, L. Overexpression of B7-H4 Is Associated with Infiltrating Immune Cells and Poor Prognosis in Metastatic Colorectal Cancer. Int. Immunopharmacol. 2021, 90, 107144. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, Z.; Zhang, C.; Che, N.; Liu, X.; Xuan, Y. B7-H4 Induces Epithelial-Mesenchymal Transition and Promotes Colorectal Cancer Stemness. Pathol. Res. Pract. 2021, 218, 153323. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, M.; Kula, A.; Mielcarska, S.; Świętochowska, E.; Waniczek, D. Prognostic Value of B7H4 Expression in Patients with Solid Cancers: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 5045. [Google Scholar] [CrossRef] [PubMed]
- Peuker, K.; Strigli, A.; Tauriello, D.V.F.; Hendricks, A.; von Schönfels, W.; Burmeister, G.; Brosch, M.; Herrmann, A.; Krüger, S.; Nitsche, J.; et al. Microbiota-Dependent Activation of the Myeloid Calcineurin-NFAT Pathway Inhibits B7H3- and B7H4-Dependent Anti-Tumor Immunity in Colorectal Cancer. Immunity 2022, 55, 701–717.e7. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhan, Y.; Ma, X.; Fang, H.; Gai, X. B7-H4 Facilitates Proliferation and Metastasis of Colorectal Carcinoma Cell through PI3K/Akt/mTOR Signaling Pathway. Clin. Exp. Med. 2020, 20, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kamai, T.; Masuda, A.; Nukui, A.; Abe, H.; Arai, K.; Yoshida, K.-I. Higher Preoperative Serum Levels of PD-L1 and B7-H4 Are Associated with Invasive and Metastatic Potential and Predictable for Poor Response to VEGF-Targeted Therapy and Unfavorable Prognosis of Renal Cell Carcinoma. Cancer Med. 2016, 5, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Krambeck, A.E.; Thompson, R.H.; Dong, H.; Lohse, C.M.; Park, E.S.; Kuntz, S.M.; Leibovich, B.C.; Blute, M.L.; Cheville, J.C.; Kwon, E.D. B7-H4 Expression in Renal Cell Carcinoma and Tumor Vasculature: Associations with Cancer Progression and Survival. Proc. Natl. Acad. Sci. USA 2006, 103, 10391–10396. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, S.; Song, M.; Liu, W.; Liu, C.; Li, Y.; Wang, M. B7-H4 Expression and Its Role in Interleukin-2/Interferon Treatment of Clear Cell Renal Cell Carcinoma. Oncol. Lett. 2014, 7, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Sato, Y.; Ohno, T.; Azuma, M.; Kume, H. Serum Soluble B7-H4 Is a Prognostic Marker for Patients with Non-Metastatic Clear Cell Renal Cell Carcinoma. PLoS ONE 2018, 13, e0199719. [Google Scholar] [CrossRef] [PubMed]
- Crispen, P.L.; Boorjian, S.A.; Lohse, C.M.; Leibovich, B.C.; Kwon, E.D. Predicting Disease Progression after Nephrectomy for Localized Renal Cell Carcinoma: The Utility of Prognostic Models and Molecular Biomarkers. Cancer 2008, 113, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, N.; Zhao, Z.; Chen, Y.; Zhang, L. Overexpression of B7-H4 Promotes Renal Cell Carcinoma Progression by Recruiting Tumor-Associated Neutrophils via Upregulation of CXCL8. Oncol. Lett. 2020, 20, 1535–1544. [Google Scholar] [CrossRef]
- Emaldi, M.; Nunes-Xavier, C.E. B7-H4 Immune Checkpoint Protein Affects Viability and Targeted Therapy of Renal Cancer Cells. Cells 2022, 11, 1448. [Google Scholar] [CrossRef]
- Mizuno, T.; Kamai, T.; Tsuzuki, T.; Nishihara, D.; Kijima, T.; Arai, K.; Yoshida, K.-I. Elevated Expression of B7 Homolog 4 Is Associated with Disease Progression in Upper Urinary Tract Urothelial Carcinoma. Cancer Immunol. Immunother. 2022, 71, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-H.; Chen, Y.-Y.; Zhu, S.-X.; Li, Y.-N.; Xu, Y.-P.; Wu, X.-J.; Guo, Y.-H.; Wang, J.-L. B7-H4 Expression in Bladder Urothelial Carcinoma and Immune Escape Mechanisms. Oncol. Lett. 2014, 8, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; Mo, N.; Zhang, L.; Yuan, X.; Lü, Z. B7-Homolog 4 Promotes Epithelial-Mesenchymal Transition and Invasion of Bladder Cancer Cells via Activation of Nuclear Factor-κB. Oncol. Res. 2018, 26, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Podojil, J.R.; Glaser, A.P.; Baker, D.; Courtois, E.T.; Fantini, D.; Yu, Y.; Eaton, V.; Sivajothi, S.; Chiang, M.; Das, A.; et al. Antibody Targeting of B7-H4 Enhances the Immune Response in Urothelial Carcinoma. Oncoimmunology 2020, 9, 1744897. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Piao, L.; Liu, S.; Cui, Y.; Xuan, Y. B7-H4 Is a Potential Prognostic Biomarker of Prostate Cancer. Exp. Mol. Pathol. 2020, 114, 104406. [Google Scholar] [CrossRef] [PubMed]
- Kgatle, M.M.; Boshomane, T.M.G.; Lawal, I.O.; Mokoala, K.M.G.; Mokgoro, N.P.; Lourens, N.; Kairemo, K.; Zeevaart, J.R.; Vorster, M.; Sathekge, M.M. Immune Checkpoints, Inhibitors and Radionuclides in Prostate Cancer: Promising Combinatorial Therapy Approach. Int. J. Mol. Sci. 2021, 22, 4109. [Google Scholar] [CrossRef] [PubMed]
- Ihle, C.L.; Provera, M.D.; Straign, D.M.; Smith, E.E.; Edgerton, S.M.; Van Bokhoven, A.; Lucia, M.S.; Owens, P. Distinct Tumor Microenvironments of Lytic and Blastic Bone Metastases in Prostate Cancer Patients. J. ImmunoTherapy Cancer 2019, 7, 293. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Zhang, Q.; Zhou, Y.; Kong, Y.; Yu, S.; Chen, J.; Zhang, Y.; Xiang, Y. Expression and Significance of Immune Checkpoints in Clear Cell Carcinoma of the Uterine Cervix. J. Immunol. Res. 2020, 2020, e1283632. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, Y.; Zhang, J.; Liu, L.; Chen, Q.; Qian, Q.; Li, S.; Zhang, Y. Roles of Immune Inhibitory Molecule B7-H4 in Cervical Cancer. Oncol. Rep. 2017, 37, 2308–2316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, T.; Xu, M.; Xiao, L.; Luo, Y.; Huang, W.; Zhang, Y.; Geng, W. B7-H4 Overexpression Impairs the Immune Response of T Cells in Human Cervical Carcinomas. Hum. Immunol. 2014, 75, 1203–1209. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, L.; Chang, X.; Pang, X.; Zhang, H.; Zhang, S. B7-H3, B7-H4, Foxp3 and IL-2 Expression in Cervical Cancer: Associations with Patient Outcome and Clinical Significance. Oncol. Rep. 2016, 35, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Gu, Y.; Zhou, Y.; Kong, Y.; Mo, S.; Yu, S.; Xiang, Y.; Chen, J. Expression of B7 Family Checkpoint Proteins in Cervical Cancer. Mod. Pathol. 2022, 35, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zong, L.; Zhang, H.; Xie, W.; Yang, F.; Sun, W.; Cui, B.; Zhang, Y. B7-H4 Expression in Precancerous Lesions of the Uterine Cervix. BioMed Res. Int. 2021, 2021, 5857092. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Yuan, C.; Xu, J.; Zhang, J.; Chen, F.; Liu, D.; Zheng, B.; Li, R.; Huang, X.; Xu, J. Role of B7-H4 in the Progression and Prognosis of Cervical Inflammation to Cancer After Human Papilloma Virus Infection. J. Biomed. Nanotechnol. 2019, 15, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, J.; Li, Z.; Chen, Y.; Zhang, Y. The B7H4-PDL1 Classifier Stratifies Immuno-Phenotype in Cervical Cancer. Cancer Cell Int. 2022, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, R.; Lin, A.; Ghazarian, M.; Yau, H.-L.; Paramathas, S.; Lang, P.A.; Schildknecht, A.; Elford, A.R.; Garcia-Batres, C.; Martin, B.; et al. B7-H4 Expression by Nonhematopoietic Cells in the Tumor Microenvironment Promotes Antitumor Immunity. Cancer Immunol. Res. 2015, 3, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wang, Y.; Shi, X.; Zong, L.; Liu, L.; Zhang, J.; Qian, Q.; Jin, J.; Ma, Y.; Cui, B.; et al. Negative Roles of B7-H3 and B7-H4 in the Microenvironment of Cervical Cancer. Exp. Cell Res. 2018, 371, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Mcmurray, H.R.; Nguyen, D.; Westbrook, T.F.; Mcance, D.J. Biology of Human Papillomaviruses. Int. J. Exp. Pathol. 2001, 82, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yang, R. RCD24, B7-H4 and PCNA Expression and Clinical Significance in Ovarian Cancer. J. BUON 2019, 24, 715–719. [Google Scholar] [PubMed]
- MacGregor, H.L.; Sayad, A.; Elia, A.; Wang, B.X.; Katz, S.R.; Shaw, P.A.; Clarke, B.A.; Crome, S.Q.; Robert-Tissot, C.; Bernardini, M.Q.; et al. High Expression of B7-H3 on Stromal Cells Defines Tumor and Stromal Compartments in Epithelial Ovarian Cancer and Is Associated with Limited Immune Activation. J. Immunother. Cancer 2019, 7, 357. [Google Scholar] [CrossRef]
- Hwang, C.; Lee, H.J.; Na, J.-Y.; Kim, K.H.; Song, Y.J.; Kim, J.Y.; Kim, K.; Shin, D.H.; Park, J.Y.; Kim, S.Y.; et al. The Stromal Tumor-Infiltrating Lymphocytes, Cancer Stemness, Epithelial-Mesenchymal Transition, and B7-H4 Expression in Ovarian Serous Carcinoma. J. Ovarian Res. 2023, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Ulrich, M.; Epp, A.; Younan, P.; Sahetya, D.; Hensley, K.; Allred, S.; Huang, L.-Y.; Hahn, J.; Gahnberg, K.; et al. SGN-B7H4V, an Investigational Vedotin ADC Directed to the Immune Checkpoint Ligand B7-H4, Shows Promising Activity in Preclinical Models. J. Immunother. Cancer 2023, 11, e007572. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, H.L.; Garcia-Batres, C.; Sayad, A.; Elia, A.; Berman, H.K.; Toker, A.; Katz, S.R.; Shaw, P.A.; Clarke, B.A.; Crome, S.Q.; et al. Tumor Cell Expression of B7-H4 Correlates with Higher Frequencies of Tumor-Infiltrating APCs and Higher CXCL17 Expression in Human Epithelial Ovarian Cancer. Oncoimmunology 2019, 8, e1665460. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Shen, W.; Zhong, Y.; Robert, C.; Bast, J.; Jazaeri, A.; Sood, A.K.; Liu, J. Expression of B7-H4 and IDO1 Is Associated with Drug Resistance and Poor Prognosis in High-Grade Serous Ovarian Carcinomas. Hum. Pathol. 2021, 113, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.M.; Kannan, L.; Geistlinger, L.; Kofia, V.; Safikhani, Z.; Gendoo, D.M.; Parmigiani, G.; Birrer, M.; Haibe-Kains, B.; Waldron, L. Consensus on Molecular Subtypes of High-Grade Serous Ovarian Carcinoma. Clin. Cancer Res. 2018, 24, 5037–5047. [Google Scholar] [CrossRef] [PubMed]
- Mach, P.; Kimmig, R.; Kasimir-Bauer, S.; Buderath, P. Association of Soluble B7-H4 and Circulating Tumor Cells in Blood of Advanced Epithelial Ovarian Cancer Patients. Front. Oncol. 2021, 11, 721067. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Fu, D.; Xi, M. Serum B7 Homologous Body 4 for the Diagnosis of Ovarian Cancer in Chinese Han Women: A Meta-Analysis. J. Cancer Res. Ther. 2018, 14, S433. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Terai, Y.; Tanabe, A.; Ono, Y.J.; Hayashi, M.; Maeda, K.; Fujiwara, S.; Ashihara, K.; Nakamura, M.; Tanaka, Y.; et al. CD24 Expression Is a Marker for Predicting Clinical Outcome and Regulates the Epithelial-Mesenchymal Transition in Ovarian Cancer via Both the Akt and ERK Pathways. Oncol. Rep. 2017, 37, 3189–3200. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Kikuchi, S.; Hishiki, A.; Shao, Y.; Heath, R.; Evison, B.J.; Actis, M.; Canman, C.E.; Hashimoto, H.; Fujii, N. A Small Molecule Inhibitor of Monoubiquitinated Proliferating Cell Nuclear Antigen (PCNA) Inhibits Repair of Interstrand DNA Cross-Link, Enhances DNA Double Strand Break, and Sensitizes Cancer Cells to Cisplatin. J. Biol. Chem. 2014, 289, 7109. [Google Scholar] [CrossRef] [PubMed]
- Tanizaki, Y.; Kobayashi, A.; Toujima, S.; Shiro, M.; Mizoguchi, M.; Mabuchi, Y.; Yagi, S.; Minami, S.; Takikawa, O.; Ino, K. Indoleamine 2,3-Dioxygenase Promotes Peritoneal Metastasis of Ovarian Cancer by Inducing an Immunosuppressive Environment. Cancer Sci. 2014, 105, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Shen, Y.; Seeger, W.L.; An, S.; Qi, R.; Sanderson, J.A.; Cai, Y. Ovarian Carcinoma-Infiltrating Regulatory T Cells Were More Potent Suppressors of CD8+ T Cell Inflammation than Their Peripheral Counterparts, a Function Dependent on TIM3 Expression. Tumor Biol. 2016, 37, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Garlanda, C.; Mantovani, A.; Riva, F. Cytokine Decoy and Scavenger Receptors as Key Regulators of Immunity and Inflammation. Cytokine 2016, 87, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xie, W.; Zhou, L. Mucosal Chemokine CXCL17: What Is Known and Not Known. Scand. J. Immunol. 2021, 93, e12965. [Google Scholar] [CrossRef]
- Toader, D.; Fessler, S.P.; Collins, S.D.; Conlon, P.R.; Bollu, R.; Catcott, K.C.; Chin, C.-N.; Dirksen, A.; Du, B.; Duvall, J.R.; et al. Discovery and Preclinical Characterization of XMT-1660, an Optimized B7-H4-Targeted Antibody–Drug Conjugate for the Treatment of Cancer. Mol. Cancer Ther. 2023, 22, 999–1012. [Google Scholar] [CrossRef]
- Xie, B.; Xia, Y.; Lin, D.; Lian, B.; Zhang, M.; Liu, L.; Qin, C.-R. Pan-Cancer Gene Analysis of m6A Modification and Immune Infiltration in Uterine Corpus Endometrial Carcinoma. Comput. Intell. Neurosci. 2022, 2022, 6530884. [Google Scholar] [CrossRef]
- Zong, L.; Yu, S.; Mo, S.; Sun, Z.; Lu, Z.; Chen, J.; Xiang, Y. B7-H4 Further Stratifies Patients With Endometrial Cancer Exhibiting a Nonspecific Molecular Profile. Arch. Pathol. Lab. Med. 2023, 147, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Gorzelnik, K.; Wasaznik-Jedras, A.; Wicherek, L.; Szubert, S. Expression of B7–H4 in Endometrial Cancer and Its Impact on Patients’ Prognosis. Ginekol. Pol. 2024, 95, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, C.; Chen, J.; Gao, X.; Xie, X.; Zhang, X. Identification of an Immune Checkpoint Gene Signature That Accurately Predicts Prognosis and Immunotherapy Response in Endometrial Carcinoma. Aging 2021, 13, 16696–16712. [Google Scholar] [CrossRef] [PubMed]
- Bregar, A.; Deshpande, A.; Grange, C.; Zi, T.; Stall, J.; Hirsch, H.; Reeves, J.; Sathyanarayanan, S.; Growdon, W.B.; Rueda, B.R. Characterization of Immune Regulatory Molecules B7-H4 and PD-L1 in Low and High Grade Endometrial Tumors. Gynecol. Oncol. 2017, 145, 446–452. [Google Scholar] [CrossRef]
- Dai, F.; Wu, J.; Deng, Z.; Li, H.; Tan, W.; Yuan, M.; Yang, D.; Liu, S.; Zheng, Y.; Hu, M.; et al. Integrated Bioinformatic Analysis of DNA Methylation and Immune Infiltration in Endometrial Cancer. BioMed Res. Int. 2022, 2022, 5119411. [Google Scholar] [CrossRef]
- Chi, J.; Liu, Y.; Yang, L.; Yang, J. Silencing of B7H4 Represses the Development of Oral Squamous Cell Carcinoma Through Promotion of M1 Macrophage Polarization. J. Oral. Maxillofac. Surg. 2022, 80, 1408–1423. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, M.; Oetting, A.; Meyer, F.; Möckelmann, N.; Droste, C.; von Bargen, C.M.; Möller-Koop, C.; Witt, M.; Borgmann, K.; Rothkamm, K.; et al. The Prognostic Impact of B7-H3 and B7-H4 in Head and Neck Squamous Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.M.; Mitani, Y.; Hoff, C.O.; Bonini, F.; Guimaraes de Sousa, L.; Marques-Piubelli, M.L.; Purushothaman, A.; Mitani, M.; Dai, H.; Lin, S.-Y.; et al. Analysis of B7-H4 Expression Across Salivary Gland Carcinomas Reveals Adenoid Cystic Carcinoma–Specific Prognostic Relevance. Mod. Pathol. 2024, 37, 100371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-W.; Wang, S.; Wu, Z.-Z.; Yang, Q.-C.; Chen, D.-R.; Wan, S.-C.; Sun, Z.-J. Overexpression of CD168 Is Related to Poor Prognosis in Oral Squamous Cell Carcinoma. Oral Dis. 2022, 28, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Wang, W.-Y.; Zhou, J.-J.; Wu, L.; Zhang, M.-J.; Yang, Q.-C.; Deng, W.-W.; Sun, Z.-J. Inhibition of DNMT1 Potentiates Antitumor Immunity in Oral Squamous Cell Carcinoma. Int. Immunopharmacol. 2022, 111, 109113. [Google Scholar] [CrossRef] [PubMed]
- Shafi, S.; Aung, T.N.; Xirou, V.; Gavrielatou, N.; Vathiotis, I.A.; Fernandez, A.; Moutafi, M.; Yaghoobi, V.; Herbst, R.S.; Liu, L.N.; et al. Quantitative Assessment of Siglec-15 Expression in Lung, Breast, Head, and Neck Squamous Cell Carcinoma and Bladder Cancer. Lab. Investig. 2022, 102, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Ye, J.; Fan, D. The B7-H4 Gene Induces Immune Escape Partly via Upregulating the PD-1/Stat3 Pathway in Non-Small Cell Lung Cancer. Hum. Immunol. 2020, 81, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, S.; Zhang, F.; Cai, L.; Xie, W. The Significance of Immune-Regulatory Molecule B7-H4 in Small Cell Lung Cancer. Ann. Palliat. Med. 2020, 9, 1953–1957. [Google Scholar] [CrossRef]
- Carvajal-Hausdorf, D.; Altan, M.; Velcheti, V.; Gettinger, S.N.; Herbst, R.S.; Rimm, D.L.; Schalper, K.A. Expression and Clinical Significance of PD-L1, B7-H3, B7-H4 and TILs in Human Small Cell Lung Cancer (SCLC). J. Immunother. Cancer 2019, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.-J.; Xia, Q.; Chen, Y.-B.; Fang, X.-F.; Li, Q.-T.; Zhu, L.-S.; Jiang, X.; Xiong, Z.-F.; Yang, S.-L. The Expression of Three Negative Co-Stimulatory B7 Family Molecules in Small Cell Lung Cancer and Their Effect on Prognosis. Front. Oncol. 2021, 11, 600238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, R.; Li, X.; Shi, Z.; Tian, H.; Feng, J.; Yu, S. B7-H4 and HHLA2, Members of B7 Family, Are Aberrantly Expressed in EGFR Mutated Lung Adenocarcinoma. Pathol. Res. Pract. 2020, 216, 153134. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Villalobos, P.; Zhang, J.; Behrens, C.; Mino, B.; Swisher, S.; Sepesi, B.; Weissferdt, A.; Kalhor, N.; Heymach, J.V.; et al. Immunohistochemical and Image Analysis-Based Study Shows That Several Immune Checkpoints Are Co-Expressed in Non–Small Cell Lung Carcinoma Tumors. J. Thorac. Oncol. 2018, 13, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shi, M.; Feng, J. Differential Expression of B7-H4, VISTA, B7-H6, HHLA2, IDO-1, PD-L1 and CD8 in EGFR Mutant and Wild-Type Lung Adenocarcinoma. Ann. Oncol. 2019, 30, v586. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, N.; Li, A.; Zhou, B.; Chen, Y.; Chen, S.; Huang, M.; Wu, F.; Zhang, L. Insulin-like Growth Factor-1 Receptor Induces Immunosuppression in Lung Cancer by Upregulating B7-H4 Expression through the MEK/ERK Signaling Pathway. Cancer Lett. 2020, 485, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, G.; Ji, C.; Lu, Q.; Qi, Y.; Tang, C.; Xiong, J.; Hu, J.; Yasar, F.B.A.; Zhang, Y.; et al. Enhanced B7-H4 Expression in Gliomas with Low PD-L1 Expression Identifies Super-Cold Tumors. J. Immunother. Cancer 2020, 8, e000154. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, C.; Li, Z.; Ai, M.; Wang, B.; Du, K.; Liu, W.; Wang, H.; Yu, P.; Chen, C.; et al. Exosomal B7–H4 from Irradiated Glioblastoma Cells Contributes to Increase FoxP3 Expression of Differentiating Th1 Cells and Promotes Tumor Growth. Redox Biol. 2022, 56, 102454. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Luo, F.; Tang, C.; Chen, D.; Qin, Z.; Hua, W.; Xu, M.; Zhong, P.; Yu, S.; Chen, D.; et al. Molecular Subgroups and B7-H4 Expression Levels Predict Responses to Dendritic Cell Vaccines in Glioblastoma: An Exploratory Randomized Phase II Clinical Trial. Cancer Immunol. Immunother. 2018, 67, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K. B7x in Cancer Immunity and Immunotherapy. Int. Immunopharmacol. 2023, 118, 110133. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune Checkpoint Therapy for Solid Tumours: Clinical Dilemmas and Future Trends. Sig. Transduct. Target. Ther. 2023, 8, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Sun, X. Development of a Novel Anti-B7-H4 Antibody Enhances Anti-Tumor Immune Response of Human T Cells. Biomed. Pharmacother. 2021, 141, 111913. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, A.; Nonomura, C.; Ashizawa, T.; Kondou, R.; Ohshima, K.; Sugino, T.; Mitsuya, K.; Hayashi, N.; Nakasu, Y.; Maruyama, K.; et al. A T-Cell–Engaging B7-H4/CD3-Bispecific Fab-scFv Antibody Targets Human Breast Cancer. Clin. Cancer Res. 2019, 25, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, Y.; Lim, S.; Park, K.; Kim, E.; Lee, H.; Yoo, J.; Pak, Y.; Kim, Y.; Ko, M.; et al. Abstract 4246: ABL103, A Novel T-Cell Engaging Bispecific Antibody, Exhibits Potent in Vitro and Vivo Antitumor Activity and Low Toxicity via B7-H4 Dependent 4-1BB Activation in Tumor Microenvironment. Cancer Res. 2022, 82, 4246. [Google Scholar] [CrossRef]
- Chang, T.-P.; Polonskaya, Z.; Luna, X.; Martomo, S.; Zhang, Z.; Ng, S.; Patel, J.P.; Lu, D. 45P A Novel Bi-Functional IL15 Cytokine Fusion Antibody Selected to Kill B7-H4 Positive Tumor Cells. Ann. Oncol. 2021, 32, S1391. [Google Scholar] [CrossRef]
- Gitto, S.B.; Whicker, M.; Davies, G.; Kumar, S.; Kinneer, K.; Xu, H.; Lewis, A.; Mamidi, S.; Medvedev, S.; Kim, H.; et al. A B7-H4-Targeting Antibody-Drug Conjugate Shows Antitumor Activity in PARPi and Platinum-Resistant Cancers with B7-H4 Expression. Clin. Cancer Res. 2024, 30, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.B.; Lanitis, E.; Dangaj, D.; Buza, E.; Poussin, M.; Stashwick, C.; Scholler, N.; Powell, D.J. Tumor Regression and Delayed Onset Toxicity Following B7-H4 CAR T Cell Therapy. Mol. Ther. 2016, 24, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Rinis, N.; Golden, J.E.; Marceau, C.D.; Carette, J.E.; Van Zandt, M.C.; Gilmore, R.; Contessa, J.N. Editing N-Glycan Site Occupancy with Small-Molecule Oligosaccharyltransferase Inhibitors. Cell Chem. Biol. 2018, 25, 1231–1241.e4. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Henry, J.T.; Lakhani, N.; Call, J.A.; Hamilton, E.P.; Colon-Otero, G.; Diamond, J.R.; O’Neil, B.; Kalyan, A.; Sonpavde, G.P.; et al. 660MO First-in-Human Study of SGN-B7H4V, a B7-H4-Directed Vedotin ADC, in Patients with Advanced Solid Tumors: Preliminary Results of a Phase I Study (SGNB7H4V-001). Ann. Oncol. 2023, 34, S464–S465. [Google Scholar] [CrossRef]
- Munari, E.; Mariotti, F.R.; Quatrini, L.; Bertoglio, P.; Tumino, N.; Vacca, P.; Eccher, A.; Ciompi, F.; Brunelli, M.; Martignoni, G.; et al. PD-1/PD-L1 in Cancer: Pathophysiological, Diagnostic and Therapeutic Aspects. Int. J. Mol. Sci. 2021, 22, 5123. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Wang, W.; Wang, Y.C.; Lin, Y.; Zhang, X.W. Diagnosis Value of Serum Soluble B7-H4 Expression in Non-Small Cell Lung Cancer. Clin. Respir. J. 2018, 12, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Kontos, F.; Michelakos, T.; Kurokawa, T.; Sadagopan, A.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3: An Attractive Target for Antibody-Based Immunotherapy. Clin. Cancer Res. 2021, 27, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Pulanco, M.C.; Madsen, A.T.; Tanwar, A.; Corrigan, D.T.; Zang, X. Recent Advancements in the B7/CD28 Immune Checkpoint Families: New Biology and Clinical Therapeutic Strategies. Cell. Mol. Immunol. 2023, 20, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Shao, Y.; Huang, M.; Liu, D.; Yu, H.; Qiu, Y. Immunometabolism in Cancer: Basic Mechanisms and New Targeting Strategy. Cell Death Discov. 2024, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- DePeaux, K.; Delgoffe, G.M. Metabolic Barriers to Cancer Immunotherapy. Nat. Rev. Immunol. 2021, 21, 785–797. [Google Scholar] [CrossRef] [PubMed]
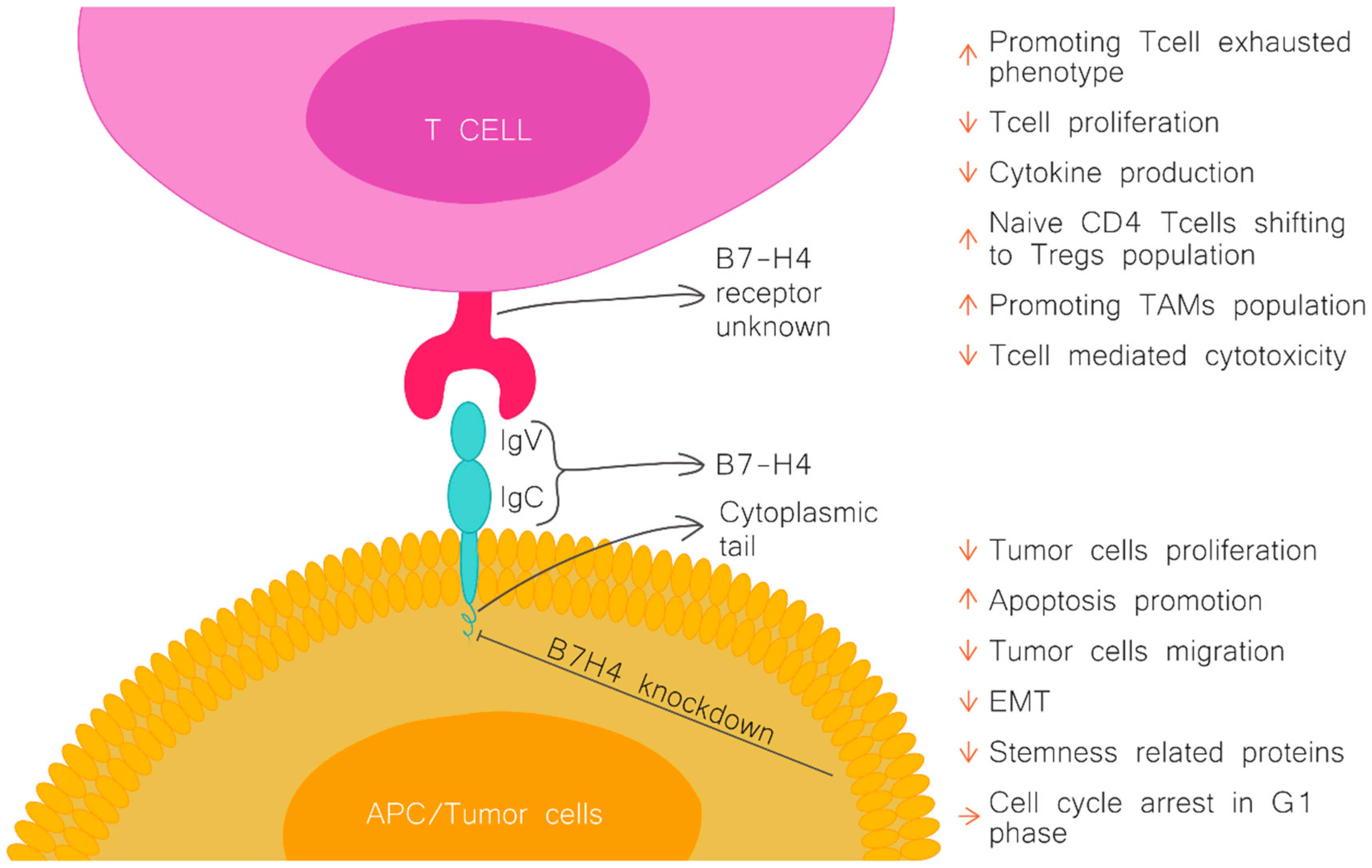
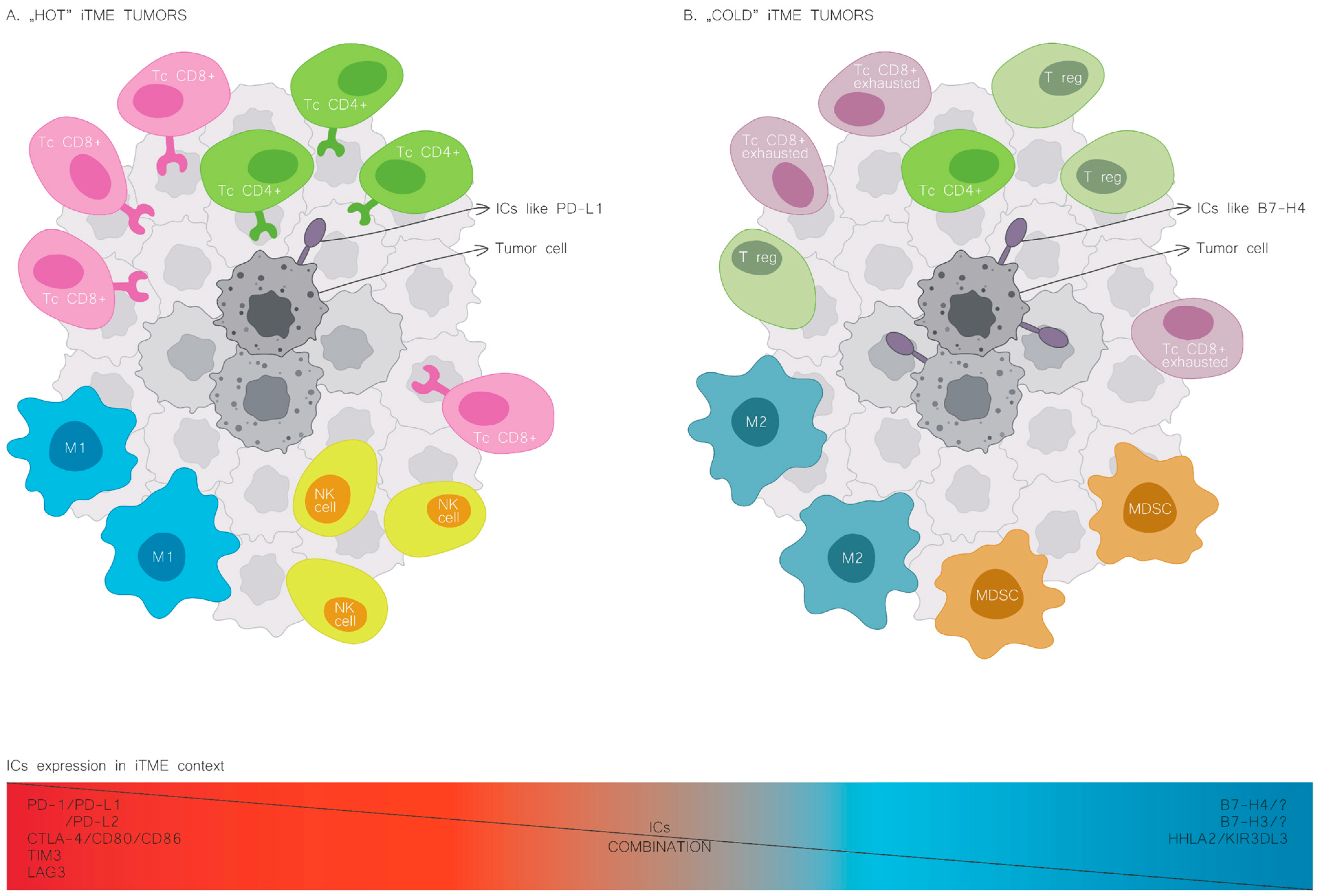
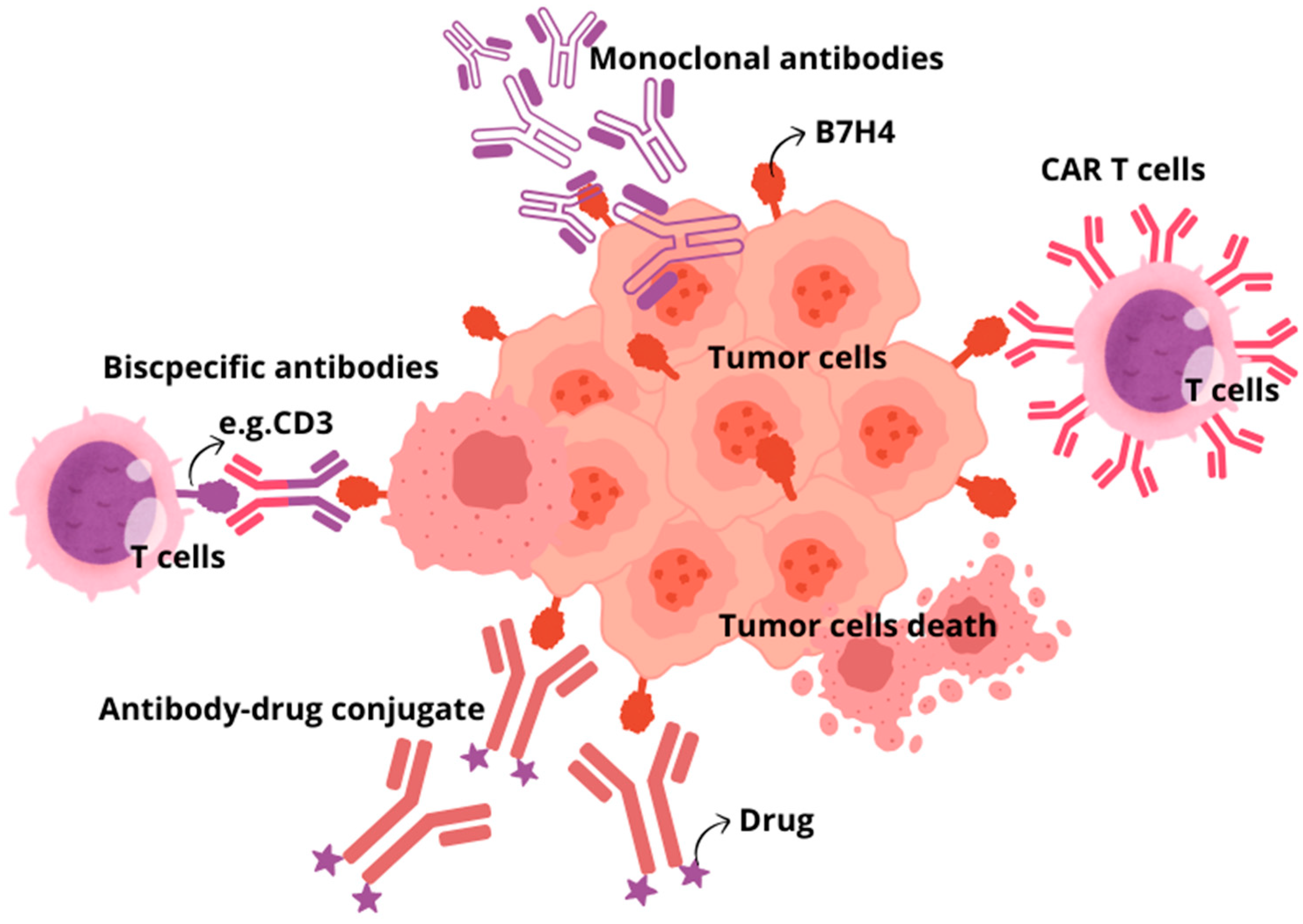
| Type of Tumour | Expression Rate | B7H4 Expression and Prognosis | Potential Mechanisms | References |
|---|---|---|---|---|
| Breast cancer | HR+/HER2+ = 25% HR−/HER2+ = 60% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Inhibitory effect on immunological responses | [30,31,34] |
| Oesophageal cancer | 53.8–95.5% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Stimulation of cell cycle progression | [20,36,37,38] |
| Gastric cancer | 44.9–80% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Inhibitory effect on immunological responses | [40,41,42,45] |
| Pancreatic cancer | 22.1–76% | Unclear | Unclear | [48,49,51,52,55,60] |
| Cholangiocarcinoma | 49.1% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Promoting tumour growth and tumour progression, inhibition of apoptosis | [61,62] |
| Gallbladder cancer | 57–69% | Unclear | Lower CD8+ TIL density in GBCs | [64,65] |
| Hepatocellular cancer | 1–73% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Inhibitory effect on immunological responses | [66,67,68,69,70,71,72] |
| Colorectal cancer | 29.1–80% | Unclear | Inhibitory effect on immunological responses | [6,74,76,78,80] |
| Renal cell carcinoma | 60% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Increase in the number of peripheral neutrophils | [84,86,87] |
| Bladder cancer | 49% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Increased ability of cancer cells to migrate and invade and increased cytotoxic activity of T cells | [92,93] |
| Prostate cancer | Unclear | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Cell cycle | [95] |
| Cervical cancer | 44.8–80.56% | Unclear | Inhibitory effect on immunological responses | [98,99,101,102,105] |
| Ovarian cancer | 94.5% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Stimulation of the expression of proteins that enhance the growth and migration of cancer cells | [109,111,114,118] |
| Endometrial cancer | 71.5% | Unclear | Unclear | [126,127,130] |
| Head and neck malignancies | Salivary gland carcinomas (SGC) = 50% Adenoid cystic carcinoma (ACC) = 94% | Unclear | Modulation of macrophage polarisation, creation of an anti-inflammatory microenvironment | [131,132,133] |
| Lung cancer | Increase | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Inhibitory effect on immunological responses | [136,137,140] |
| Small cell lung carcinoma | 2.6–6.8% | Unclear | Unclear | [138,139,140] |
| Lung adenocarcinoma | 44.9% | Unclear | Unclear | [141,142,143] |
| Central nervous system malignancies | Glioma = 54.1% | Unfavourable prognosis in the case of elevated B7H4 expression in tumour tissues | Decreased expression of checkpoint genes | [145,146,147] |
| Type of Tumour | B7H4 Expression and Immune Infiltration | References |
|---|---|---|
| Breast cancer | Increase in regulatory lymphocytes Decrease in cytotoxic lymphocytes | [34] |
| Oesophageal cancer | Increase in Foxp3+T lymphocytes, CD68+ macrophages Decrease in TIL CD3+, CD8+ | [20,35,37] |
| Gastric cancer | Increase in Foxp3+ Tregs, macrophages, neutrophils Decrease in CD8+ lymphocytes | [40,45] |
| Cholangiocarcinoma | Increase in NA Decrease in CD8+ lymphocytes in the tumour stroma | [61] |
| Gallbladder cancer | Increase in NA Decrease in CD8+ TILs in GBC | [64] |
| Colorectal cancer | Increase in T CD3+, TAM Decrease in IL-9, IL-18, T CD8+ | [6,75,79,82] |
| Renal cell carcinoma | Increase in peripheral neutrophils Decrease in NA | [87] |
| Bladder cancer | Increase in NA Decrease in cytotoxic lymphocytes | [92] |
| Cervical cancer | Increase in Tregs, CD25+FOXP3+T cells, IL-10 and TGF-β1 Decrease in T CD8+, IFN-γ, IL-2 | [100,101,103,105,107] |
| Ovarian cancer | Increase in increased density of stromal immune infiltrations, CD11c+HLA-DRhigh APCs, IL6, IL-10, TGFB1, IFNG mRNA expression, ACKR2 mRNA, CCL2, CCL4, CCL5, CCL8, CXCL17, CXCL10, CXCL11, CXCL17 Decrease in NA | [111,113,114] |
| Endometrial cancer | Increase in resting memory CD4+ T cells Decrease in CD8+ cells, activated memory CD4+ T cells | [126,129,130] |
| Lung cancer | Increase in NA Decrease in CD8+ T | [137] |
| Glioma | Increase in Tregs Decrease in TILs, TAMs | [145,146] |
| Target | Drug | Cancer Type | Phase | NTC Number | Estimated Primary Completion |
|---|---|---|---|---|---|
| B7H4 | ADC:XMT-1660 | Breast, ovarian, endometrial cancer | I | NCT05377996 | 2026-12 |
| B7H4 | ADC:SGN-B7H4V | Solid tumours | I | NCT05194072 | 2025-06-30 |
| B7H4, TOP1 | ADC:AZD8205 | Solid tumours | I, II | NCT05123482 | 2025-06-30 |
| B7H4, PD-1 | ADC:BG-C9074 and Tislelizumab | Solid tumours | I | NCT06233942 | 2027-09-28 |
| B7H4, PD-1 | FPA150 and Pembrolizumab | Solid tumours | I | NCT03514121 | 2024-03-14-results submitted, yet NA |
| B7H4 and CD3 | bispecific antibody GEN1047 | Solid tumours | II | NCT05180474 | 2026-01-31 |
| 4-1BB and B7H4 | bispecific antibody ABL103 | Solid tumours | I | NCT06126666 | 2024-11-15 |
| B7H4 and CD3 | bispecific antibody PF-07260437 | Breast, ovarian, endometrial cancer | I | NCT05067972 | 2023-10-17 Terminated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawidowicz, M.; Kot, A.; Mielcarska, S.; Psykała, K.; Kula, A.; Waniczek, D.; Świętochowska, E. B7H4 Role in Solid Cancers: A Review of the Literature. Cancers 2024, 16, 2519. https://doi.org/10.3390/cancers16142519
Dawidowicz M, Kot A, Mielcarska S, Psykała K, Kula A, Waniczek D, Świętochowska E. B7H4 Role in Solid Cancers: A Review of the Literature. Cancers. 2024; 16(14):2519. https://doi.org/10.3390/cancers16142519
Chicago/Turabian StyleDawidowicz, Miriam, Anna Kot, Sylwia Mielcarska, Katarzyna Psykała, Agnieszka Kula, Dariusz Waniczek, and Elżbieta Świętochowska. 2024. "B7H4 Role in Solid Cancers: A Review of the Literature" Cancers 16, no. 14: 2519. https://doi.org/10.3390/cancers16142519
APA StyleDawidowicz, M., Kot, A., Mielcarska, S., Psykała, K., Kula, A., Waniczek, D., & Świętochowska, E. (2024). B7H4 Role in Solid Cancers: A Review of the Literature. Cancers, 16(14), 2519. https://doi.org/10.3390/cancers16142519






