The Impact of Pain Education Interventions for Cancer Survivors and Caregivers: A Systematic Review with Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
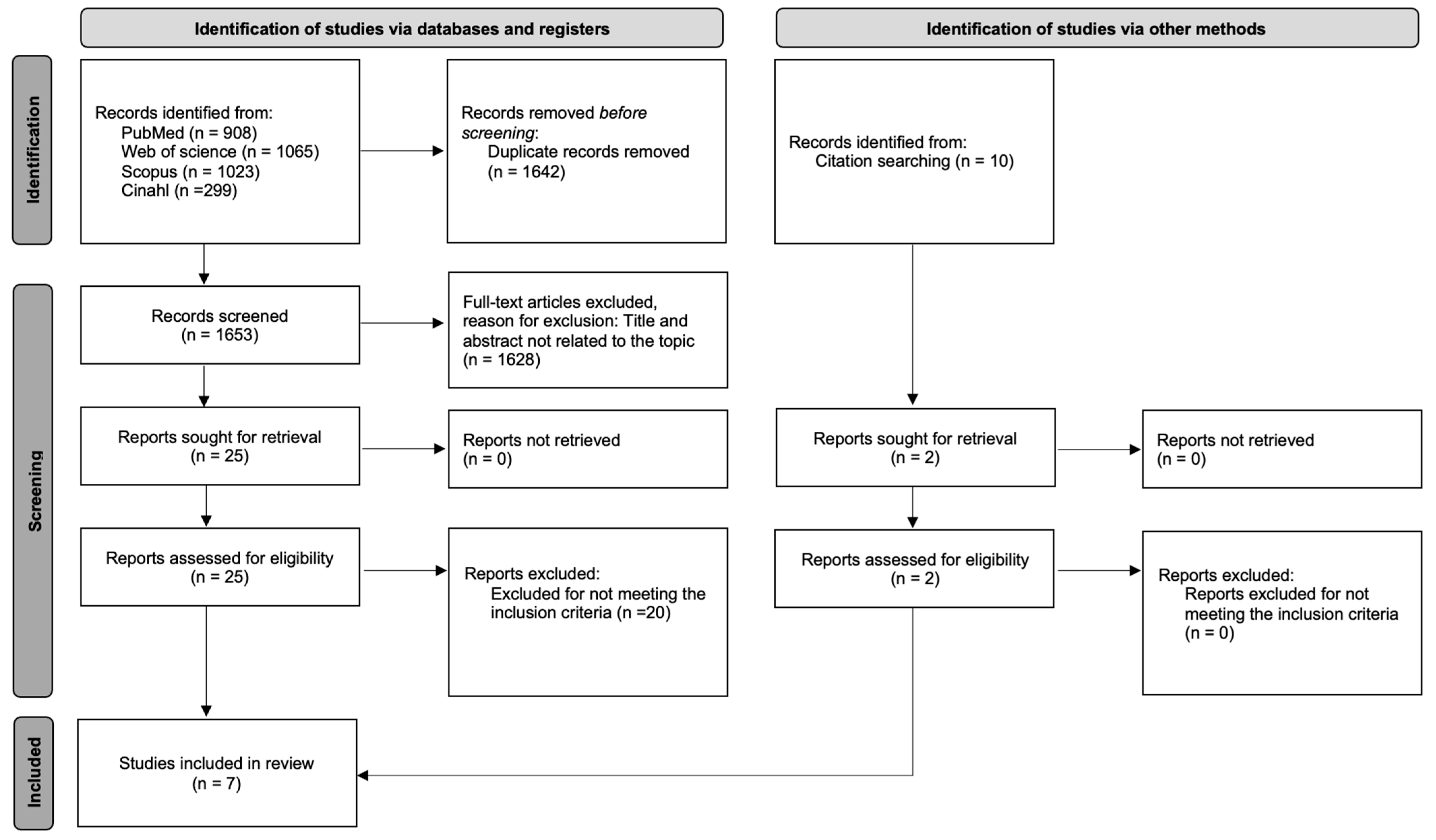
2.1. Search Strategy and Eligibility Criteria
2.2. Study Selection and Data Extraction
2.3. Assessment Quality and Risk of Bias in Included Studies
2.4. Meta-Analysis
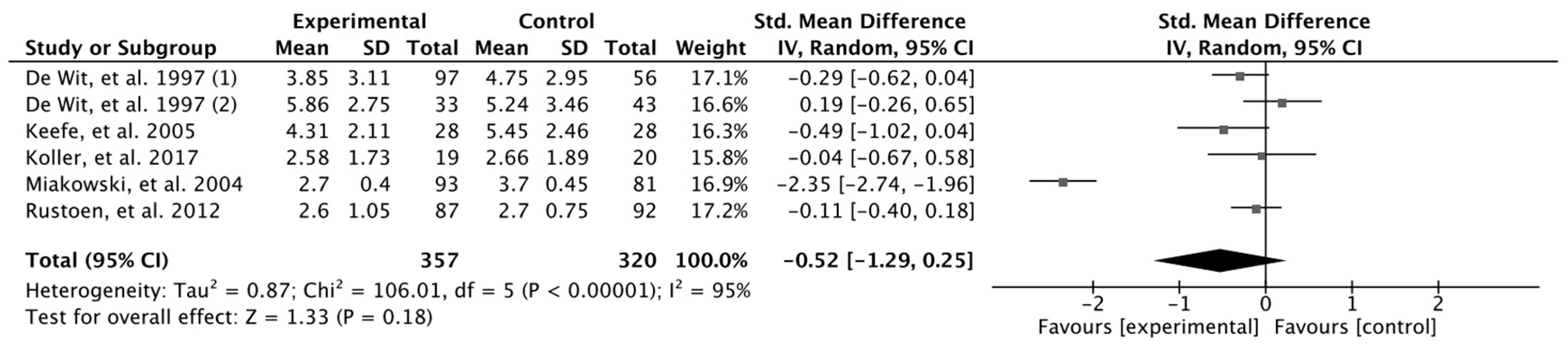
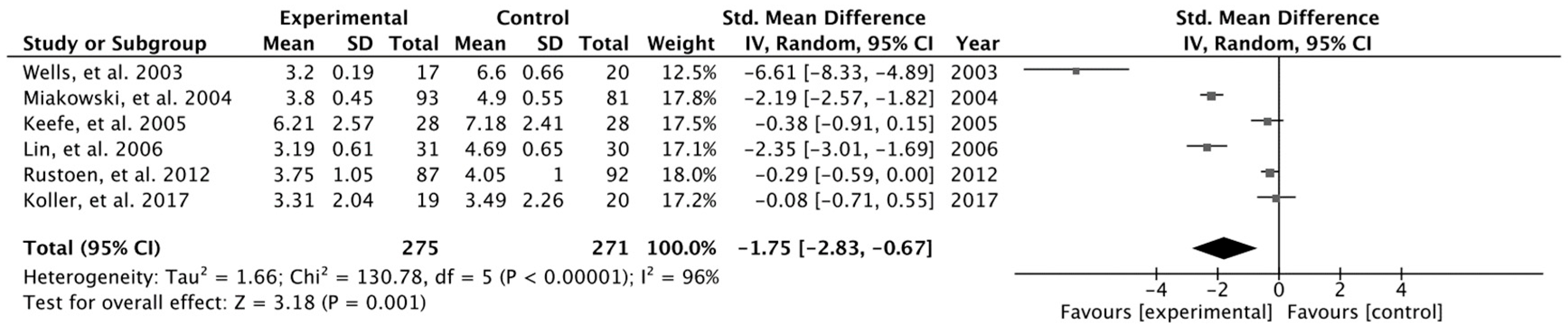
3. Results
Results Obtained in Meta-Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Adam, R.; Bond, C.; Murchie, P. Educational interventions for cancer pain. A systematic review of systematic reviews with nested narrative review of randomized controlled trials. Patient Educ. Couns. 2015, 98, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.B.; Hansen, J.A.; Moskowitz, M.; Todd, B.L.; Feuerstein, M. It’s not over when it’s over: Long-term symptoms in cancer survivors—A systematic review. Int. J. Psychiatry Med. 2010, 40, 163–181. [Google Scholar] [CrossRef]
- Nijs, J.; Lahousse, A.; Fernández-De-Las-Peñas, C.; Madeleine, P.; Fontaine, C.; Nishigami, T.; Desmedt, C.; Vanhoeij, M.; Mostaqim, K.; Cuesta-Vargas, A.I.; et al. Towards precision pain medicine for pain after cancer: The Cancer Pain Phenotyping Network multidisciplinary international guidelines for pain phenotyping using nociplastic pain criteria. Br. J. Anaesth. 2023, 130, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Joypaul, S.; Kelly, F.; McMillan, S.S.; King, M.A. Multi-disciplinary interventions for chronic pain involving education: A systematic review. PLoS ONE 2019, 14, e0223306. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Shin, S.J.; Kim, S.C.; An, S.; Rha, S.Y.; Ahn, J.B.; Cho, B.C.; Choi, H.J.; Sohn, J.H.; Kim, H.S.; et al. Randomized controlled trial of standardized education and telemonitoring for pain in outpatients with advanced solid tumors. Support. Care Cancer 2013, 21, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Oldenmenger, W.H.; Smitt, P.A.S.; van Montfort, C.A.; de Raaf, P.J.; van der Rijt, C.C. A combined pain consultation and pain education program decreases average and current pain and decreases interference in daily life by pain in oncology outpatients: A randomized controlled trial. Pain 2011, 152, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Oldenmenger, W.H.; Geerling, J.I.; Mostovaya, I.; Vissers, K.C.; de Graeff, A.; Reyners, A.K.; van der Linden, Y.M. A systematic review of the effectiveness of patient-based educational interventions to improve cancer-related pain. Cancer Treat. Rev. 2018, 63, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Latter, S.; Hopkinson, J.B.; Richardson, A.; Hughes, J.A.; Lowson, E.; Edwards, D. How can we help family carers manage pain medicines for patients with advanced cancer? A systematic review of intervention studies. BMJ Support. Palliat. Care 2016, 6, 263–275. [Google Scholar] [CrossRef]
- I Bennett, M.; Flemming, K.; Closs, S.J. Education in cancer pain management. Curr. Opin. Support. Palliat. Care 2011, 5, 20–24. [Google Scholar] [CrossRef]
- Rischer, J.B.; Childress, S.B. Implementation of the AHCPR clinical practice guideline on the management of cancer pain. J. Pharm. Care Pain Symptom Control 1998, 6, 79–90. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. Available online: www.york.ac.uk/inst/crd (accessed on 3 September 2023).
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Saunders, L.D.; Soomro, G.M.; Buckingham, J.; Jamtvedt, G.; Raina, P. Assessing the Methodological Quality of Nonrandomized Intervention Studies. West. J. Nurs. Res. 2003, 25, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Andrews, J.; Guyatt, G.; Oxman, A.D.; Alderson, P.; Dahm, P.; Falck-Ytter, Y.; Nasser, M.; Meerpohl, J.; Post, P.N.; Kunz, R.; et al. GRADE guidelines: 14. Going from evidence to recommendations: The significance and presentation of recommendations. J. Clin. Epidemiol. 2013, 66, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- de Wit, R.; van Dam, F.; Zandbelt, L.; van Buuren, A.; van der Heijden, K.; Leenhouts, G.; Loonstra, S. A Pain Education Program for chronic cancer pain patients: Follow-up results from a randomized controlled trial. Pain 1997, 73, 55–69. [Google Scholar] [CrossRef]
- Wells, N.; Hepworth, J.T.; Murphy, B.A.; Wujcik, D.; Johnson, R. Improving Cancer Pain Management Through Patient and Family Education. J. Pain Symptom Manag. 2003, 25, 344–356. [Google Scholar] [CrossRef]
- Miaskowski, C.; Dodd, M.; West, C.; Schumacher, K.; Paul, S.M.; Tripathy, D.; Koo, P. Randomized Clinical Trial of the Effectiveness of a Self-Care Intervention to Improve Cancer Pain Management. J. Clin. Oncol. 2004, 22, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Keefe, F.J.; Ahles, T.A.; Sutton, L.; Dalton, J.; Baucom, D.; Pope, M.S.; Knowles, V.; McKinstry, E.; Furstenberg, C.; Syrjala, K.; et al. Partner-Guided Cancer Pain Management at the End of Life: A Preliminary Study. J. Pain Symptom Manag. 2005, 29, 263–272. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chou, P.-L.; Wu, S.-L.; Chang, Y.-C.; Lai, Y.-L. Long-term effectiveness of a patient and family pain education program on overcoming barriers to management of cancer pain. Pain 2006, 122, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Rustøen, T.; Valeberg, B.T.; Kolstad, E.; Wist, E.; Paul, S.; Miaskowski, C. A Randomized Clinical Trial of the Efficacy of a Self-care Intervention to Improve Cancer Pain Management. Cancer Nurs. 2014, 37, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Miaskowski, C.; De Geest, S.; Opitz, O.; Spichiger, E. Results of a randomized controlled pilot study of a self-management intervention for cancer pain. Eur. J. Oncol. Nurs. 2013, 17, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hors-Fraile, S.; Rivera-Romero, O.; Schneider, F.; Fernandez-Luque, L.; Luna-Perejon, F.; Civit-Balcells, A.; de Vries, H. Analyzing recommender systems for health promotion using a multidisciplinary taxonomy: A scoping review. Int. J. Med. Inform. 2018, 114, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Vanlint, S.; Stocks, N.; Mittinty, M.N.; Moseley, G.L. Exploring effect of pain education on chronic pain patients’ expectation of recovery and pain intensity. Scand. J. Pain 2018, 18, 211–219. [Google Scholar] [CrossRef]
- Paice, J.A.; Ferrell, B. The management of cancer pain. CA Cancer J. Clin. 2011, 61, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Allard, P.; Maunsell, E.; Labbé, J.; Dorval, M. Educational Interventions to Improve Cancer Pain Control: A Systematic Review. J. Palliat. Med. 2001, 4, 191–203. [Google Scholar] [CrossRef]
- Makhlouf, S.M.; Pini, S.; Ahmed, S.; Bennett, M.I. Managing Pain in People with Cancer—A Systematic Review of the Attitudes and Knowledge of Professionals, Patients, Caregivers and Public. J. Cancer Educ. 2020, 35, 214–240. [Google Scholar] [CrossRef]
- Champarnaud, M.; Villars, H.; Girard, P.; Brechemier, D.; Balardy, L.; Nourhashemi, F. Effectiveness of Therapeutic Patient Education Interventions for Older Adults with Cancer: A Systematic Review. J. Nutr. Health Aging 2020, 24, 772–782. [Google Scholar] [CrossRef]
- Steves, S.L.; Scafide, K.N. Multimedia in preoperative patient education for adults undergoing cancer surgery: A systematic review. Eur. J. Oncol. Nurs. 2021, 52, 101981. [Google Scholar] [CrossRef] [PubMed]
- Sayin, Y.; Aksoy, G. The effect of analgesic education on pain in patients undergoing breast surgery: Within 24 hours after the operation. J. Clin. Nurs. 2012, 21, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Rigdon, A.S. Development of Patient Education for Older Adults Receiving Chemotherapy. Clin. J. Oncol. Nurs. 2010, 14, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Pontin, J.C.B.; Di Gioia, K.C.S.; Dias, A.S.; Teramatsu, C.T.; Matuti, G.d.S.; Mafra, A.D.L. Positive effects of a pain education program on patients with chronic pain: Observational study. Braz. J. Pain 2021, 4, 130–135. [Google Scholar] [CrossRef]
- Fisher, J.D.; Fisher, W.A. Changing AIDS-risk behavior. Psychol. Bull. 1992, 111, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Wijma, A.J.; Leysen, L.; Pas, R.; Willaert, W.; Hoelen, W.; Ickmans, K.; van Wilgen, C.P. Explaining pain following cancer: A practical guide for clinicians. Braz. J. Phys. Ther. 2018, 23, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Prevost, V.; Delorme, C.; Grach, M.-C.; Chvetzoff, G.; Hureau, M. Therapeutic Education in Improving Cancer Pain Management: A Synthesis of Available Studies. Am. J. Hosp. Palliat. Med. 2016, 33, 599–612. [Google Scholar] [CrossRef]
- Chiauzzi, E.; Pujol, L.A.; Wood, M.; Bond, K.; Black, R.; Yiu, E.; Zacharoff, K. painACTION-back pain: A self-management website for people with chronic back pain. Pain Med. 2010, 11, 1044–1058. [Google Scholar] [CrossRef]
| Author, Year, Country | Study Sample (n) | Sample (% Men) | Sample Age (Year ± SD) | Etiology (%) | Extent of Disease (%) | Treatment Estatus (%) | Caregiver: Relation-ship (%) | Quality Assessment Downs and Black (Risk of Bias) |
|---|---|---|---|---|---|---|---|---|
| De Wit et al. [20] (1997), Netherlands | RCT:313 EG1: with district nurse (53) EG2: without district nurse (106) CG1: with district nurse (51) CG2: without district nurse (103) | 37.4 | 55.5 ± 12.4 | H&N: 18 (5.8) Digestive: 38 (12.1) Respiratory: 34 (10.9) Breast: 94 (30) Bone: 43 (13.7) Genitourinary: 74 (23.6) Others: 35 (11.2) | Local: 45 (14.4) Regional: 60 (19.2) Metastatic: 182 (58.1) Unknown: 9 (2.9) Not applicable: 17 (5.4) | Surgery: 50 (18) ChT: 112 (36) RT: 39 (12) HT: 21 (6) No treatment: 70 (22) Other/Unknow: 21 (7) | Informal caregiver | 21 (Some concerns of bias) |
| Wells et al. [21] (2003), USA | RCT: 64 EG1: pain hot line (21) EG2: weekly calls (19) CG (24) | 66 | 53 ± 14.5 | H&N: 21(39) | No metastatic disease: 23(43) Local metastasis: 12(22) Distant metastasis: 18(35) | NR | Informal caregiver: Spouse (64) Adult child (14) Sibling (8) | 20 (High risk of bias) |
| Miaskowski et al. [22] (2004), USA | RCT: 212 EG (93) CG (81) | 51 EG: 31.2 CG: 27.2 | EG: 60 ± 11.8 CG: 58.8 ± 12.9 | Breast: 90 (52.02) Prostate: 22 (12.72) Respiratory: 22 (12.72) Other: 38 (22.54) | Metastatic: 173 (100) | ChT: 70 (40.22) RT: 29 (16.66) HT: 54 (31.03) Biotherapy: 2 (1.15) No treatment: 18 (10.64) | Informal caregiver | 22 (High risk of bias) |
| Keefe et al. [23] (2005), USA | RCT: 78 EG: 41 CG: 37 | 56.1 | 60.49 | NR | Metastatic: 78 (100) | Palliative care: 78 (100) | Informal caregivers: Spouses (54) daughters (14) unknown (32) | 21 (Low risk of bias) |
| Lin et al. [24] (2006), Taiwan | RCT: 61 EG: 31 CG: 30 | 39.64 EG: 38.71 CG: 40 | EG: 59.45 ± 16.90 CG: 55 ± 14.38 | NR | Metastatic: 45 (73.77) | ChT: 9 (14.75) RT: 25 (40.98) ChT + RT: 4 (6.55) No treatment: 23 (37.70) | Informal caregiver | 18 (High risk of bias) |
| Rustoen et al. [25] (2012), USA | RCT: 179 EG: 87 CG: 92 | 51.39 EG: 47.1 CG: 55.4 | EG: 64.32 ± 11.4 CG: 67.38 ± 11.4 | Breast: 66 (36.87) Prostate: 65 (36.31) Colon: 18 (10.06) Other: 30(16.76) Gynecological: 13 (33.3) Other: 13 (33.3) | Metastatic: 179 (100) | ChT: 62 (34.63) RT: 58 (32.40) HT: 48 (26.82) 15 (38.4) Other: 4 (10.4) | Informal caregiver | 19 (Some concerns of bias) |
| Koller et al. [26] (2017), Germany | Pilot RCT: 39 EG: 20 CG: 19 | 51.1 EG: 40 CG: 63.8 | 56.6 ± 10.6 | Gastrointes-tinal: 13 (33.3) Breast and gynecological: 13 (33.3) Other: 13 (33.3) | NR | Curative: 20 (51.3) Palliative: 15 (38.4) Other 4 (13.3) | Informal caregiver | 21 (Some concerns of bias) |
| Author, (Year) | Experimental Intervention/ Components | Comparative Intervention | Face-to-Face vs. Distance Supervised vs. Non-Supervised Individual vs. Group | Total Sessions; Days/Week; Total Minutes | Outcomes | Main Results |
|---|---|---|---|---|---|---|
| De Wit et al. [20] (1997) | Taxonomy 1. Pain knowledge 2. Advising changing routines 3. Advising medication use 4. Barriers for pain management Specific Pain Topic Definition, causes, control (pharmaco-logical, analgesia and other techniques,), non-adherence, side effects, misconceptions, and pain levels recording. | Usual care | Mixed Supervised Individual | 3; every 3 days; 15–60 | Pain experience (MPQ-DLV) Present and average pain intensity (NRS) Pain knowledge (PKQ-DLV) QoL (EORTC QLQ-C30) | p < 0.05 for NPRS and PKQ-DLV in favor of EG1 and EG2 p > 0.05 for the rest of outcomes |
| Wells et al. [21] (2003) | Taxonomy 1. Pain knowledge 2. Advising medication use 3. Barriers for pain management Specific Pain Topic Control (pharmaco-logical, analgesia), non-adherence, side effects, and pain levels recording. | Usual care | Mixed Supervised Individual | EG1: 1, 1× Week; 15–45 EG2: 1, 1× Week; 15–45 EG3: 4, 1× Week; 15 | Pain experience (BPI-SF) Barriers to pain (BQ-r) Family pain beliefs (FPQ) Medication Use (PMI-r) | p < 0.05 for FPQ in favor of EG p > 0.05 for the rest of outcomes |
| Miaskow-ski et al. [22] (2004) | Taxonomy 1. Pain knowledge 2. Advising medication use 3. Barriers for pain management Specific Pain Topic Reported needs, communic-ating skills, control (analgesia), side effects, and pain levels recording. | Usual care | Mixed Supervised Individual | 6; 1× Week; NR | Least, average and worst pain intensity (NRS) Medication use | p < 0.05 for NRS and medication use in favor of EG |
| Keefe et al. [23] (2005) | Taxonomy 1. Pain knowledge 2. Advising changing routines 3. Barriers for pain management Specific Pain Topic Reported needs, control (pharmaco-logical, analgesia), side effects, coping strategies (pleasant imagery, activity pacing, activity-rest cycling), communica-ting skills, and relief barriers. | Usual care | Face-to-face Supervised Individual | 3, biweekly; 45–60 | Pain experience (BPI) QoL (FACT-G, V.4) Partner self-efficacy (CPSS) Caregiver strain (CSI) Partners’ mood (PMS-B) | p < 0.05 for CPSS and CSI p > 0.05 for the rest of outcomes |
| Lin et al. [24] (2006) | Taxonomy 1. Pain knowledge 2. Advising medication use 3. Barriers for pain management Specific Pain Topic Control (analgesia), side effects, and pain levels recording. | Usual care | Mixed Supervised Individual | 3; weekly-biweekly; 30–40 | Pain experience (BPI) Barriers to pain (BQT) Medication use (KPS) | p < 0.05 for BPI, BQT in favor of EG p > 0.05 for the rest of outcomes |
| Rustoen et al. [25] (2012) | Taxonomy 1. Pain knowledge 2. Advising changing routine Specific Pain Topic Reported needs, control (analgesia), side effects, communica-ting skills, and pain levels recording. | Usual care | Mixed Supervised Individual | 6; weekly; NR | Pain experience (PES) Least, average and worst pain intensity (NRS) Medication use | (p < 0.001). p > 0.05 for PES p > 0.05 for the rest of outcomes |
| Koller et al. [26] (2017) | Taxonomy 1. Pain knowledge 2. Advising changing routines Specific Pain Topic Reported needs, misconcep-tions, control (analgesia), side effects, and communica-ting skills. | Usual care | Face-to-face Supervised Individual | 1; NR; NR | Average and worst pain intensity (NRS) Knowledge pain (PPQ) Self-efficacy (PSQ) Barriers to cancer (BQ) QoL (MOS-SF) | p > 0.05 for PPQ p < 0.05 for the rest of outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Hernández, S.; Heredia-Ciuró, A.; Martín-Núñez, J.; Calvache-Mateo, A.; Navas-Otero, A.; López-López, L.; Valenza, M.C. The Impact of Pain Education Interventions for Cancer Survivors and Caregivers: A Systematic Review with Meta-Analysis. Cancers 2024, 16, 2468. https://doi.org/10.3390/cancers16132468
Hernández-Hernández S, Heredia-Ciuró A, Martín-Núñez J, Calvache-Mateo A, Navas-Otero A, López-López L, Valenza MC. The Impact of Pain Education Interventions for Cancer Survivors and Caregivers: A Systematic Review with Meta-Analysis. Cancers. 2024; 16(13):2468. https://doi.org/10.3390/cancers16132468
Chicago/Turabian StyleHernández-Hernández, Sofía, Alejandro Heredia-Ciuró, Javier Martín-Núñez, Andrés Calvache-Mateo, Alba Navas-Otero, Laura López-López, and Marie Carmen Valenza. 2024. "The Impact of Pain Education Interventions for Cancer Survivors and Caregivers: A Systematic Review with Meta-Analysis" Cancers 16, no. 13: 2468. https://doi.org/10.3390/cancers16132468
APA StyleHernández-Hernández, S., Heredia-Ciuró, A., Martín-Núñez, J., Calvache-Mateo, A., Navas-Otero, A., López-López, L., & Valenza, M. C. (2024). The Impact of Pain Education Interventions for Cancer Survivors and Caregivers: A Systematic Review with Meta-Analysis. Cancers, 16(13), 2468. https://doi.org/10.3390/cancers16132468