1. Introduction
Sarcomas are malignant tumors distinguished by their mesenchymal differentiation [
1] with an incidence of 5 cases per 100,000, classifying them as rare diseases [
2]. The misdiagnosis and subsequent unplanned whoops resection (UE) of sarcomas, under the presumption of benignity, constitutes a significant clinical challenge, particularly in the case of smaller, superficial sarcomas in younger patients [
3,
4]. An average rate of 33% for UE among sarcoma patients, with observed rates ranging from 18% to 53%, was reported in a comprehensive review encompassing 33 studies and underscored the complexity and variability of sarcoma management practices [
5]. However, an extensive study from Japan including a nationwide cancer registry with 8761 patients reported a rate of only 11% for UE [
6]. The term UE itself encompasses a broad spectrum of scenarios in the literature, lacking a universally accepted (and standardized) definition. Studies have variously described UEs as the removal of a tumor without adequate preoperative diagnostics and consideration of surgical margins or any surgery conducted without specialized evaluation and planning based on the surgeon’s initial diagnosis. This diversity reflects the multifaceted nature of sarcoma diagnosis and the critical need for precise planning to avoid adverse outcomes [
5,
7,
8,
9].
The reasons for UE of sarcomas are manifold, primarily based on four key aspects. The overall rarity of sarcomas and, additionally, their subdivision into approximately 70 subgroups, each with distinct clinical and therapeutic differences; the intrinsic complexity of sarcomas, due to which, conventional diagnostic criteria for malignant tumors are not always directly applicable; the complex diagnostic process involving conventional macroscopy, immunohistochemistry, and molecular genetics, the latter requiring high professional expertise; and the significant disparity in expertise observed in rare diseases like sarcomas [
2]. Another contributing factor may arise from the often-prolonged diagnostic intervals between the first physician visit and the engagement of an expert [
10].
The impact on the clinical outcome following UE has not been conclusively studied. It is assumed that UEs are linked to reduced local recurrence-free survival (LRFS) compared to planned oncologic resections (PEs), while no disparity is evidenced concerning metastasis-free survival (MFS) and cancer-specific survival (CSS) [
4,
11,
12,
13]. Furthermore, there is an established correlation between residual tumor presence in the re-resected specimen and reduced LRFS, as well as overall survival (OS) [
13,
14,
15,
16,
17]. Given the significant prevalence of residual tumors observed in 31–74% of cases following UE [
5], it is reasonable to anticipate a reduction in LRFS and OS post-UE.
There is no uniform agreement on how to best approach patients after UE [
18,
19,
20]. The UK guidelines recommend considering re-resection for UE with positive resection margins (R1/R2), highlighting the procedure’s potential to improve OS rates and extend MSFS [
18,
19,
21]. Research, including a study by Nakamura et al., has shown similar survival and local recurrence rates between PE and UE, followed by subsequent re-resection [
22]. Despite these benefits, corrective surgeries often require more complex reconstructive techniques, such as skin grafts, flaps, or even limb amputation [
8], raising the critical question of whether re-resection should be universally applied to all UEs or only a select subset [
20]. This unresolved dilemma highlights a significant gap in our current treatment paradigms, underscoring the pressing need for innovative research approaches that can elucidate and guide these crucial decisions in sarcoma care.
While retrospective studies certainly have provided valuable insights into the outcomes of surgical interventions for sarcomas, their inherent limitations, such as the inability to establish causality and control for confounding factors, have left critical questions unanswered. The gold standard of clinical studies, a randomized controlled trial (RCT), faces unsurmountable ethical hurdles when it comes to comparing UE versus PE, due to the direct impact on patient care and treatment decisions [
23]. This critical juncture in sarcoma research underscores the urgent need for alternative methodologies that can offer the precision and reliability of RCTs without their ethical implications.
Target Trial Emulation (TTE) has emerged as a novel methodological framework that addresses these challenges [
23,
24,
25]. By emulating the design principles of RCTs and utilizing real-world-time data, TTE allows for the ethical and rigorous exploration of treatment outcomes, effectively bypassing the ethical concerns tied to randomizing surgical treatment options [
25]. Leveraging real-world-time data to approximate the causal inference of RCTs, TTE can also minimize potential selection biases typically associated with observational studies [
25]. This innovative approach not only bridges the methodological divide but also opens up new avenues for exploring the nuanced impacts of surgical strategies on sarcoma patient outcomes. Adopting TTE, this represents a pivotal advancement in clinical research, enabling the investigation of UEs versus PEs with a level of scientific rigor previously deemed unattainable.
In brief, this study aims to assess the association between UE (compared to PE) and LRFS, MFS, CSS, and OS by emulating a target trial. By pioneering a methodological alternative to traditional RCT’s, we aim to shed new light on the implications of surgical strategies for sarcoma, potentially guiding more effective treatment pathways. This exploration promises to not only advance our understanding but also stimulate further research into the nuanced impacts of surgical decisions on patient outcomes in sarcoma care.
2. Materials and Methods
To uphold the quality in sarcoma care and to foster interdisciplinary exchanges, the Swiss Sarcoma Network (SSN) (
https://www.swiss-sarcoma.net/, accessed on 31 May 2024) collects patient data longitudinally across the therapeutic continuum of soft tissues and bone tumors, creating a multi-center, high-quality real-world-time (RWT) data warehouse [
26,
27]. The current data repository, Adjumed.ch (Adjumed Services AG, Zurich, Switzerland;
www.adjumed.ch, accessed on 10 October 2023), hosts the dataset, which is anticipated to transition to a new management system, Sarconnector
® (BF&PH, Zurich, Switzerland) to function as a dynamic real-world-time data (RWT) warehouse for automated analysis [
28].
The SSN data warehouse encompasses the data from 2104 patients registered by SSN members from 2018 to 2023, as illustrated in
Figure 1. All sarcoma patients who underwent either UE or PE and received treatment (whether initial or follow-up) at the University Hospital Lucerne (LUKS) or the Cantonal Hospital Winterthur (KSW) were consecutively included (
n = 477). In 87 cases, the inclusion criteria were not met: no index surgery was planned (
n = 85), death occurred before the index surgery (
n = 1), and no data on the index surgery were available (
n = 1). Exclusion criteria were a history of any malignant neoplasm within the past 5 years (
n = 36), any history of leukemia (
n = 2), and age below 16 years (
n = 10). Furthermore, desmoid tumors were not included in this study. The final cohort for analyzing the outcome of UE versus PE included 429 patients.
The observation period began at the point of the initial surgical intervention, either UE or PE, referred to as time zero, and ended with the occurrence of the predefined outcomes (local recurrence, metastasis, or death) or date of last patient contact. Due to the real-world-time structure of our data, time zero—the point of initial surgical intervention—can occur before the official start of the study inclusion period. This situation arises when a patient with a recurrence is enrolled in the study, potentially leading to an overestimation of the incidence of local recurrence in the presented cohort. Tumor reappearance at the original site was captured as local recurrence, tumor spread to a different site as metastasis, and mortality was subdivided into all-cause and death due to sarcoma. All consecutive patients were presented to the SSN-MDTB (Multidisciplinary Tumor Board) to determine their recommended individualized treatment strategy.
Utilizing the methodology of TTE, this study incorporated the principles of RCT to real-world-time data [
23,
25]. This approach involved the prior development of a target trial protocol (
Table 1), creating a multivariable Cox regression model and implementing propensity score weighting to approximate the randomization process of a RCT. Hazard ratios (HRs) and 95% confidence intervals (CIs) are presented. The proportional hazards assumption in the Cox proportional hazards regression was verified using Schoenfeld residuals. The linearity assumption for the included covariates was checked using Martingale residuals. Regarding the heterogeneity of the study population, adjustments were made for known prognostic factors in a multivariable Cox regression model, including the tumor biological behavior, grade, size, and sarcoma classification, as well as the anatomical region of the tumor. Sarcoma classification is divided into axial (including head, neck, trunk, visceral, intraperitoneal, and retroperitoneal) and appendicular (encompassing upper and lower extremity). Biological behavior serves as an index for the inherent biological characteristics and clinical behavior, encompassing their growth pattern, invasiveness, metastatic potential, and potential response to treatment. These include three stages: benign, intermediate, and malignant, with benign soft tissue tumors not represented in this study. The propensity score was designed to equalize the distribution of baseline variables across the treatment arms—UE and PE—by being assigned in weights of the inverse probability of treatment assignment based on the aforementioned covariates.
To ensure the statistical analysis’s reliability, sensitivity analyses were performed, adjusting for additional variables such as age, sex, tumor volume (cm
3), and resection status. The influence of chemotherapy and radiotherapy with curative intent on the outcome was also examined. Additionally, the subsets of patients without atypical lipomatous tumor (ALT) and dermatofibrosarcoma protuberans (DFSP) were analyzed. Because 76 cases had time zero prior to 2018 and were partly subsequently captured, an additional sensitivity analysis was designed to include only cases with time zero from 2018 onwards (See
Appendix A Table A1,
Table A2,
Table A3 and
Table A4).
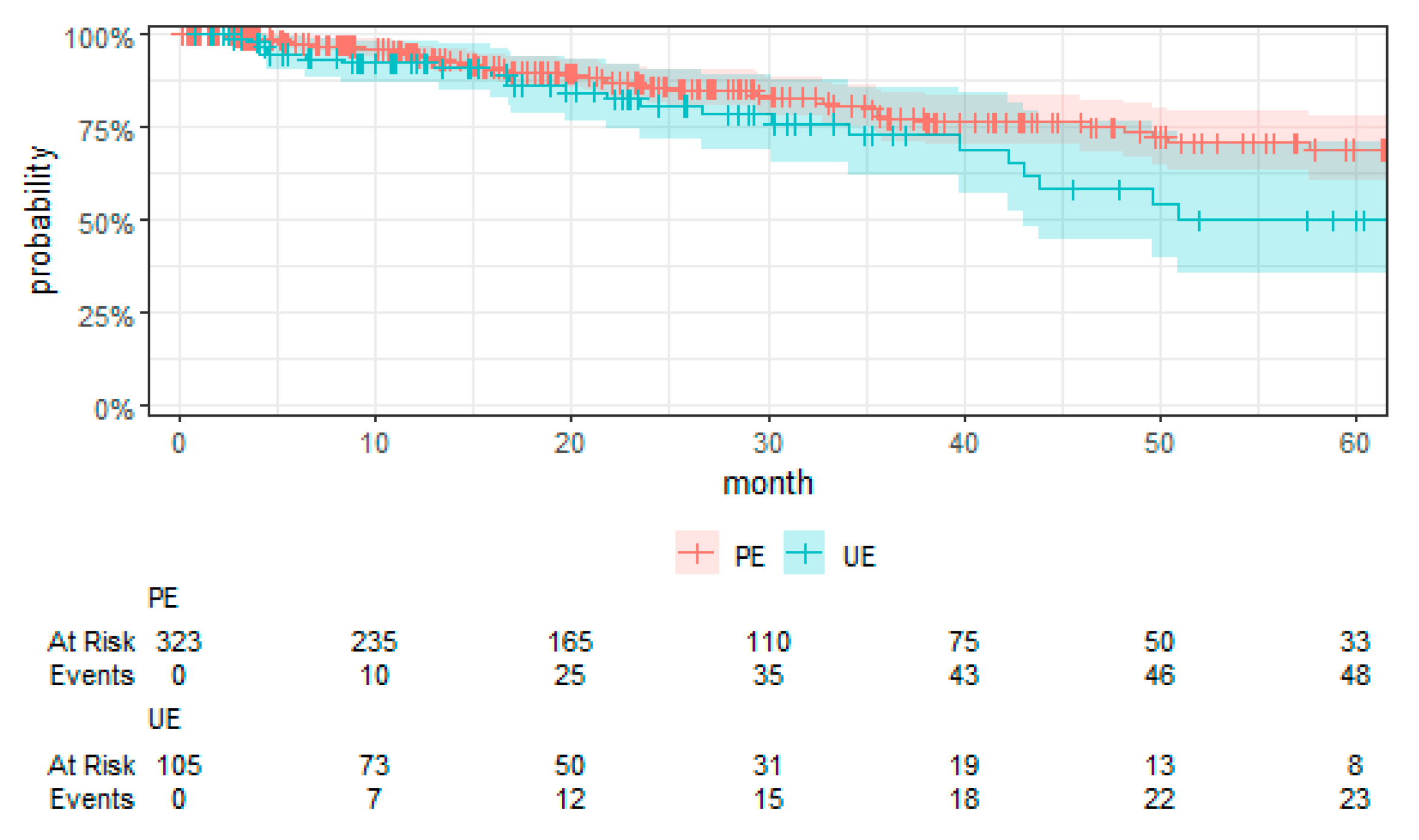
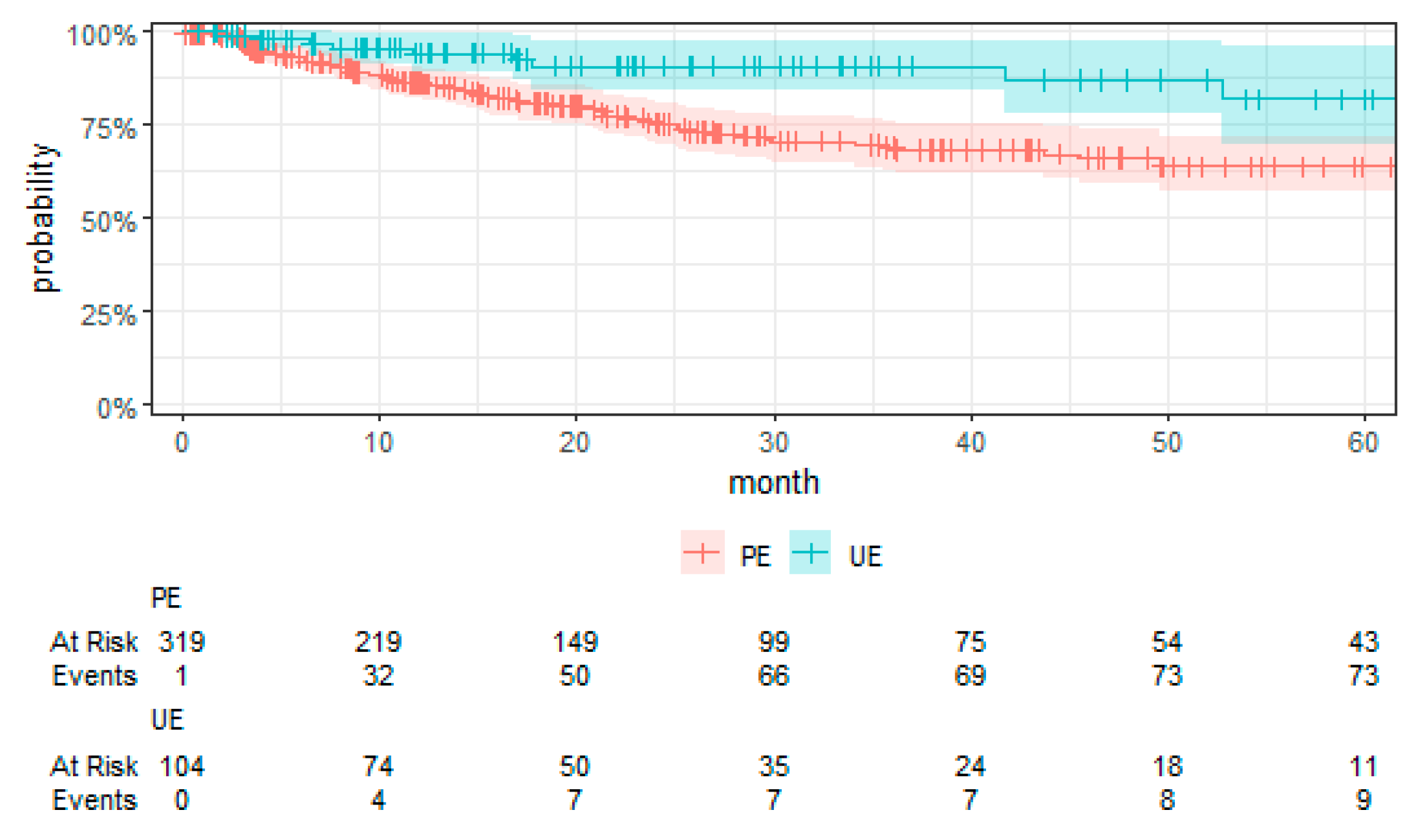
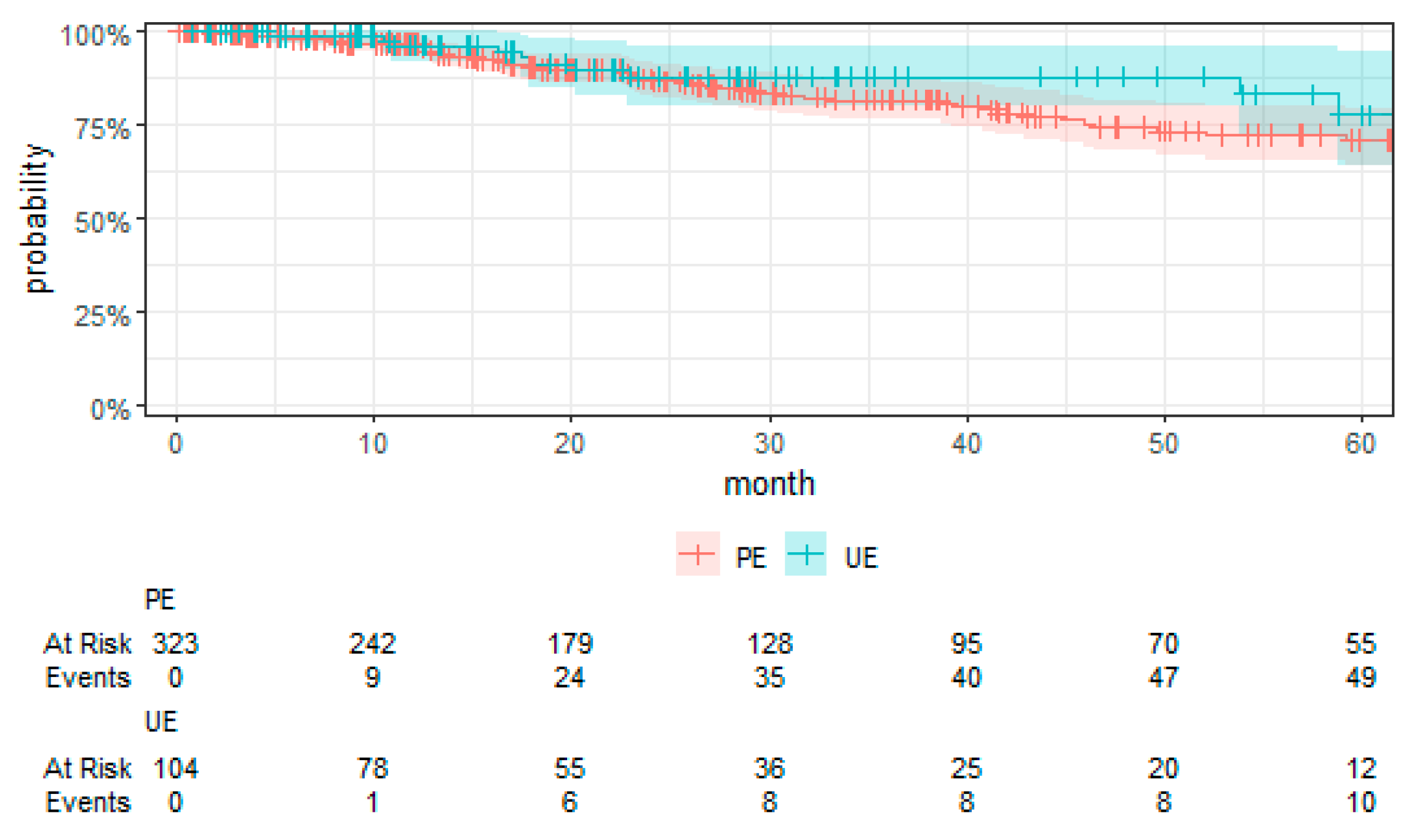
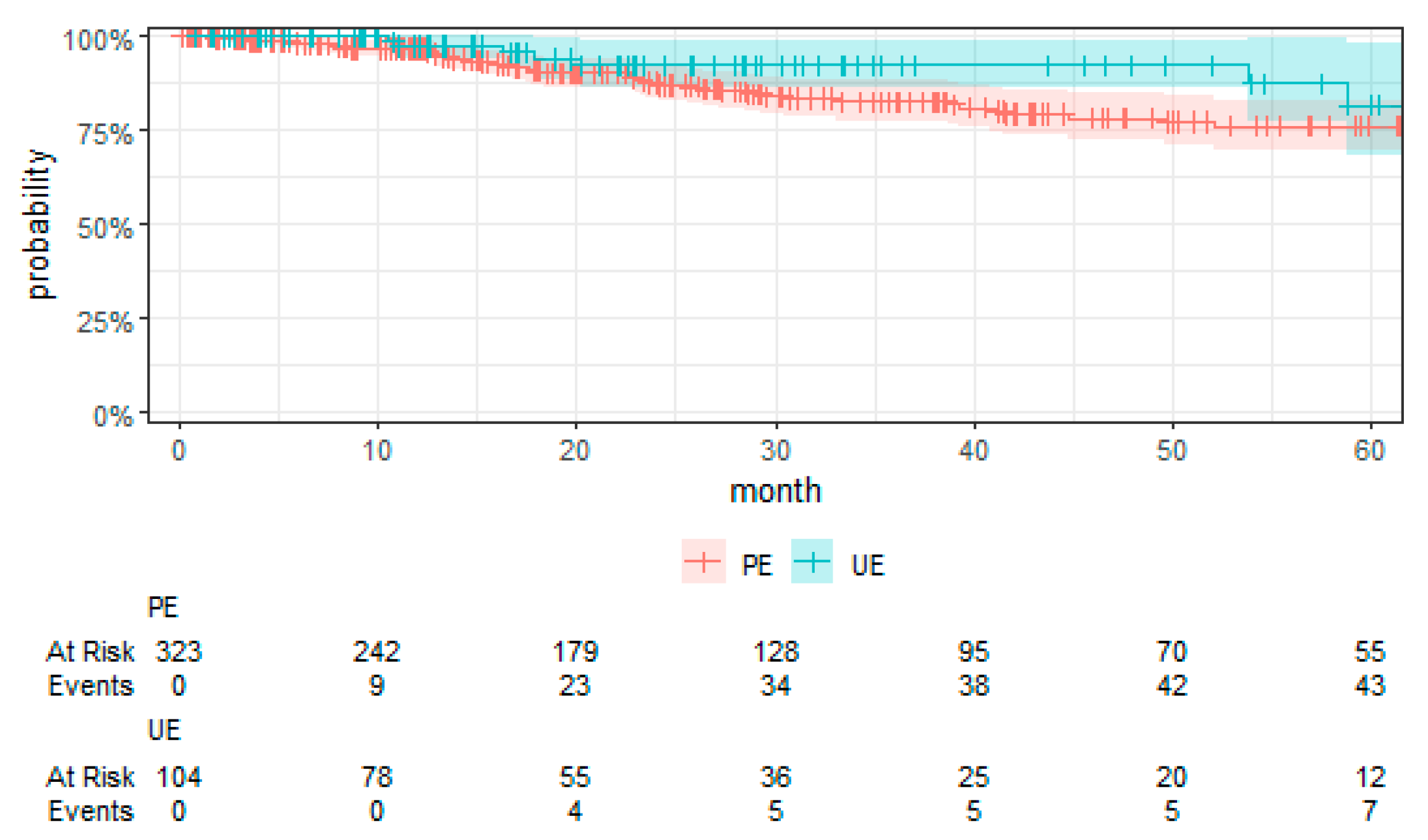
Kaplan–Meier curves were employed to visually compare the time-to-event data between the UE and PE groups.
Statistical analysis was done using R statistical software (Posit, PBC, Boston, MA, USA, Version 4.3.2) [
29]. A
p-value < 0.05 was considered statistically significant.
4. Discussion
For the first time, employing TTE to overcome the limitations associated with RCTs, this study presents a comprehensive evaluation of surgical strategies for sarcoma treatment. Our analysis revealed a notably higher risk of local recurrence in patients undergoing UEs compared to those receiving PEs, with a hazard ratio that underscores the significance of the initial surgical management on disease control. We found no significant differences in MFS and OS between the UE and PE groups. These findings suggest that, despite the adverse impact of UEs on local control, subsequent therapeutic interventions might mitigate the short-term risks of metastasis and mortality. Moreover, the tumor grade emerged as a significant prognostic factor, affirming its established role in influencing sarcoma outcomes. High-grade tumors were associated with increased risks of local recurrence, metastasis, and death, highlighting the critical importance of tumor biology in patient prognosis. These key findings contribute to the ongoing discourse on optimal sarcoma management strategies, emphasizing the need for precision in surgical planning and the potential benefits of incorporating TTE into oncological research.
Our study elucidates the complex interplay between surgical strategies and sarcoma outcomes, revealing a notably higher risk of local recurrence for patients undergoing UEs compared to PEs. This finding aligns with the literature but notably is in the upper end of the typical 5-year reported local recurrence rates (8% to 17%), pointing to a potential for non-referral selection bias in our RWT cohort [
4,
11,
12,
13,
30,
31,
32,
33,
34]. Despite these concerns, our use of TTE effectively navigates these challenges by approximating the rigor of RCTs, providing a robust framework for comparing UE and PE outcomes despite inherent limitations such as selection bias [
24,
25,
35].
Contrary to conventional expectations, our analysis found no significant differences in MFS, CSS, and OS between the UE and PE groups. This deviation from the expected outcomes suggests that subsequent therapeutic interventions may mitigate the adverse impact of UEs on short-term outcomes. The role of tumor grade as a prognostic factor further complicates the relationship between surgical strategy and sarcoma outcomes, with high-grade tumors associated with increased risks irrespective of the initial surgical approach [
36,
37,
38].
The observed variations in the follow-up treatments for UE patients reflect the individualized nature of sarcoma management post-surgery [
8,
9,
18,
20,
33]. This diversity in treatment strategies, alongside the differential outcomes reported, underscores the importance of considering both biological aggressiveness and post-surgical care in patient prognosis and treatment planning [
36,
37,
38,
39,
40,
41].
In light of our findings, the elevated risk of local recurrence post-UE and the nuanced interplay between surgical strategy and tumor biology underscore the need for refined surgical guidelines and patient management strategies in sarcoma care. These insights, alongside the observed disparities in the existing literature, underscore the critical areas for future research, particularly in exploring how individualized treatment strategies post-surgery can influence short-term outcomes. By highlighting these novel aspects, our study points to the significance of integrating the multidisciplinary network approach into the decision-making process, urging a shift towards more personalized and evidence-based approaches in sarcoma treatment planning.
Employing TTE has enabled us to navigate the ethical and logistical hurdles that preclude RCTs in sarcoma research, allowing for a rigorous comparison of UE and PE. This study’s insights highlight TTE’s utility in generating evidence-based recommendations for sarcoma management, particularly by enhancing our understanding of the differential impacts of surgical strategies [
23,
24,
25]. Our findings advocate for TTE’s broader application, potentially enriching clinical guidelines and decision-making processes in sarcoma care. Using this methodology, we have demonstrated not just a way to address observational studies’ methodological challenges in sarcoma research but also a means to propel sarcoma research towards more personalized and evidence-based treatment strategies, aligning with findings from the existing literature [
3,
4,
5,
6] and setting a precedent for future studies to follow.
While our study leverages the prospective collection of data within the SSN to enhance the robustness of our findings, the retrospective analysis of these data introduces inherent limitations. Notably, including patients diagnosed before 2018 who later presented with recurrences may augment the observed rate of local recurrence, reflecting a form of non-referral selection bias. This aspect necessitates cautious interpretation of our results, particularly concerning the generalizability of our findings across all sarcoma subtypes and treatment modalities. Furthermore, despite the methodological rigor provided by TTE, challenges such as selection bias and the complete balancing of cohort characteristics remain. Additionally, with a median follow-up of 1.9 years, statements about long-term prognoses are hardly possible. These limitations highlight the need for future prospective studies to validate our findings and explore the comprehensive impact of surgical strategies on sarcoma outcomes in a more controlled setting.
The significant risk of local recurrence following UE highlights the critical need for rigorous surgical margin evaluation and, when necessary, individualized re-resection based on data evidence [
18,
19,
20,
21,
33]. This emphasizes the essential role of a multidisciplinary approach in sarcoma management, where decisions are informed by a comprehensive understanding of tumor biology, potentially enhanced by our application of TTE. TTE’s ability to simulate RCT-like conditions provides a robust basis for refining treatment guidelines, facilitating personalized care that integrates tumor-specific biological characteristics [
23,
24,
25].
Looking forward, it is imperative that future research undertakes prospective studies to validate our findings, with a particular focus on the role of tumor biology in determining treatment efficacy and patient outcomes [
36,
37,
38,
39,
40,
41]. Additionally, examining how surgical choices affect patient quality of life will be crucial. Such studies should extend beyond traditional survival metrics to capture the broader impact of treatment strategies on the well-being of sarcoma patients, aligning with the shift towards patient-centered care in oncology [
8,
9,
18,
33]. This comprehensive approach promises not only to enhance clinical outcomes but also to improve the overall quality of life for individuals facing sarcomas, advocating for treatment plans that are as unique as the patients themselves [
20,
22].
Ultimately, our study calls for an integration of clinical expertise and multidisciplinary perspectives in developing evidence-based, patient-specific strategies for sarcoma treatment. By embracing the insights provided by TTE and prioritizing the exploration of tumor biology alongside patient-centric considerations, we can advance towards more effective and nuanced care for sarcoma patients.