Prognostic Evaluation of Piezo2 Channels in Mammary Gland Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Piezo Channels
1.2. Piezo and Cancer
1.3. Piezo and Mammary Gland Carcinoma
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemical Assay
2.3. Data Analysis
3. Results
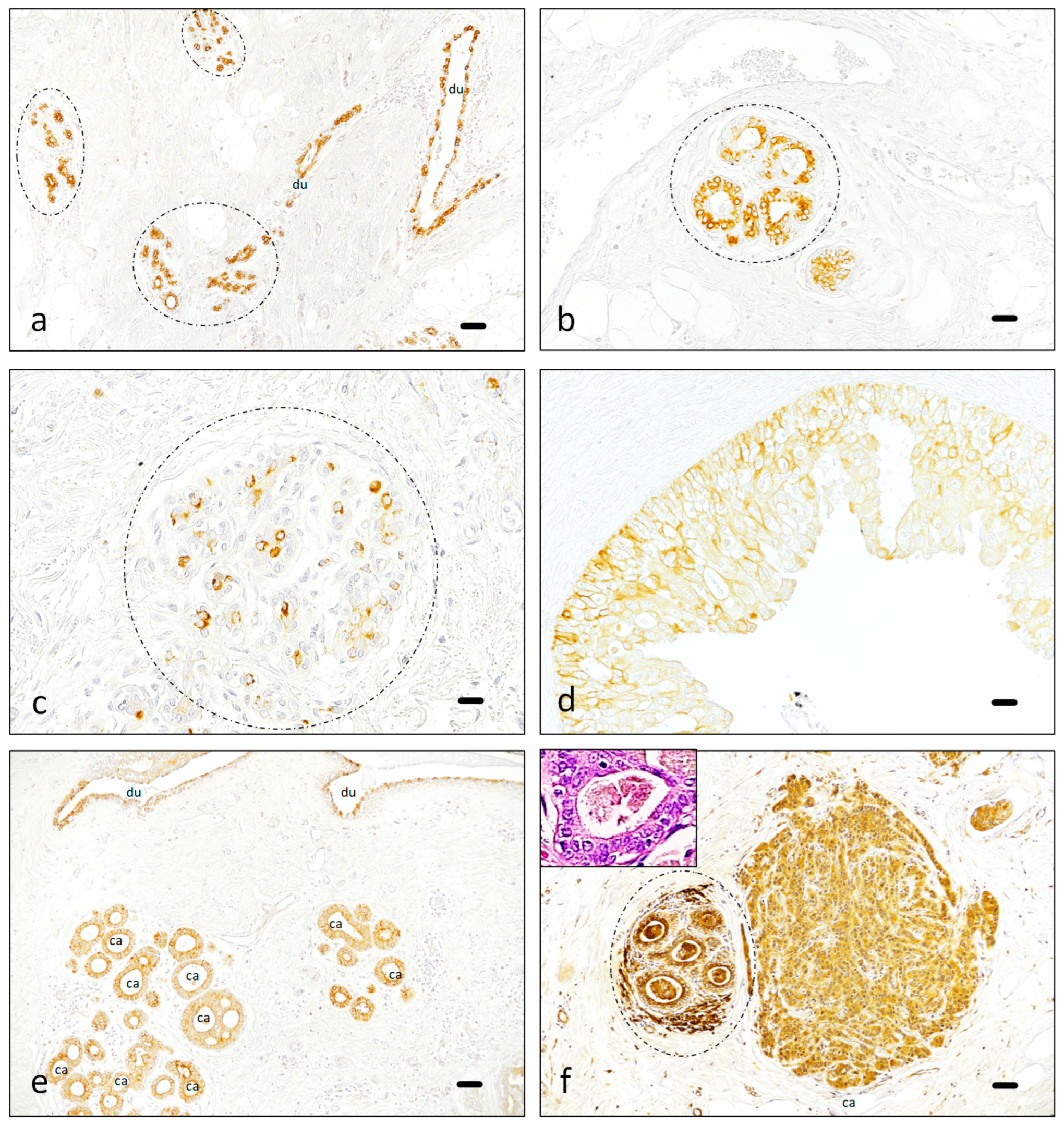
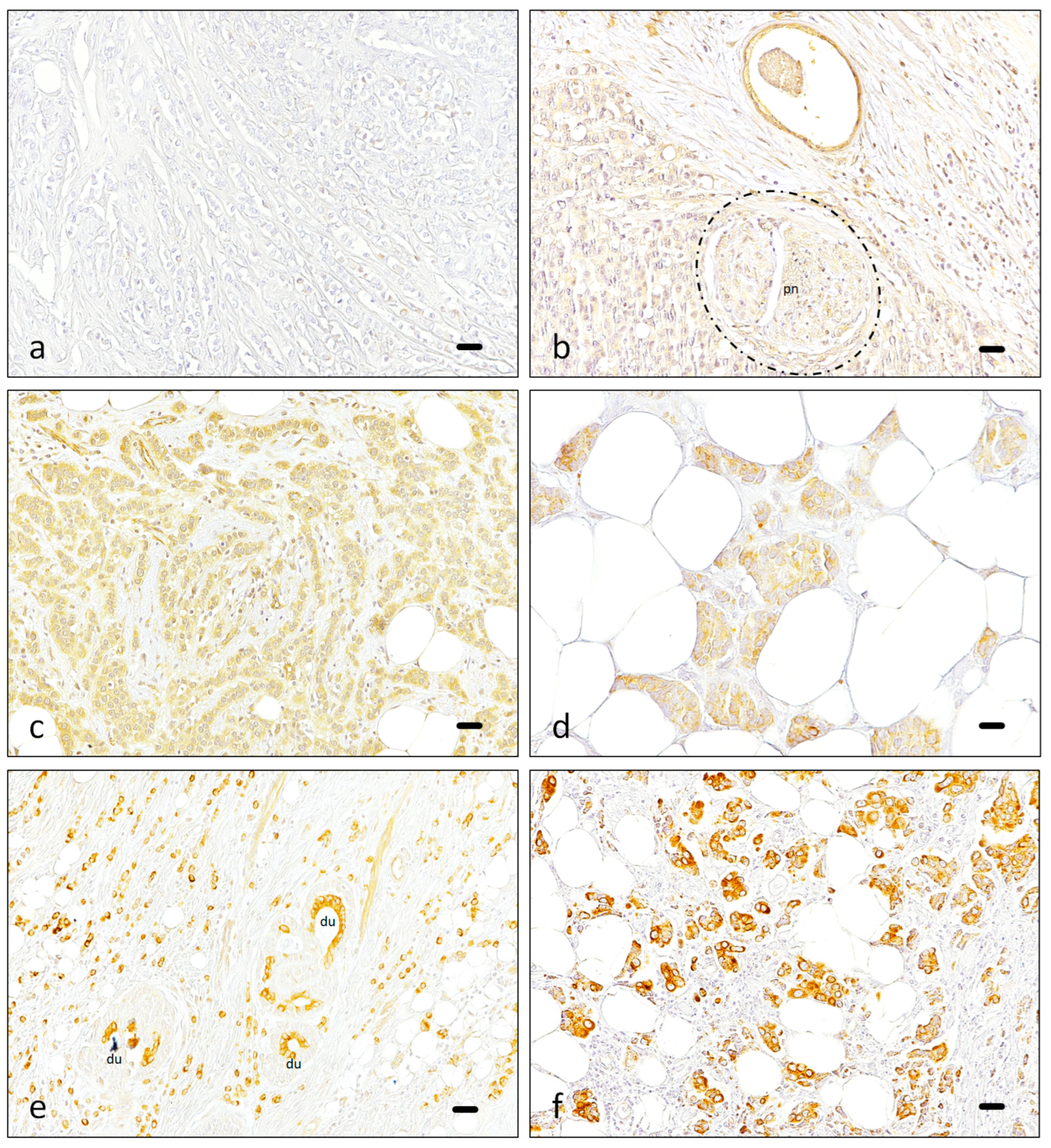
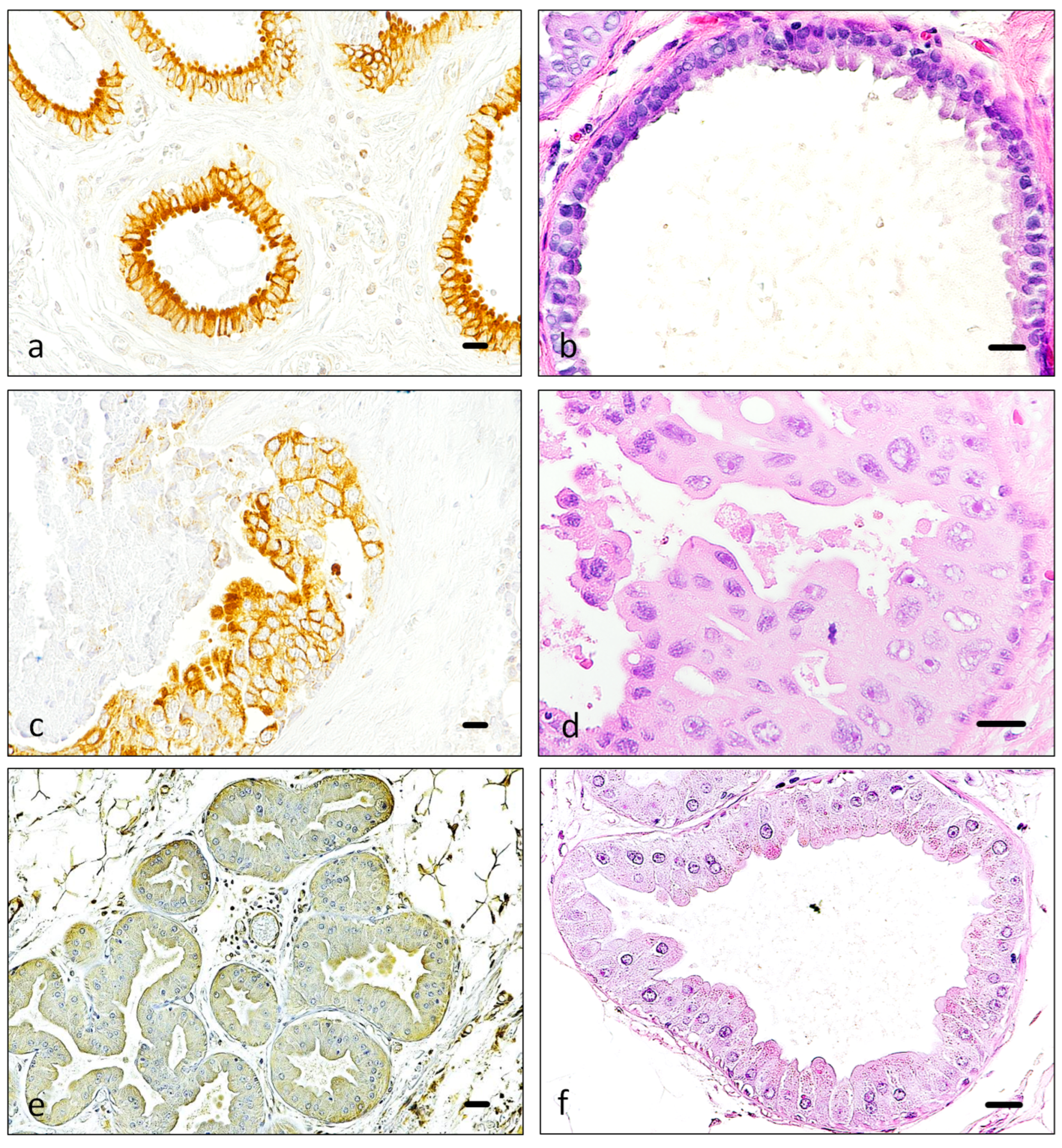
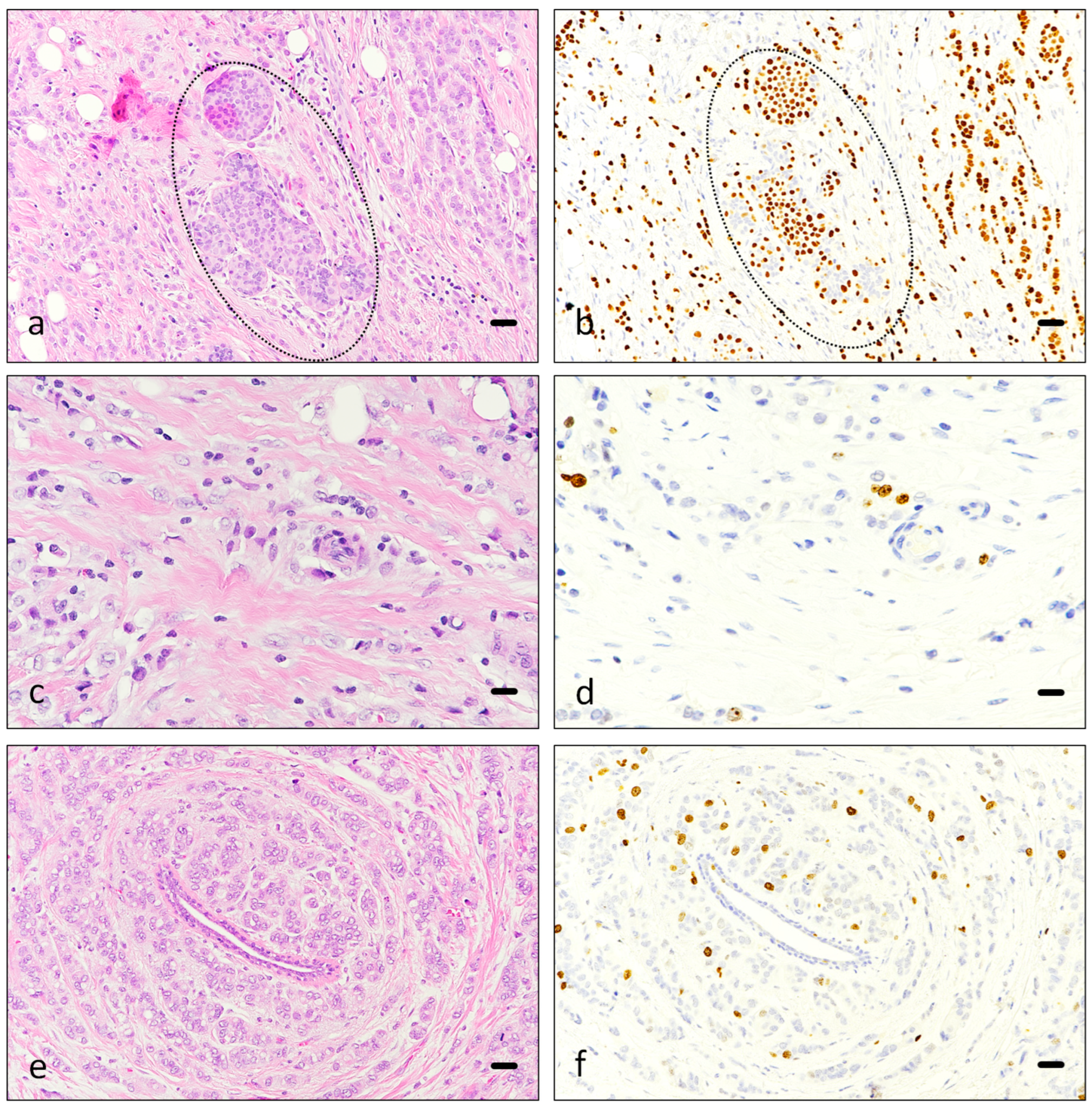
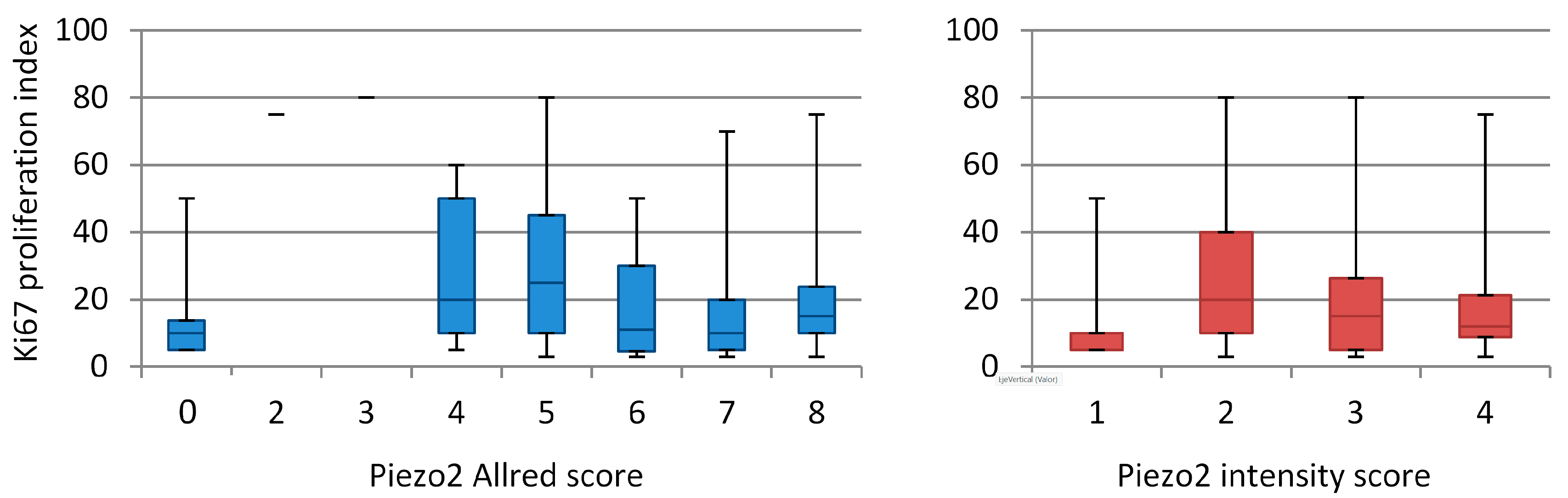
3.1. Immunohistochemistry
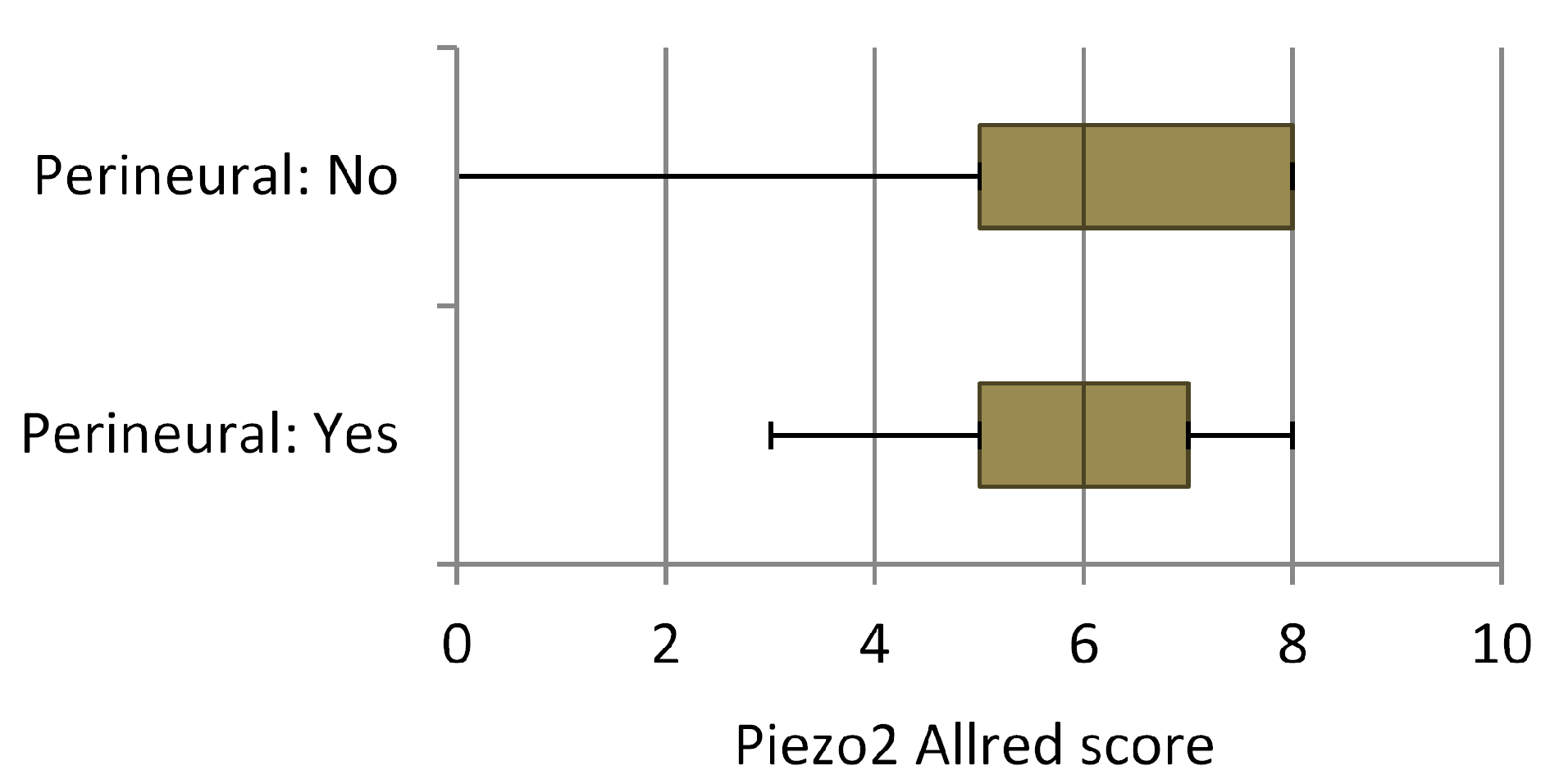
3.2. Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lewis, A.H.; Grandl, J. Touch, tension, and transduction—The function and regulation of Piezo ion channels. Trends Biochem. Sci. 2017, 42, 57–71. [Google Scholar] [CrossRef] [PubMed]
- McHugh, B.J.; Buttery, R.; Lad, Y.; Banks, S.; Haslett, C.; Sethi, T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J. Cell Sci. 2010, 123, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Olson, W.; Dong, P.; Fleming, M.; Luo, W. The specification and wiring of mammalian cutaneous low-threshold mechanoreceptors. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Bagriantsev, S.N.; Gracheva, E.O.; Gallagher, P.G. Piezo proteins: Regulators of mechanosensation and other cellular processes. J. Biol. Chem. 2014, 289, 31673–31681. [Google Scholar] [CrossRef] [PubMed]
- Gaub, B.M.; Müller, D. Mechanical stimulation of Piezo1 receptors depends on extracellular matrix proteins and directionality of force. Nano Lett. 2017, 17, 2064–2072. [Google Scholar] [CrossRef]
- Pardo-Pastor, C.; Rubio-Moscardo, F.; Vogel-González, M.; Serra, S.A.; Afthinos, A.; Mrkonjic, S.; Destaing, O.; Abenza, J.F.; Fernández-Fernández, J.M.; Trepat, X.; et al. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc. Natl. Acad. Sci. USA 2018, 115, 1925–1930. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Yang, X.; Zhou, G.; Wang, L.; Xiao, B. Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin-β-catenin mechanotransduction complex. Cell Rep. 2022, 38, 110342. [Google Scholar] [CrossRef]
- García-Mesa, Y.; García-Piqueras, J.; García, B.; Feito, J.; Cabo, R.; Cobo, J.; Vega, J.A.; García-Suárez, O. Merkel cells and Meissner’s corpuscles in human digital skin display Piezo2 immunoreactivity. J. Anat. 2017, 231, 978–989. [Google Scholar] [CrossRef]
- Pethö, Z.; Najder, K.; Bulk, E.; Schwab, A. Mechanosensitive ion channels push cancer progression. Cell Calcium 2019, 80, 79–90. [Google Scholar] [CrossRef]
- De Felice, D.; Alaimo, A. Mechanosensitive Piezo channels in cancer: Focus on altered calcium signaling in cancer cells and in tumor progression. Cancers 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sun, Z.; Zhang, X.; Niu, K.; Wang, Y.; Zheng, J.; Li, H.; Liu, Y. Loss of stretch-activated channels, Piezo´s, accelerates non-small cell lung cancer progression and cell migration. Biosci. Rep. 2019, 39, BSR20181679. [Google Scholar] [CrossRef] [PubMed]
- Etem, E.O.; Ceylan, G.G.; Özaydin, S.; Ceylan, C.; Özercan, I.; Kuloglu, T. The increased expression of Piezo1 and Piezo2 ion channels in human and mouse bladder carcinoma. Adv. Clin. Exp. Med. 2018, 27, 1025–1031. [Google Scholar] [CrossRef]
- Shang, H.; Xu, A.; Yan, H.; Xu, D.; Zhang, J.; Fang, X. PIEZO2 prometes cell proliferation and metastasis in colon carcinoma through the SLIT2/ROBO1/VEGFC pathway. Adv. Clin. Exp. Med. 2023, 32, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Li, S.; Hu, Z. Upregulation of Piezo1 is a novel prognostic indicator in glioma patients. Cancer Manag. Res. 2020, 12, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, X.; van Wijnbergen, J.W.M.; Yuan, L.; Liu, Y.; Zhang, C.; Jia, W. Identification of Piezo1 as a potential prognostic marker in gliomas. Sci. Rep. 2020, 10, 16121. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Liu, G.; Zhang, X.; Zhi, L.; Zhao, J.; Wang, G. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J. Cancer Res. Clin. Oncol. 2020, 146, 1139–1152. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Zhang, D.; Men, H.; Huo, L.; Geng, Q.; Wang, S.; Gao, Y.; Zhang, W.; Zhang, Y.; et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development though the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int. J. Oncol. 2019, 55, 629–644. [Google Scholar] [CrossRef]
- Hasegawa, K.; Fujii, S.; Matsumoto, S.; Tajiri, Y.; Kikucho, A.; Kiyoshima, T. YAP signaling induces Piezo1 to promote oral squamous cell carcinoma cell proliferation. J. Pathol. 2021, 253, 80–93. [Google Scholar] [CrossRef]
- Kuriyama, M.; Hirose, H.; Masuda, T.; Shudou, M.; Arafiles, J.V.V.; Imanishi, M.; Maekawa, M.; Hara, Y.; Futaki, S. Piezo1 activation using Yoda1 inhibits macropinocytosis in A431 human epidermoid carcinoma cells. Sci. Rep. 2022, 12, 6322. [Google Scholar] [CrossRef]
- Gutiérrez-Villanueva, M.; García-Mesa, Y.; García-Piqueras, J.; Cobo, R.; García-Suárez, O.; Vega, J.A.; Feito, J. The sensory innervation of the human nipple. Ann. Anat. 2020, 229, 151456. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Liu, J.; Ding, B.; Jin, L.; Xu, L.; Li, X.; Chen, J.; Fan, W. Five miRNAs-mediated Piezo2 downregulation, accompanied with activation of Hedgehog signaling pathway, predicts poor prognosis of breast cancer. Aging 2019, 11, 2628–2652. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rezania, S.; Kammerer, S.; Sokolowski, A.; Devaney, T.; Gorischek, A.; Jahn, S.; Hackl, H.; Groschner, K.; Windpassinger, C.; et al. Piezo1 forms mechanosensitive ion channels in the human MCF-7 breast cancer cell line. Sci. Rep. 2015, 5, 8364. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhang, Q.; Tan, Y.; Lam, K.H.; Zheng, H.; Qian, M. Non-contact high-frequency ultrasound microbeam stimulation: A novel finding and potential causes of cell responses. IEEE Trans. Biomed. Eng. 2020, 67, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, E.; Takabe, K.; Vujcic, M.; Gottlieb, P.A.; Dai, T.; Mercado-Perez, A.; Beyder, A.; Wang, Q.; Opyrchal, M. Mechano-sensing cannel PIEZO2 enhances invasive phenotype in triple-negative breast cancer. Int. J. Mol. Sci. 2022, 23, 9909. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; El-Sayed, M.E.; Lee, A.H.S.; Elston, C.W.; Grainge, M.J.; Hodi, Z.; Blamey, R.W.; Ellis, I.O. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J. Clin. Oncol. 2008, 26, 3153–3158. [Google Scholar] [CrossRef]
- Vasconcelos, I.; Hussainzada, A.; Berger, S.; Fietze, E.; Linke, J.; Siedentopf, F.; Schoenegg, W. The St. Gallen surrogate classification for breast cancer subtypes successfully predicts tumor presenting features, nodal involvement, recurrence patterns and disease free survival. Breast 2016, 29, 181–185. [Google Scholar] [CrossRef]
- Takahashi, H.; Oshi, M.; Asaoka, M.; Yan, L.; Endo, I.; Takabe, K. Molecular biological features of Nottingham histological grade 3 breast cancers. Ann. Surg. Oncol. 2020, 27, 4475–4485. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a prognostic biomarker in invasive breast cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Mrouj, K.; Andrés-Sánchez, N.; Dubra, G.; Singh, P.; Sobecki, M.; Chahar, D.; Al Ghoul, E.; Aznar, A.B.; Prieto, S.; Pirot, N.; et al. Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2026507118. [Google Scholar] [CrossRef] [PubMed]
- Lashen, A.G.; Toss, M.S.; Ghannam, S.F.; Makhlouf, S.; Green, A.; Mongan, N.P.; Rakha, E. Expression, assessment and significance of Ki67 expresssion in breast cancer: An update. J. Clin. Pathol. 2023, 76, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Q. Prognostic indicators for gastrointestinal stromal tumors: A review. Transl. Oncol. 2020, 13, 100812. [Google Scholar] [CrossRef]
- Couvelard, A.; Cazes, A.; Cros, J. Updates in histopathological classification and tissue biomarkers of digestive neuroendocrine neoplasms: What the clinician should know. Best. Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101795. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.M.J.; Hubner, R.A.; Valle, J.W.; McNamara, M.G. Selection of chemotherapy in advanced poorly differentiated extra-pulmonary neuroendocrine carcinoma. Cancers 2023, 15, 4951. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; He, D.-S.; Tang, R.-X.; Ren, F.-H.; Chen, G. Ki-67 is a valuable prognostic factor in gliomas: Evidence from a systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2015, 16, 411–420. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, Z.; Fu, T.; Jin, X.; Yu, T.; Liang, Y.; Zhao, X.; Huang, L. Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: Evidence from a systematic meta-analysis. BMC Cancer 2014, 14, 153. [Google Scholar] [CrossRef]
- Choong, P.F.; Akerman, M.; Willén, H.; Andersson, C.; Gustafson, P.; Baldetorp, B.; Fernö, M.; Alvegard, T.; Rydholm, A. Prognostic value of Ki-67 expression in 182 soft tissue sarcomas. Proliferation—A marker of metastasis? APMIS 1994, 102, 915–924. [Google Scholar] [CrossRef]
- Ladstein, R.G.; Bachmann, I.M.; Straume, O.; Akslen, L.A. Ki-67 expresssion is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BMC Cancer 2010, 10, 140. [Google Scholar] [CrossRef]
- Mahadevappa, A.; Krishna, S.M.; Vimala, M.G. Diagnostic and prognostic significance of Ki-67 immunohistochemical expression in surface epitelial ovarian carcinoma. J. Clin. Diagn. Res. 2017, 11, EC08–EC12. [Google Scholar] [CrossRef]
- Li, Y.; Yue, L.; Li, Y.; Zhang, Q.; Liang, X. Prognostic value of Ki-67 in nasopharyngeal carcinoma: A meta-analysis. Biosci. Rep. 2021, 41, BSR20203334. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ren, T.; Li, J.; Wang, N.; Xu, L.; Guo, Q.; Zhang, H.; Ma, J. The poor prognosis of lacrimal gland adenocarcinoma: A clinical study and literature review. J. Cancer Res. Clin. Oncol. 2024, 150, 26. [Google Scholar] [CrossRef] [PubMed]
- González-Castrillón, L.M.; Wurmser, M.; Öhlund, D.; Wilson, S.I. Dysregulation of core neurodevelopmental pathways-a common feature of cancers with perineural invasion. Front. Genet. 2023, 14, 1181775. [Google Scholar] [CrossRef]
- Hosoya, K.; Wakahara, M.; Ikeda, K.; Umekita, Y. Perineural invasion predicts unfavorable prognosis in patients with invasive breast cancer. Cancer Diagn. Progn. 2023, 3, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.J.; Miranda, G.; Amaro, T.; Salgado, M.; Mesquita, A. Prognostic value of tumor budding for early breast cancer. Biomedicines 2023, 11, 2906. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.N.; Moura, R.S.; Correia-Pinto, J.; Nogueira-Silva, C. Intraluminal chloride regulates lung branching morphogenesis: Involvement of PIEZO1/PIEZO2. Respir. Res. 2023, 24, 42. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, F.; Laforge, J.; Pflieger, J.-F. Influence of the vestibular system on the neonatal motor behaviors in the gray short-tailed opossum (Monodelphis domestica). IBRO Neurosci. Rep. 2023, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef] [PubMed]
- García-Piqueras, J.; García-Mesa, Y.; Cárcaba, L.; Feito, J.; Torres-Parejo, I.; Martín-Biedma, B.; Cobo, J.; García-Suárez, O.; Vega, J.A. Ageing of the somatosensory system at the periphery: Age-related changes in cutaneous mechanoreceptors. J. Anat. 2019, 234, 839–852. [Google Scholar] [CrossRef]
- Ilic, I.R.; Stojanovic, N.M.; Radulovic, N.S.; Zivkovic, V.V.; Randjelovic, P.J.; Petrovic, A.S.; Bozic, M.; Ilic, R.S. The quantitative ER immunohistochemical analysis in breast cancer: Detecting the 3 + 0, 4 + 0, and 5 + 0 Allred score cases. Medicina 2019, 55, 461. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Qu, Y.; Han, B.; Yu, Y.; Yao, W.; Bose, S.; Karlan, B.Y.; Giuliano, A.E.; Cui, X. Evaluation of MCF10A as a reliable model for normal human mammary epithelial cells. PLoS ONE 2015, 10, e0131285. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.; Li, C. The prognostic value of Piezo1 in breast cancer patients vith various clinicopathological features. Anticancer Drugs 2021, 32, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J. miR-10b-5p-mediated upregulation of PIEZO1 predicts poor prognosis and links to purine metabolism in breast cancer. Genomics 2022, 114, 110351. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Cai, G.; Ho, K.K.Y.; Wen, K.; Tong, Z.; Deng, L.; Liu, A.P. Compression enhances invasive phenotype and matrix degradation of breast cancer cells via Piezo1 activation. BMC Mol. Cell Biol. 2022, 23, 1. [Google Scholar] [CrossRef]
- Karska, J.; Kowalski, S.; Saczko, J.; Moisescu, M.G.; Kulbacka, J. Mechanosensitive ion channels and their role in cancer cells. Membranes 2023, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-Z.; Zhou, T.; Xu, J.-Q.; Wang, Y.-X.; Sun, M.-M.; He, Y.-J.; Pan, S.-W.; Xiong, W.; Peng, Z.-K.; Gao, X.-H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 13. [Google Scholar] [CrossRef]
- Shin, K.C.; Park, H.J.; Kim, J.G.; Lee, I.H.; Cho, H.; Park, C.; Sung, T.S.; Koh, S.D.; Park, S.W.; Bae, Y.M. The Piezo2 ion channel is mechanically activated by low-threshold positive pressure. Sci. Rep. 2019, 9, 6446. [Google Scholar] [CrossRef]
- Amemiya, Y.; Maki, M.; Shibata, H.; Takahara, T. New insights into the regulation of mTOR signaling via Ca2+-binding proteins. Int. J. Mol. Sci. 2023, 24, 3923. [Google Scholar] [CrossRef]
- Bong, A.H.L.; Hua, T.; So, C.L.; Peters, A.A.; Robitaille, M.; Tan, Y.Y.; Roberts-Thomson, S.J.; Monteith, G.R. AKT regulation of ORAI1-mediated calcium influx in breast cancer cells. Cancers 2022, 14, 4794. [Google Scholar] [CrossRef]
- Glogowska, E.; Arhatte, M.; Chatelain, F.C.; Lesage, F.; Xu, A.; Grashoff, C.; Discher, D.E.; Patel, A.; Honoré, E. Piezo1 and Piezo2 foster mechanical gating of K2P channels. Cell Rep. 2021, 37, 110070. [Google Scholar] [CrossRef]
- Szczot, M.; Nickolls, A.R.; Lam, R.M.; Chesler, A.T. The form and function of PIEZO2. Annu. Rev. Biochem. 2021, 90, 507–534. [Google Scholar] [CrossRef]
- Human Protein Atlas Home Page, sections PIEZO2 and PIEZO1. Available online: https://www.proteinatlas.org/ (accessed on 9 June 2024).
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Rossi, L.; Laas, E.; Mallon, P.; Vincent-Salomon, A.; Guinebretiere, J.-M.; Lerebours, F.; Rouzier, R.; Pierga, J.-Y.; Reyal, F. Prognostic impact of discrepant Ki67 and mitotic index on hormone receptor-positive, HER2-negative breast carcinoma. Br. J. Cancer 2015, 113, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- de Azambuja, E.; Cardoso, F.; de Castro, G., Jr.; Colozza, M.; Mano, M.S.; Durbecq, V.; Sotiriou, C.; Larsimont, D.; Piccart-Gebhart, M.J.; Paesmans, M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br. J. Cancer 2007, 96, 1504–1513. [Google Scholar] [CrossRef]
- Alamoodi, M. Factors affecting pathological complete response in locally advanced breast cancer cases receiving neoadjunvant therapy: A comprehensive literature review. Eur. J. Breast Health 2023, 20, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Dowsett, M.; Pineda, S.; Wale, C.; Salter, J.; Quinn, E.; Zabaglo, L.; Mallon, E.; Green, A.R.; Ellis, I.O.; et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J. Clin. Oncol. 2011, 29, 4273–4278. [Google Scholar] [CrossRef]
- Lashen, A.G.; Toss, M.S.; Katayama, A.; Cogna, R.; Mongan, N.P.; Rakha, E.A. Assessment of proliferation in breast cancer: Cell cycle or mitosis? An observational study. Histopathology 2021, 79, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Aykut, B.; Chen, R.; Kim, J.I.; Wu, D.; Shadaloey, S.A.A.; Abengozar, R.; Preiss, P.; Saxena, A.; Pushalkar, S.; Leinwand, J.; et al. Targeting Piezo1 unleashes innate immunity against cancer and infectious disease. Sci. Immunol. 2020, 5, eabb5168. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in breast cancer: Updated recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Soliman, N.A.; Yussif, S.M. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 2016, 13, 496–504. [Google Scholar] [CrossRef] [PubMed]
- de Gregorio, A.; Friedl, T.W.P.; Hering, E.; Widschwendter, P.; de Gregorio, N.; Bekes, I.; Janni, W.; Dayan, D.; Huober, J.B. Ki67 as proliferative marker in patients with early breast cancer and its association with clinicopathological factors. Oncology 2021, 99, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Aristei, C.; Tomatis, M.; Ponti, A.; Marotti, L.; Cardoso, M.J.; Cheung, K.L.; Curigliano, G.; De Vries, J.; Santini, D.; Sardanelli, F.; et al. Treatment and outcomes in breast cancer patients: A cross section study from the EUSOMA breast centre network. Eur. J. Cancer 2024, 196, 113438. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.M.; Rendi, M.H.; Frederick, P.D.; Onega, T.; Allison, K.H.; Mercan, E.; Brunyé, T.T.; Shapiro, L.; Weaver, D.L.; Elmore, J.G. Breast cancer prognostic factors in the digital era: Comparison of Nottingham grade using whole slide images and glass slides. J. Pathol. Inform. 2019, 10, 11. [Google Scholar] [CrossRef]
| a | ||
| Histology | Number | Percent |
| Ductal | 105 | 84 |
| Lobulillar | 9 | 7.2 |
| Mucinous | 4 | 3.2 |
| Turbomolecular | 2 | 1.6 |
| Dutal/lobulillar | 1 | 0.8 |
| Dutal + tubular | 1 | 0.8 |
| Medullary | 1 | 0.8 |
| Micropapillary | 1 | 0.8 |
| Solid papillary | 1 | 0.8 |
| b | ||
| Stage | Number | Percent |
| pTis | 8 | 6.4 |
| pT1a | 2 | 1.6 |
| pT1b | 18 | 14.4 |
| pT1c | 62 | 49.6 |
| pT2 | 31 | 24.8 |
| pT3 | 2 | 1.6 |
| pT4 | 1 | 0.8 |
| a | Nottingham grade | Frequency | Percent |
| Grade 1 | 32 | 29.36 | |
| Grade 2 | 43 | 39.45 | |
| Grade 3 | 34 | 31.19 | |
| b | Tubule formation | Frequency | Percent |
| Score 1 | 8 | 7.34 | |
| Score 2 | 20 | 18.35 | |
| Score 3 | 81 | 74.31 | |
| c | Pleomorphism | Frequency | Percent |
| Score 1 | 13 | 11.93 | |
| Score 2 | 68 | 62.39 | |
| Score 3 | 28 | 25.69 | |
| d | Mitoses | Frequency | Percent |
| Score 1 | 66 | 60.55 | |
| Score 2 | 26 | 23.85 | |
| Score 3 | 17 | 15.6 |
| Estrogen Receptor | Frequency | Percent | Progesterone Receptor | Frequency | Percent |
| Positive | 106 | 84.8% | Positive | 85 | 68% |
| Negative | 19 | 15.2% | Negative | 45 | 32% |
| Hormone Receptors | Frequency | Percent | Her2 | Frequency | Percent |
| Positive | 108 | 86.4% | Positive | 36 | 29% |
| Negative | 17 | 13.6% | Negative | 88 | 71% |
| St. Gallen Classification | Frequency | Percent |
|---|---|---|
| Luminal A | 69 | 55.2% |
| Luminal B Her2− | 15 | 12% |
| Luminal B Her2+ | 24 | 19.2% |
| Her2+ non luminal | 11 | 8.8% |
| Basal-like | 6 | 4.8% |
| Cells Expressing | <1% (1) | 1–10% (2) | 11–33% (3) | 34–66% (4) | >66% (5) |
|---|---|---|---|---|---|
| Low expression (1) | 1 | 2 | 9 | 14 | 17 |
| Medium expression (2) | 0 | 0 | 6 | 6 | 17 |
| Intense expression (3) | 0 | 0 | 0 | 3 | 36 |
| Normal Gland | Cancer | ||||
|---|---|---|---|---|---|
| Value | Frequency | Percent | Value | Frequency | Percent |
| Score 0 | 1 | 1.6% | Score 0 | 5 | 8.2% |
| Score 1 | 16 | 26.2% | Score 1 | 22 | 36.1% |
| Score 2 | 12 | 19.7% | Score 2 | 18 | 29.5% |
| Score 3 | 32 | 52.5% | Score 3 | 16 | 26.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Sanz, R.; Rodrigues-Françoso, A.; García-Mesa, Y.; García-Alonso, F.J.; Gómez-Muñoz, M.A.; Malmierca-González, S.; Salazar-Blázquez, R.; García-Suárez, O.; Feito, J. Prognostic Evaluation of Piezo2 Channels in Mammary Gland Carcinoma. Cancers 2024, 16, 2413. https://doi.org/10.3390/cancers16132413
Martín-Sanz R, Rodrigues-Françoso A, García-Mesa Y, García-Alonso FJ, Gómez-Muñoz MA, Malmierca-González S, Salazar-Blázquez R, García-Suárez O, Feito J. Prognostic Evaluation of Piezo2 Channels in Mammary Gland Carcinoma. Cancers. 2024; 16(13):2413. https://doi.org/10.3390/cancers16132413
Chicago/Turabian StyleMartín-Sanz, Raquel, Aline Rodrigues-Françoso, Yolanda García-Mesa, Francisco Javier García-Alonso, María Asunción Gómez-Muñoz, Sandra Malmierca-González, Rocío Salazar-Blázquez, Olivia García-Suárez, and Jorge Feito. 2024. "Prognostic Evaluation of Piezo2 Channels in Mammary Gland Carcinoma" Cancers 16, no. 13: 2413. https://doi.org/10.3390/cancers16132413
APA StyleMartín-Sanz, R., Rodrigues-Françoso, A., García-Mesa, Y., García-Alonso, F. J., Gómez-Muñoz, M. A., Malmierca-González, S., Salazar-Blázquez, R., García-Suárez, O., & Feito, J. (2024). Prognostic Evaluation of Piezo2 Channels in Mammary Gland Carcinoma. Cancers, 16(13), 2413. https://doi.org/10.3390/cancers16132413







