Clinical Characteristics and Special Considerations in the Management of Rare Melanoma Subtypes
Abstract
Simple Summary
Abstract
1. Introduction
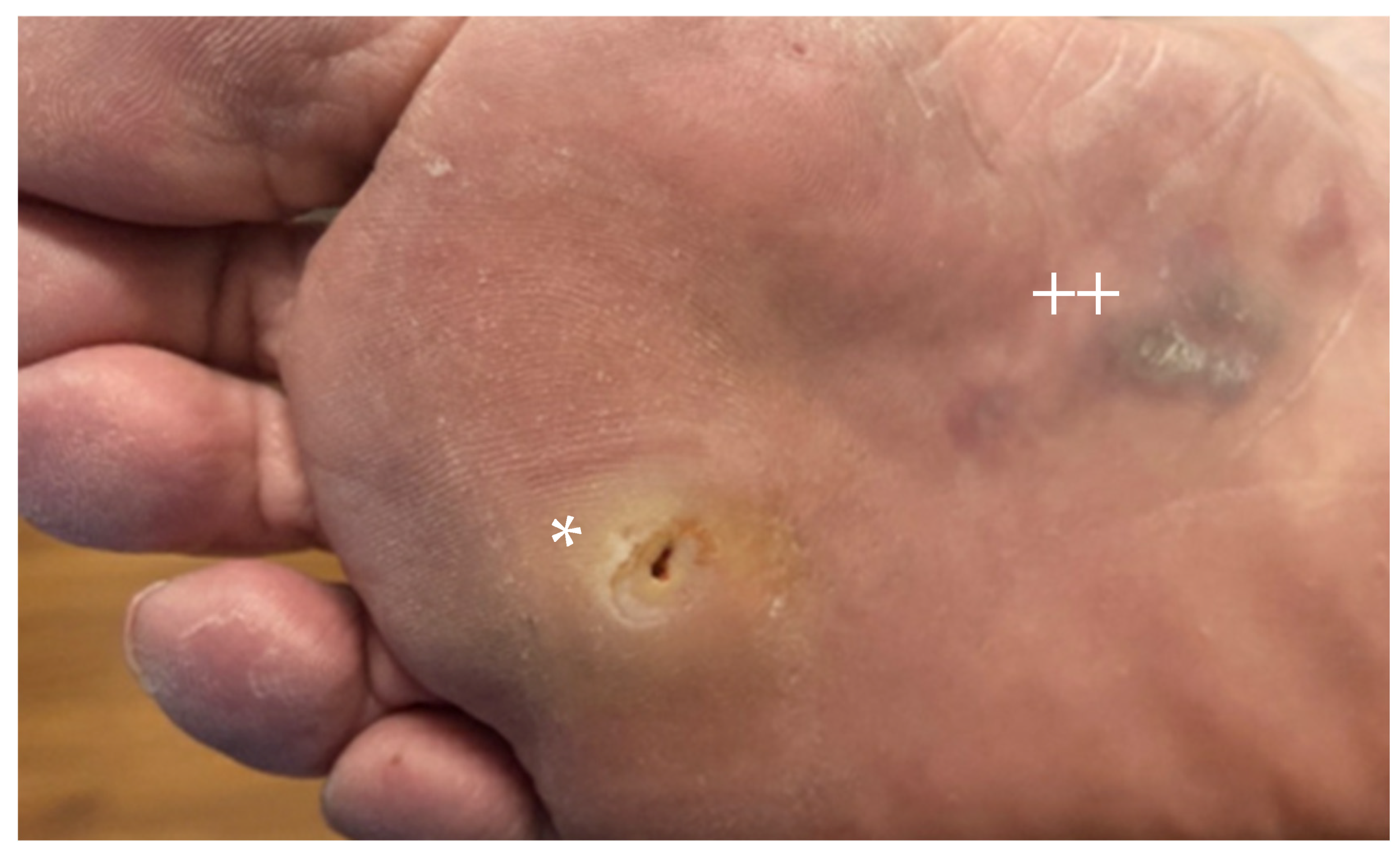
2. Acral Melanoma
2.1. Epidemiology
2.2. Presentation and Histopathology
2.3. Treatment for Localized Disease
2.4. Advanced Disease
3. Mucosal Melanoma
3.1. Epidemiology
3.2. Presentation and Histopathology
3.3. Localized Disease
3.4. Advanced Disease
4. Uveal Melanoma
4.1. Epidemiology
4.2. Presentation and Histopathology
4.3. Localized Disease
4.4. Advanced Disease
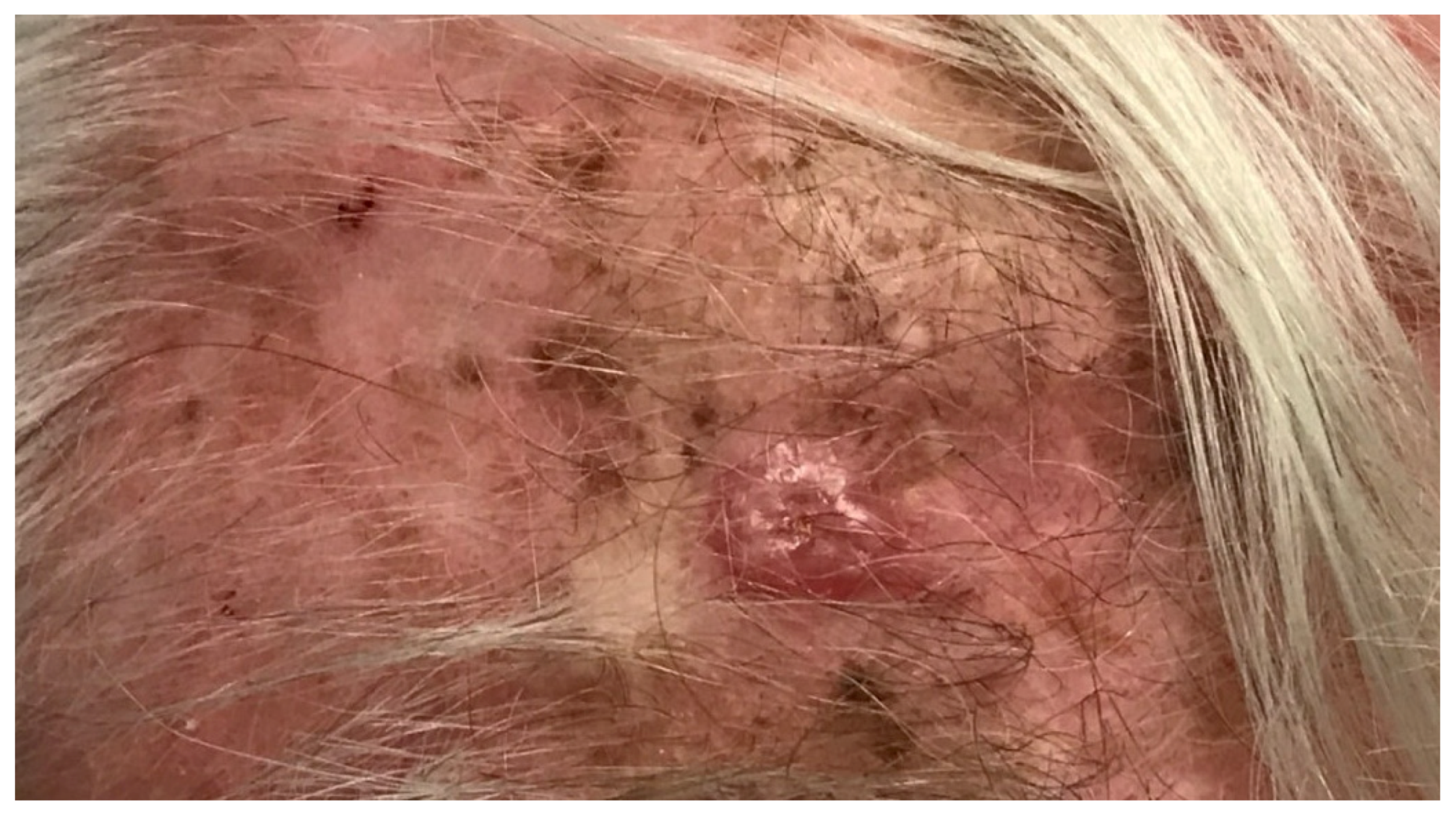
5. Desmoplastic Melanoma
5.1. Epidemiology
5.2. Diagnosis and Histopathology
5.3. Localized Disease Considerations
5.4. Advanced Disease
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- George, D.D.; Armenio, V.A.; Katz, S.C. Combinatorial immunotherapy for melanoma. Cancer Gene Ther. 2017, 24, 141–147. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Bradford, P.T.; Goldstein, A.M.; McMaster, M.L.; Tucker, M.A. Acral Lentiginous Melanoma: Incidence and Survival Patterns in the United States, 1986–2005. Arch. Dermatol. 2009, 145, 427–434. [Google Scholar] [CrossRef]
- Slingluff, C.L.; Vollmer, R.; Seigler, H.F. Acral Melanoma: A Review of 185 Patients with Identification of Prognostic Variables. J. Surg. Oncol. 1990, 45, 91–98. [Google Scholar] [CrossRef]
- Klemen, N.D.; Wang, M.; Rubinstein, J.C.; Olino, K.; Clune, J.; Ariyan, S.; Cha, C.; Weiss, S.A.; Kluger, H.M.; Sznol, M. Survival after checkpoint inhibitors for metastatic acral, mucosal, and uveal melanoma. J. Immunother. Cancer 2020, 8, e000341. [Google Scholar] [CrossRef]
- van Not, O.J.; de Meza, M.M.; van den Eertwegh, A.J.; Haanen, J.B.; Blank, C.U.; Aarts, M.J.; van den Berkmortel, F.W.; van Breeschoten, J.; de Groot, J.W.B.; Hospers, G.A.; et al. Response to immune checkpoint inhibitors in acral melanoma: A nationwide cohort study. Eur. J. Cancer 2022, 167, 70–80. [Google Scholar] [CrossRef]
- Patrick, R.J.; Fenske, N.A.; Messina, J.L. Primary mucosal melanoma. J. Am. Acad. Dermatol. 2007, 56, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Seetharamu, N.; Ott, P.A.; Pavlick, A.C. Mucosal melanomas: A case-based review of the literature. Oncologist 2010, 15, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.M.; King, J.B.; White, A.; Singh, S.D.; Lichtenfeld, J.L. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010–2019. Prev. Med. 2023, 175, e107692. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Li, S.; Sheng, X.; Si, L.; Cui, C.; Han, M.; Guo, J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer 2011, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- De Wet, J.; Tod, B.; Visser, W.I.; Jordaan, H.F.; Schneider, J.W. Clinical and pathologic feastures of acral melanoma in a South African population: A retrospective study. S. Afr. Med. J. 2018, 108, 777–781. [Google Scholar] [CrossRef]
- Hudson, D.A.; Krige, J.E. Melanoma in Black South Africans. J. Am. Coll. Surg. 1995, 180, 65–71. [Google Scholar]
- Mendes, Q.G.L.; Koifman, S. Socioeconomic status as a predictor of melanoma survival in a series of 1083 cases from Brazil: Just a marker of health services accessibility? Melanoma Res. 2013, 23, 199–205. [Google Scholar] [CrossRef]
- Darmawan, C.C.; Jo, G.; Montenegro, S.E.; Kwak, Y.; Cheol, L.; Cho, K.H.; Mun, J.-H. Early detection of acral melanoma: A review of clinical, dermoscopic, histopathologic, and molecular characteristics. J. Am. Acad. Dermatol. 2019, 81, 805–812. [Google Scholar] [CrossRef]
- Susok, L.; Gambichler, T. Caucasians with acral lentiginous melanoma have the same outcome as patients with stage- and limb-matched superficial spreading melanoma. J. Cancer Res. Clin. Oncol. 2022, 148, 497–502. [Google Scholar] [CrossRef]
- Smalley, K.S.M.; Teer, J.K.; Chen, Y.A.; Wu, J.-Y.; Yao, J.; Koomen, J.M.; Chen, W.-S.; Rodriguez-Waitkus, P.; Karreth, F.A.; Messina, J.L. A Mutational Survey of Acral Nevi. JAMA Dermatol. 2021, 157, 831–835. [Google Scholar] [CrossRef]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [PubMed]
- Weitman, E.S.; Perez, M.C.; Lee, D.; Kim, Y.; Fulp, W.; Sondak, V.K.; Sarnaik, A.A.; Gonzalez, R.J.; Cruse, C.W.; Messina, J.L.; et al. Re-biopsy of partially sampled thin melanoma impacts sentinel lymph node sampling as well as surgical margins. Melanoma Manag. 2019, 6, MMT17. [Google Scholar] [CrossRef] [PubMed]
- Scolyer, R.A.; Thompson, J.F.; McCarthy, S.W.; Strutton, G.M.; Elder, D.E. Incomplete biopsy of melanocytic lesions can impair the accuracy of pathologic diagnosis. Australas J. Dermatol. 2006, 47, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Gumaste, P.V.; Fleming, N.H.; Silva, I.; Shapiro, R.L.; Berman, R.S.; Zhong, J.; Osman, I.; Stein, J.A. Analysis of recurrence patterns in acral versus nonacral melanoma: Should histologic subtype influence treatment guidelines? J. Natl. Compr. Cancer Netw. 2014, 12, 1706–1712. [Google Scholar] [CrossRef]
- Kolla, A.M.; Vitiello, G.A.; Friedman, E.B.; Sun, J.; Potdar, A.; Daou, H.; Farrow, N.E.; Farley, C.R.; Vetto, J.T.; Han, D.; et al. Acral Lentiginous Melanoma: A United States Multi-Center Substage Survival Analysis. Cancer Control 2021, 28, 10732748211053567. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Soong, S.-J.; Smith, T.; Ross, M.I.; Urist, M.M.; Karakousis, C.P.; Temple, W.J.; Mihm, M.C.; Barnhill, R.L.; Jewell, W.R.; et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann. Surg. Oncol. 2001, 8, 101–108. [Google Scholar] [PubMed]
- Balch, C.M.; Urist, M.M.; Karakousis, C.P.; Smith, T.J.; Temple, W.J.; Drzewiecki, K.; Jewell, W.R.; Bartolucci, A.A.; Mihm, M.C.; Barnhill, R.; et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial. Ann. Surg. 1993, 218, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Cohn-Cedermark, G.; Rutqvist, L.E.; Andersson, R.; Breivald, M.; Ingvar, C.; Johansson, H.; Jönsson, P.E.; Krysander, L.; Lindholm, C.; Ringborg, U. Long term results of a randomized study by the Swedish Melanoma Study Group on 2-cm versus 5-cm resection margins for patients with cutaneous melanoma with a tumor thickness of 0.8–2.0 mm. Cancer 2000, 89, 1495–1501. [Google Scholar] [CrossRef]
- Karakousis, C.P.; Balch, C.M.; Urist, M.M.; Ross, M.M.; Smith, T.J.; Bartolucci, A.A. Local recurrence in malignant melanoma: Long-term results of the multiinstitutional randomized surgical trial. Ann. Surg. Oncol. 1996, 3, 446–452. [Google Scholar] [CrossRef]
- Khayat, D.; Rixe, O.; Martin, G.; Soubrane, C.; Banzet, M.; Bazex, J.A.; Lauret, P.; Vérola, O.; Auclerc, G.; Harper, P.; et al. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick). Cancer 2003, 97, 1941–1946. [Google Scholar] [CrossRef]
- Lens, M.B.; Nathan, P.; Bataille, V. Excision margins for primary cutaneous melanoma: Updated pooled analysis of randomized controlled trials. Arch. Surg. 2007, 142, 885–891. [Google Scholar] [CrossRef]
- Ringborg, U.; Andersson, R.; Eldh, J.; Glaumann, B.; Hafström, L.; Jacobsson, S.; Jönsson, P.-E.; Johansson, H.; Krysander, L.; Lagerlöf, B.; et al. Resection margins of 2 versus 5 cm for cutaneous malignant melanoma with a tumor thickness of 0.8 to 2.0 mm: Randomized study by the Swedish Melanoma Study Group. Cancer 1996, 77, 1809–1814. [Google Scholar] [CrossRef]
- Thomas, J.M.; Newton-Bishop, J.; A’Hern, R.; Coombes, G.; Timmons, M.; Evans, J.; Cook, M.; Theaker, J.; Fallowfield, M.; O’Neill, T.; et al. Excision margins in high-risk malignant melanoma. N. Engl. J. Med. 2004, 350, 757–766. [Google Scholar] [CrossRef]
- Veronesi, U.; Cascinelli, N.; Adamus, J.; Balch, C.; Bandiera, D.; Barchuk, A.; Bufalino, R.; Craig, P.; De Marsillac, J.; Durand, J.; et al. Thin stage I primary cutaneous malignant melanoma. Comparison of excision with margins of 1 or 3 cm. N. Engl. J. Med. 1988, 318, 1159–1162. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohara, K.; Kishi, A.; Teramoto, Y.; Sato, S.; Fujisawa, Y.; Fujimoto, M.; Otsuka, F.; Hayashi, N.; Yamazaki, N.; et al. Effects of non-amputative wide local excision on the local control and prognosis of in situ and invasive subungual melanoma. J. Dermatol. 2015, 42, 861–866. [Google Scholar] [CrossRef]
- Cheraghlou, S.; Ugwu, N.; Girardi, M. Sentinel lymph node biopsy positivity in patients with acral lentiginous and other subtypes of cutaneous melanoma. JAMA Dermatol. 2022, 158, 51–58. [Google Scholar] [CrossRef]
- Yeh, I.; Jorgenson, E.; Shen, L.; Xu, M.; North, J.P.; Shain, A.H.; Reuss, D.; Wu, H.; Robinson, W.A.; Olshen, A.; et al. Targeted Genomic Profiling of Acral Melanoma. J. Natl. Cancer Inst. 2019, 111, 1068–1077. [Google Scholar] [CrossRef]
- Curtin, J.A.; Fridlyand, J.; Kageshita, T.; Patel, H.N.; Busam, K.J.; Kutzner, H.; Cho, K.-H.; Aiba, S.; Bröcker, E.-B.; LeBoit, P.E.; et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005, 353, 2135–2147. [Google Scholar] [CrossRef]
- Furney, S.J.; Turajlic, S.; Stamp, G.; Thomas, J.M.; Hayes, A.; Strauss, D.; Gavrielides, M.; Xing, W.; Gore, M.; Larkin, J.; et al. The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res. 2014, 27, 835–838. [Google Scholar] [CrossRef]
- Bai, X.; Mao, L.L.; Chi, Z.H.; Sheng, X.N.; Cui, C.L.; Kong, Y.; Dai, J.; Wang, X.; Li, S.M.; Tang, B.X.; et al. BRAF inhibitors: Efficacious and tolerable in BRAF-mutant acral and mucosal melanoma. Neoplasma 2017, 64, 626–632. [Google Scholar] [CrossRef]
- Bhave, P.; Ahmed, T.; Lo, S.N.; Shoushtari, A.; Zaremba, A.; Versluis, J.M.; Mangana, J.; Weichenthal, M.; Si, L.; Lesimple, T.; et al. Efficacy of anti-PD-1 and ipilimumab alone or in combination in acral melanoma. J. Immunother. Cancer 2022, 10, e004668. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Munhoz, R.R.; Kuk, D.; Ott, P.A.; Johnson, D.B.; Tsai, K.K.; Rapisuwon, S.; Eroglu, Z.; Sullivan, R.J.; Luke, J.J.; et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 2016, 122, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Saberian, C.; Ludford, K.; Roszik, J.; Gruschkus, S.; Johnson, D.H.; Bernatchez, C. Analysis of tumor mutation burden (TMB), PD-L1 status and clinical outcomes with checkpoint inhibitors (CPI) in acral melanoma (AM). Pigment Cell Melanoma Res. 2019, 33, 226–227. [Google Scholar]
- Zheng, Q.; Li, J.; Zhang, H.; Wang, Y.; Zhang, S. Immune checkpoint inhibitors in advanced acral melanoma: A systematic review. Front. Oncol. 2020, 10, 602705. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Zhang, X.; Shu, Y.; Pan, H.; Wu, D.; Liu, J.; Lou, F.; Mao, L.; Wang, X.; Wen, X.; et al. A Phase Ib Study of Pembrolizumab as Second-Line Therapy for Chinese Patients with Advanced or Metastatic Melanoma (KEYNOTE-151). Transl. Oncol. 2019, 12, 828–835. [Google Scholar] [CrossRef]
- Nathan, P.; Ascierto, P.A.; Haanen, J.; Espinosa, E.; Demidov, L.; Garbe, C.; Guida, M.; Lorigan, P.; Chiarion-Sileni, V.; Gogas, H.; et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: A single-arm, open-label, phase II study (CheckMate 172). Eur. J. Cancer 2019, 119, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Namikawa, K.; Yoshino, K.; Yoshikawa, S.; Uchi, H.; Goto, K.; Nakamura, Y.; Fukushima, S.; Kiniwa, Y.; Takenouchi, T.; et al. Anti-PD1 checkpoint inhibitor therapy in acral melanoma: A multicenter study of 193 Japanese patients. Ann. Oncol. 2020, 31, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, X.; Yang, Y.; Xu, W.; Tian, H.; Lian, B.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; et al. Apatinib combined with camrelizumab in advanced acral melanoma patients: An open-label, single-arm phase 2 trial. Eur. J. Cancer 2023, 182, 57–65. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Kiniwa, Y.; Kato, H.; Yamasaki, O.; Yoshikawa, S.; Maekawa, T.; Matsushita, S.; Takenouchi, T.; Inozume, T.; et al. Efficacy comparison between anti-PD-1 antibody monotherapy and anti-PD-1 plus anti-CTLA-4 combination therapy as first-line immunotherapy for advanced acral melanoma: A retrospective, multicenter study of 254 Japanese patients. Eur. J. Cancer 2022, 176, 78–87. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Hu, T.; Sun, W.; Xu, Y.; Qu, X.; Jin, Y.; Luo, Z.; Chen, Y. Combination of pembrolizumab plus temozolomide therapy in unresectable and advanced melanoma: A multicenter retrospective analysis in China. Ann. Transl. Med. 2021, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Lian, B.; Li, C. Camrelizumab Plus Apatinib and Temozolomide as First-Line Treatment in Patients with Advanced Acral Melanoma. JAMA Oncol. 2023, 9, 1099–1107. [Google Scholar] [CrossRef]
- Li, S.; Sheng, X.; Si, L.; Cui, C.; Kong, Y.; Mao, L.; Lian, B.; Tang, B.; Yan, X.; Wang, X.; et al. Outcomes and Predictive Factors of Isolated Limb Infusion for Patients with In-transit Melanoma in China. Ann. Surg. Oncol. 2018, 25, 885–893. [Google Scholar] [CrossRef]
- Franke, V.; Smeets, P.M.G.; van der Wal, J.E.; van Akkooi, A.C.J. Complete response to talimogene laherparepvec in a primary acral lentiginous melanoma. Melanoma Res. 2020, 30, 548–551. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Spencer, S.A.; Lydiatt, W. Mucosal Melanoma: A Clinically and Biologically Unique Disease Entity. J. Natl. Compr. Cancer Network. 2012, 10, 345–356. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Postow, M.A.; Hamid, O.; Carvajal, R.D. Mucosal melanoma: Pathogenesis, clinical behavior, and management. Curr. Oncol. Rep. 2012, 14, 441–448. [Google Scholar] [CrossRef]
- Nomura, M.; Oze, I.; Masuishi, T.; Yokota, T.; Satake, H.; Iwasawa, S.; Kato, K.; Andoh, M. Multicenter prospective phase II trial of nivolumab in patients with unresectable or metastatic mucosal melanoma. Int. J. Clin. Oncol. 2020, 25, 972–977. [Google Scholar] [CrossRef]
- Amin, M.; Edge, S.; Greene, F. Cancer Staging Manual, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Cui, C.; Lian, B.; Zhou, L.; Song, X.; Zhang, X.; Wu, D.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; et al. Multifactorial Analysis of Prognostic Factors and Survival Rates Among 706 Mucosal Melanoma Patients. Ann. Surg. Oncol. 2018, 25, 2184–2192. [Google Scholar] [CrossRef]
- Cui, C.; Lian, B.; Zhang, X.; Wu, D.; Li, K.; Si, L.; Yang, Y.; Tian, H.; Zhou, L.; Chi, Z.; et al. An Evidence-Based Staging System for Mucosal Melanoma: A Proposal. Ann. Surg. Oncol. 2022, 29, 5221–5234. [Google Scholar] [CrossRef]
- Newell, F.; Kong, Y.; Wilmott, J.S.; Johansson, P.A.; Ferguson, P.M.; Cui, C.; Li, Z.; Kazakoff, S.H.; Burke, H.; Dodds, T.J.; et al. Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets. Nat. Commun. 2019, 10, 3163. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Furney, S.J.; Turajlic, S.; Stamp, G.; Nohadani, M.; Carlisle, A.; Thomas, J.M.; Hayes, A.; Strauss, D.; Gore, M.; Oord, J.V.D.; et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J. Pathol. 2013, 230, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, J.; Guo, J.; Si, L.; Bai, X. Evolving treatment approaches to mucosal melanoma. Curr. Oncol. Rep. 2022, 24, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Nassar, K.W.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Biol. 2020, 61, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Schoenewolf, N.L.; Bull, C.; Belloni, B.; Holzmann, D.; Tonolla, S.; Lang, R.; Mihic-Probst, D.; Andres, C.; Dummer, R. Sinonasal, genital and acrolentiginous melanomas show distinct characteristics of KIT expression and mutations. Eur. J. Cancer 2012, 48, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.J.; Shia, J.; Hwu, W.J.; Busam, K.J.; Paty, P.B.; Guillem, J.G.; Coit, D.G.; Wong, W.D.; Weiser, M.R. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann. Surg. 2006, 244, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Pessaux, P.; Pocard, M.; Elias, D.; Duvillard, P.; Avril, M.; Zimmerman, P.; Lasser, P. Surgical management of primary anorectal melanoma. Br. J. Surg. 2004, 91, 1183–1187. [Google Scholar] [CrossRef]
- Nilsson, P.J.; Ragnarsson-Olding, B.K. Importance of clear resection margins in anorectal malignant melanoma. Br. J. Surg. 2010, 97, 98–103. [Google Scholar] [CrossRef]
- Oldenburg, M.S.; Price, D.L. The Utility of Sentinel Node Biopsy for Sinonasal Melanoma. J. Neurol. Surg. B Skull. Base 2017, 78, 425–429. [Google Scholar] [CrossRef]
- Olsha, O.; Mintz, A.; Gimon, Z.; Deutch, R.G.; Rabin, I.; Halevy, A.; Reissman, P. Anal melanoma in the era of sentinel lymph node mapping: A diagnostic and therapeutic challenge. Tech. Coloproctol. 2005, 9, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Kuk, D.; Shoushtari, A.N.; Barker, C.A.; Panageas, K.S.; Munhoz, R.R.; Momtaz, P.; Ariyan, C.E.; Brady, M.S.; Coit, D.G.; Bogatch, K.; et al. Prognosis of Mucosal, Uveal, Acral, Nonacral Cutaneous, and Unknown Primary Melanoma from the Time of First Metastasis. Oncologist 2016, 21, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Mignard, C.; Deschamps Huvier, A.; Gillibert, A.; Duval Modeste, A.B.; Dutriaux, C.; Khammari, A.; Avril, M.F.; Kramkimel, N.; Mortier, L.; Marcant, P.; et al. Efficacy of Immunotherapy in Patients with Metastatic Mucosal or Uveal Melanoma. J. Oncol. 2018, 2018, 1908065. [Google Scholar] [CrossRef]
- Postow, M.A.; Luke, J.J.; Bluth, M.J.; Ramaiya, N.; Panageas, K.S.; Lawrence, D.P.; Ibrahim, N.; Flaherty, K.T.; Sullivan, R.J.; Ott, P.A.; et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist 2013, 18, 726–732. [Google Scholar] [CrossRef]
- D’angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination with Ipilimumab in Patients with Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Moya-Plana, A.; Herrera Gómez, R.G.; Rossoni, C.; Dercle, L.; Ammari, S.; Girault, I.; Roy, S.; Scoazec, J.Y.; Vagner, S.; Janot, F.; et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanom. Cancer Immunol. Immunother. 2019, 68, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Namikawa, K.; Reijers, I.L.M.; Buchbinder, E.I.; Soon, J.A.; Zaremba, A. Single-agent anti-PD-1 or combined with ipilimumab in patients with mucosal melanoma: An international, retrospective, cohort study. Ann. Oncol. 2022, 33, 968–980. [Google Scholar] [CrossRef]
- Ho, J.; Mattei, J.; Tetzlaff, M.; Williams, M.D.; Davies, M.A.; Diab, A.; Oliva, I.C.G.; McQuade, J.; Patel, S.P.; Tawbi, H.; et al. Neoadjuvant checkpoint inhibitor immunotherapy for resectable mucosal melanoma. Front. Oncol. 2022, 12, 1001150. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Si, L.; Kong, Y.; Flaherty, K.T.; Xu, X.; Zhu, Y.; Corless, C.L.; Li, L.; Li, H.; Sheng, X.; et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J. Clin. Oncol. 2011, 29, 2904–2909. [Google Scholar] [CrossRef]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef]
- Wang, X.; Cui, C.; Lian, B.; Si, L.; Chi, Z.; Sheng, X.; Kong, Y.; Mao, L.; Bai, X.; Tang, B.; et al. Axitinib in Combination with Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients with Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J. Clin. Oncol. 2019, 37, 2987–2999. [Google Scholar]
- Yonekawa, Y.; Kim, I.K. Epidemiology and Management of Uveal Melanoma. Hematol. Oncol. Clin. N Am. 2012, 26, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: A relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lou, L.; Wang, Y.; Miao, Q.; Jin, K.; Chen, M.; Ye, J. Epidemiological Study of Uveal Melanoma from US Surveillance, Epidemiology, and End Results Program (2010–2015). J. Ophthalmol. 2020, 2020, 3614039. [Google Scholar] [CrossRef]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.O.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Rishi, P.; Koundanya, V.V.; Shields, C.L. Using risk factors for detection and prognostication of uveal melanoma. Ind. J. Ophthalmol. 2015, 63, 110–116. [Google Scholar] [CrossRef]
- Karlsson, J.; Nilsson, L.M.; Mitra, S.; Alsén, S.; Shelke, G.V.; Sah, V.R.; Forsberg, E.M.V.; Stierner, U.; All-Eriksson, C.; Einarsdottir, B.; et al. Molecular profiling of driver events in metastatic uveal melanoma. Nat. Commun. 2020, 11, 1894. [Google Scholar] [CrossRef]
- Broman, K.K.; Zager, J.S. Intra-arterial perfusion-based therapies for regionally metastatic cutaneous and uveal melanoma. Melanoma Manag. 2019, 6, MMT26. [Google Scholar] [CrossRef]
- Nichols, E.E.; Richmond, A.; Daniels, A.B. Tumor Characteristics, Genetics, Management, and the Risk of Metastasis in Uveal Melanoma. Semin. Ophthalmol. 2016, 31, 304–309. [Google Scholar] [CrossRef]
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Survival Rates in Patients After Treatment for Metastasis from Uveal Melanoma. JAMA Ophthalmol. 2018, 136, 981–986. [Google Scholar] [CrossRef]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L. The Coms randomized trial of iodine 125 brachytherapy for choroidal melanoma. Evid. -Based Eye Care 2004, 5, 164–166. [Google Scholar] [CrossRef]
- Ny, L.; Jespersen, H.; Karlsson, J.; Alsén, S.; Filges, S.; All-Eriksson, C.; Andersson, B.; Carneiro, A.; Helgadottir, H.; Levin, M.; et al. The PEMDAC phase 2 study of pembrolizumab and entinostat in patients with metastatic uveal melanoma. Nat. Commun. 2021, 12, 5155. [Google Scholar] [CrossRef] [PubMed]
- Meijer, T.S.; Burgmans, M.C.; de Leede, E.M.; de Geus-Oei, L.-F.; Boekestijn, H.J.M. Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Ocular Melanoma Metastases Confined to the Liver: A Prospective Phase II Study. Ann. Surg. Oncol. 2020, 28, 1130–1141. [Google Scholar] [CrossRef]
- Nathan, P.; Needham, A.; Corrie, P.G.; Danson, S.; Evans, J.; Ochsenreither, S.; Kumar, S.; Goodman, A.; Larkin, J.M.G.; Karydis, I.; et al. LBA73SELPAC: A 3 arm randomised phase II study of the MEK inhibitor selumetinib alone or in combination with paclitaxel (PT) in metastatic uveal melanoma (UM). Ann. Oncol. 2019, 30, v908–v910. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in Combination with Dacarbazine in Patients with Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Sosman, J.A.; Quevedo, J.F.; Milhem, M.M.; Joshua, A.M.; Kudchadkar, R.R.; Linette, G.P.; Gajewski, T.F.; Lutzky, J.; Lawson, D.H.; et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: A randomized clinical trial. JAMA 2014, 311, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Ben-Shabat, I.; Hansson, C.; Eilard, M.S.; Cahlin, C.; Rizell, M.; Lindnér, P.; Mattsson, J.; Bagge, R.O. Isolated hepatic perfusion as a treatment for liver metastases of uveal melanoma. J. Vis. Exp. 2015, 95, 52490. [Google Scholar]
- Huibers, A.; DePalo, D.K.; Perez, M.C.; Zager, J.S.; Olofsson Bagge, R. Isolated hyperthermic perfusions for cutaneous melanoma in-transit metastasis of the limb and uveal melanoma metastasis to the liver. Clin. Exp. Metastasis 2023. [Google Scholar] [CrossRef]
- Alexander, H.; Wen, D.; Chu, M.; Han, C.; Hadden, P.; Thomas, R.; Bartlett, A. Selective internal radiation therapy for hepatic metastases of uveal melanoma: A systematic review. Br. J. Radiol. 2022, 95, 20210200. [Google Scholar] [CrossRef] [PubMed]
- Tulokas, S.; Mäenpää, H.; Peltola, E.; Kivelä, T.; Vihinen, P.; Virta, A.; Mäkelä, S.; Kallio, R.; Hernberg, M. Selective internal radiation therapy (SIRT) as treatment for hepatic metastases of uveal melanoma: A Finnish nation-wide retrospective experience. Acta. Oncol. 2018, 57, 1373–1380. [Google Scholar] [CrossRef]
- Estler, A.; Artzner, C.; Bitzer, M.; Nikolaou, K.; Hoffmann, R.; Hepp, T.; Hagen, F.; Eigentler, T.; Forschner, A.; Grözinger, G. Efficacy and tolerability of chemosaturation in patients with hepatic metastases from uveal melanoma. Acta Radiol. 2022, 63, 577–585. [Google Scholar] [CrossRef]
- Tong, T.M.L.; Samim, M.; Kapiteijn, E.; Meijer, T.S.; Speetjens, F.M.; Brüning, R.; Schroeder, T.H.; El-Sanosy, S.; Maschke, H.; Wacker, F.K.; et al. Predictive Parameters in Patients Undergoing Percutaneous Hepatic Perfusion with Melphalan for Unresectable Liver Metastases from Uveal Melanoma: A Retrospective Pooled Analysis. Cardiovasc. Intervent. Radiol. 2022, 45, 1304–1313. [Google Scholar] [CrossRef]
- Artzner, C.; Mossakowski, O.; Hefferman, G.; Grosse, U.; Hoffmann, R.; Forschner, A.; Eigentler, T.; Syha, R.; Grözinger, G. Chemosaturation with percutaneous hepatic perfusion of melphalan for liver-dominant metastatic uveal melanoma: A single center experience. Cancer Imaging. 2019, 19, 31. [Google Scholar] [CrossRef]
- Brüning, R.; Tiede, M.; Schneider, M.; Wohlmuth, P.; Weilert, H.; Oldhafer, K.; Stang, A. Unresectable Hepatic Metastasis of Uveal Melanoma: Hepatic Chemosaturation with High-Dose Melphalan-Long-Term Overall Survival Negatively Correlates with Tumor Burden. Radiol. Res. Pract. 2020, 2020, 5672048. [Google Scholar] [CrossRef]
- Modi, S.; Gibson, T.; Vigneswaran, G.; Patel, S.; Wheater, M.; Karydis, I.; Gupta, S.; Takhar, A.; Pearce, N.; Ottensmeier, C.; et al. Chemosaturation with percutaneous hepatic perfusion of melphalan for metastatic uveal melanoma. Melanoma Res. 2022, 32, 103–111. [Google Scholar] [CrossRef]
- Dewald, C.L.A.; Warnke, M.M.; Bruning, R.; Schneider, M.A.; Wohlmuth, P.; Hinrichs, J.B. Percutaneous Hepatic Perfusion (PHP) with Melphalan in Liver-Dominant Metastatic Uveal Melanoma: The German Experience. Cancers 2021, 14, 118. [Google Scholar] [CrossRef]
- Karydis, I.; Gangi, A.; Wheater, M.J.; Choi, J.; Wilson, I.; Thomas, K.; Pearce, N.; Takhar, A.; Gupta, S.; Hardman, D.; et al. Percutaneous hepatic perfusion with melphalan in uveal melanoma: A safe and effective treatment modality in an orphan disease. J. Surg. Oncol. May 2018, 117, 1170–1178. [Google Scholar] [CrossRef]
- Bethlehem, M.S.; Katsarelias, D.; Olofsson Bagge, R. Meta-Analysis of Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver Metastases. Cancers 2021, 13, 4726. [Google Scholar] [CrossRef]
- Hughes, M.S.; Zager, J.; Faries, M.; Alexander, H.R.; Royal, R.E.; Wood, B.; Choi, J.; McCluskey, K.; Whitman, E.; Agarwala, S.; et al. Results of a Randomized Controlled Multicenter Phase III Trial of Percutaneous Hepatic Perfusion Compared with Best Available Care for Patients with Melanoma Liver Metastases. Ann. Surg. Oncol. 2016, 23, 1309–1319. [Google Scholar] [CrossRef]
- Zager, J.S.; Orloff, M.M.; Ferrucci, P.F.; Glazer, E.S.; Ejaz, A.; Richtig, E.; Ochsenreither, S.; Lowe, M.C.; Reddy, S.A.; Beasley, G.; et al. FOCUS phase 3 trial results: Percutaneous hepatic perfusion (PHP) with melphalan for patients with ocular melanoma liver metastases (PHP-OCM-301/301A). J. Clin. Oncol. 2022, 40 (16 Suppl.), 9510. [Google Scholar] [CrossRef]
- Kolb, M.; Forschner, A.; Artzner, C.; Grözinger, G.; Said, I.; Dittmann, H.; Seith, F. Selective Internal Radiotherapy (SIRT) and Chemosaturation Percutaneous Hepatic Perfusion (CS-PHP) for Metastasized Uveal Melanoma: A Retrospective Comparative Study. Cancers 2023, 15, 4942. [Google Scholar] [CrossRef]
- DeWane, M.E.; Kelsey, A.; Oliviero, M.; Rabinovitz, H.; Grant-Kels, J.M. Melanoma on chronically sun-damaged skin: Lentigo maligna and desmoplastic melanoma. J. Am. Acad. Dermatol. 2019, 81, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, N.G.; Han, D. Desmoplastic melanoma. J. Surg. Oncol. 2019, 119, 208–215. [Google Scholar] [CrossRef]
- Busam, K.J. Cutaneous desmoplastic melanoma. Adv. Anat. Pathol. 2005, 12, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, G.; Zhao, X.; Rao, N.G.; Messina, J.L.; Marzban, S.S.; Sarnaik, A.A.; Cruse, C.W.; Sondak, V.K.; Zager, J.S. Clinicopathologic predictors of survival in patients with desmoplastic melanoma. PLoS ONE 2015, 10, e0119716. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.; Caudell, J.J.; Han, D.; Zager, J.S.; Yu, D.; Cruse, C.W.; Marzban, S.S.; Messina, J.L.; Trotti, A.M.; Sondak, V.K.; et al. Radiotherapy influences local control in patients with desmoplastic melanoma. Cancer 2014, 120, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Williams, G.; Gyorki, D.; Kelly, J.; Stretch, J.; Varey, A.; Hong, A.; Scolyer, R.; Thompson, J. Desmoplastic melanoma: A review of its pathology and clinical behaviour, and of management recommendations in published guidelines. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1290–1298. [Google Scholar] [CrossRef]
- Maurichi, A.; Miceli, R.; Camerini, T.; Contiero, P.; Patuzzo, R.; Tragni, G.; Crippa, F.; Romanidis, K.; Ruggeri, R.; Carbone, A.; et al. Pure desmoplastic melanoma: A melanoma with distinctive clinical behavior. Ann. Surg. 2010, 252, 1052–1057. [Google Scholar] [CrossRef]
- Hawkins, W.G.; Busam, K.J.; Ben-Porat, L.; Panageas, K.S.; Coit, D.G.; Gyorki, D.E.; Linehan, D.C.; Brady, M.S. Desmoplastic melanoma: A pathologically and clinically distinct form of cutaneous melanoma. Ann. Surg. Oncol. 2005, 12, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Nepote, A.; Avallone, G.; Ribero, S.; Cavallo, F.; Roccuzzo, G.; Mastorino, L.; Conforti, C.; Paruzzo, L.; Poletto, S.; Schianca, F.C.; et al. Current Controversis and Challenges on BRAF V600K-Mutant Cutaneous Melanoma. J. Clin. Med. 2022, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Hruby, G.; Scolyer, R.A.; Murali, R.; Hong, A.; FitzGerald, P.; Pham, T.T.; Quinn, M.J.; Thompson, J.F. Desmoplastic neurotropic melanoma: A clinicopathologic analysis of 128 cases. Cancer 2008, 113, 2770–2778. [Google Scholar] [CrossRef] [PubMed]
- Guadagnolo, B.A.; Prieto, V.; Weber, R.; Ross, M.I.; Zagars, G.K. The role of adjuvant radiotherapy in the local management of desmoplastic melanoma. Cancer 2014, 120, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Ross, M.I.; Prieto, V.G.; Ballo, M.T.; Johnson, M.M.; Mansfield, P.F.; Lee, J.E.; Cormier, J.N.; Gershenwald, J.E. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer 2006, 106, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zager, J.S.; Yu, D.; Zhao, X.; Walls, B.; Marzban, S.S.; Rao, N.G.; Sondak, V.K.; Messina, J.L. Desmoplastic melanoma: Is there a role for sentinel lymph node biopsy? Ann. Surg. Oncol. 2013, 20, 2345–2351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laeijendecker, A.E.; Sharouni, M.E.; Sigurdsson, V.; van Diest, P.J. Desmoplastic melanoma: The role of pure and mixed subtype in sentinel lymph node biopsy. Cancer Med. 2020, 9, 671–677. [Google Scholar] [CrossRef]
- Eroglu, Z.; Zaretsky, J.M.; Hu-Lieskovan, S.; Kim, D.W.; Algazi, A.; Johnson, D.B.; Liniker, E.; Kong, B.; Munhoz, R.; Rapisuwon, S.; et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature 2018, 553, 347–350. [Google Scholar] [CrossRef]
- Farshidfar, F.; Rhrissorrakrai, K.; Levovitz, C.; Peng, C.; Knight, J.; Bacchiocchi, A.; Su, J.; Yin, M.; Sznol, M.; Ariyan, S.; et al. Integrative molecular and clinical profiling of acral melanoma links focal amplification of 22q11.21 to metastasis. Nat. Commun. 2022, 13, 898. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shannon, A.B.; Zager, J.S.; Perez, M.C. Clinical Characteristics and Special Considerations in the Management of Rare Melanoma Subtypes. Cancers 2024, 16, 2395. https://doi.org/10.3390/cancers16132395
Shannon AB, Zager JS, Perez MC. Clinical Characteristics and Special Considerations in the Management of Rare Melanoma Subtypes. Cancers. 2024; 16(13):2395. https://doi.org/10.3390/cancers16132395
Chicago/Turabian StyleShannon, Adrienne B., Jonathan S. Zager, and Matthew C. Perez. 2024. "Clinical Characteristics and Special Considerations in the Management of Rare Melanoma Subtypes" Cancers 16, no. 13: 2395. https://doi.org/10.3390/cancers16132395
APA StyleShannon, A. B., Zager, J. S., & Perez, M. C. (2024). Clinical Characteristics and Special Considerations in the Management of Rare Melanoma Subtypes. Cancers, 16(13), 2395. https://doi.org/10.3390/cancers16132395




