Interest of Integrated Whole-Body PET/MR Imaging in Gastroenteropancreatic Neuroendocrine Neoplasms: A Retro-Prospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
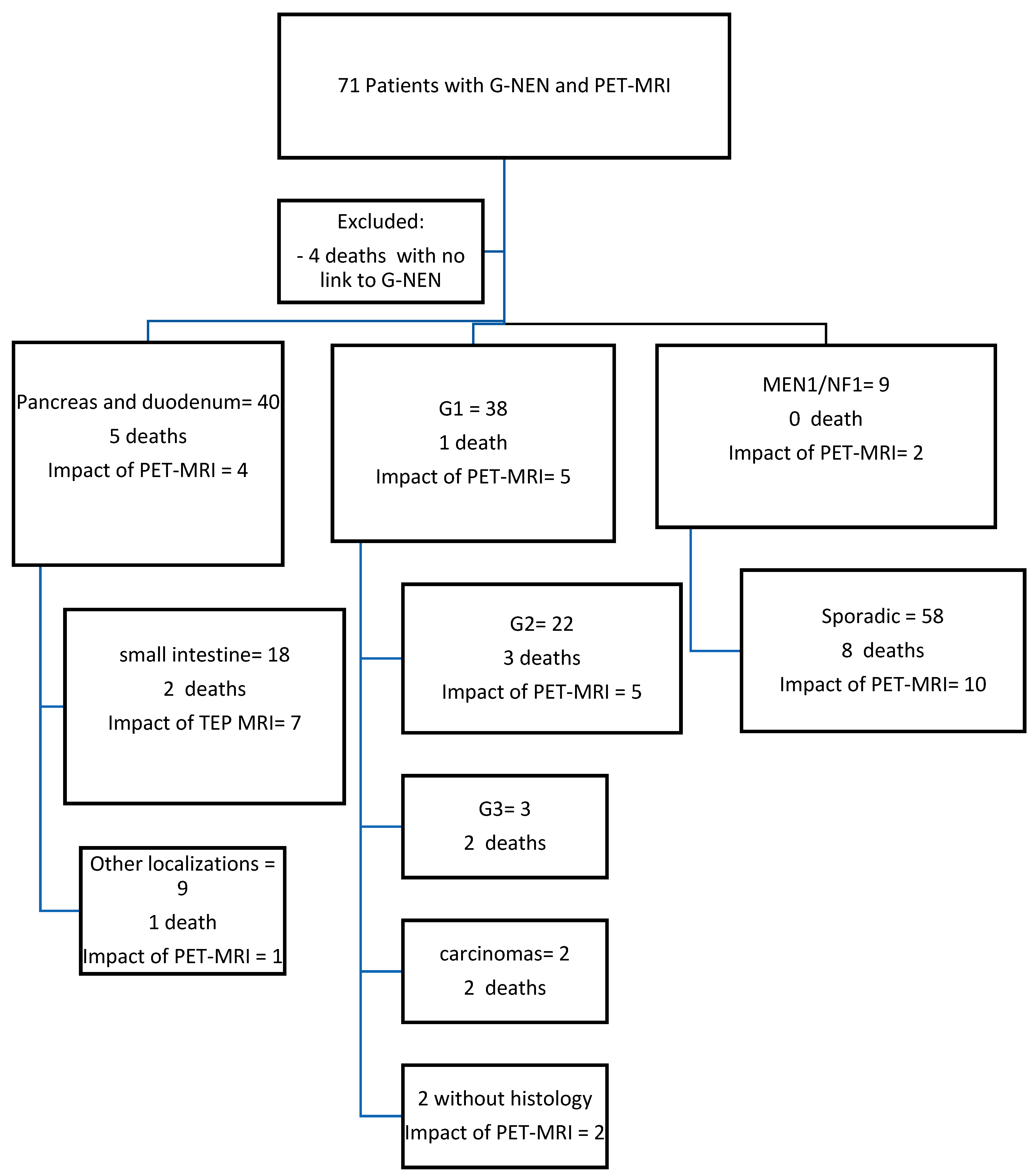
2.1. Population and Study Design
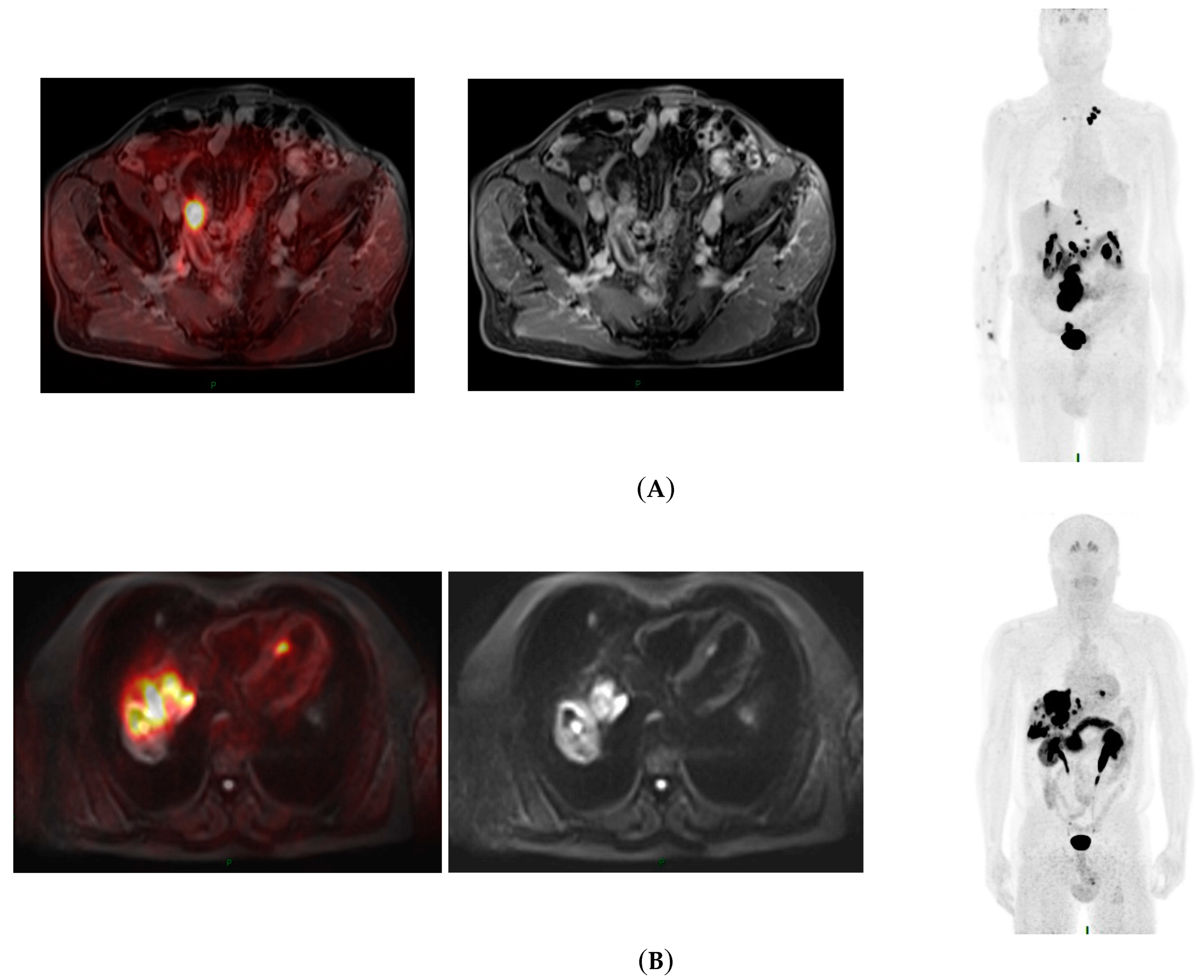
2.2. PET-MRI Acquisition Protocol and Data Collection
2.3. Endpoints and Statistical Analysis
3. Results
3.1. Study Participants
3.2. Impact of PET-MRI at Baseline
3.3. Impact of PET-MRI during the Follow-Up
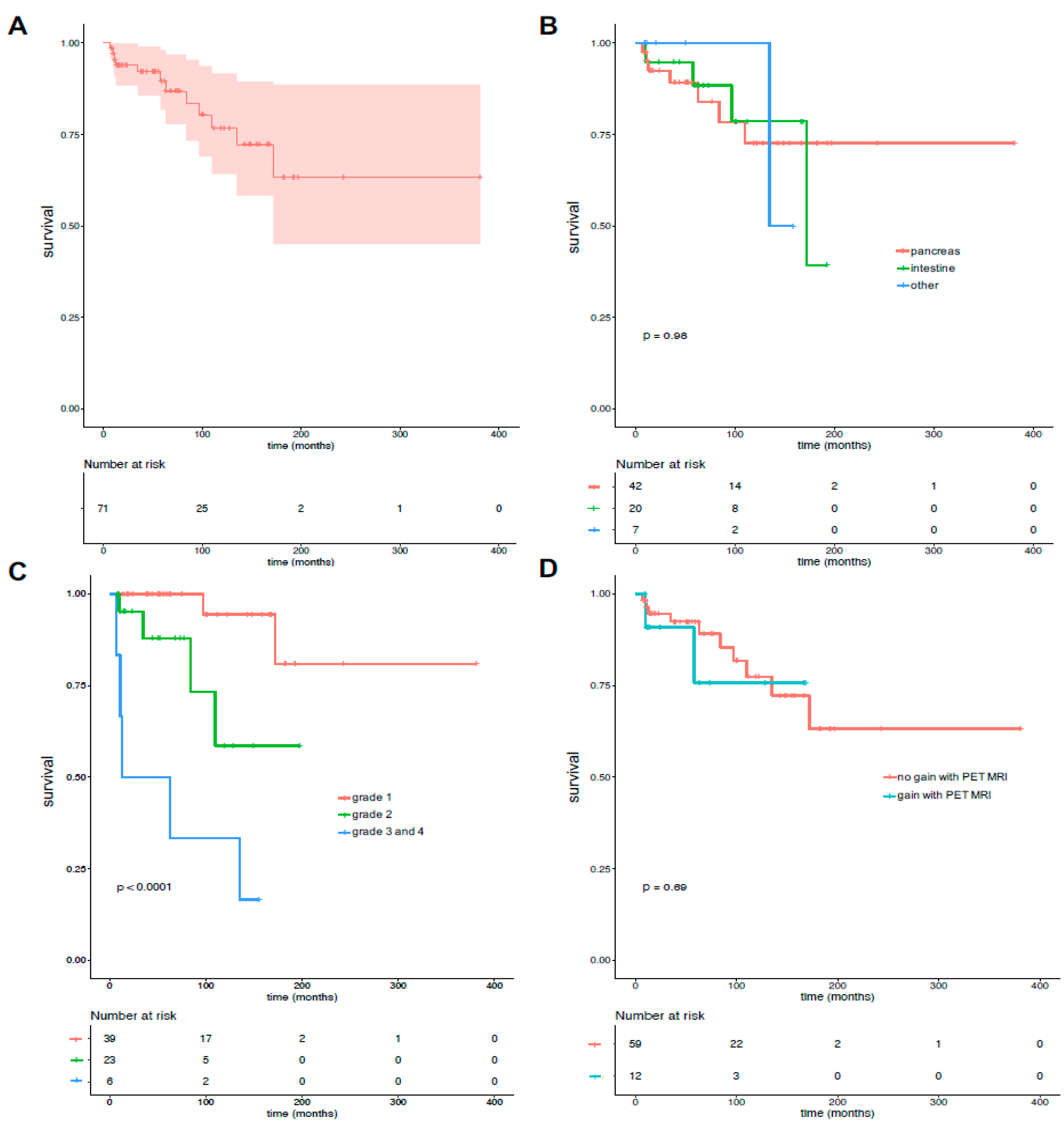
3.4. Overall Survival and Factors Associated with Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Patients | Age | Sex F:1/M:0 | Primary Localization | Meta | Associated Cancer | Genetic Syndrome | Histo | Histology Grading | Ki 67% | Follow-Up Duration (Month) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | 1 | pancreas | 0 | 0 | MEN1 | 1 | G1 | 1% | 183 |
| 2 | 67 | 0 | duodenum | 1 | 0 | 1 | G3 | 60% | 11 | |
| 3 | 80 | 0 | duodeno-pancreas | 0 | 1 | 1 | G2 | 10–15% | 11 | |
| 4 | 62 | 0 | small intestine | 1 | 0 | 1 | G2 | 5–10% | 9 | |
| 5 | 31 | 1 | pancreas | 0 | 0 | MEN1 | 1 | G1 | ≤1% | 14 |
| 6 | 81 | 0 | pancreas | 1 | 1 | 1 | G1 | NM | 182 | |
| 7 | 62 | 1 | appendix | 0 | 1 | 1 | G2 | 5% | 11 | |
| 8 | 75 | 1 | pancreas | 1 | 0 | 1 | G2 | 5–20% | 128 | |
| 9 | 32 | 1 | duodenum | 1 | 0 | NF1 | 1 | G2 | 3% | 52 |
| 10 | 87 | 1 | small intestine | 1 | 0 | 1 | G1 | <2% | 101 | |
| 11 | 55 | 1 | pancreas | 1 | 0 | 1 | G2 | 6% | 44 | |
| 12 | 68 | 0 | small intestine | 1 | 0 | 1 | G1 | ND | 62 | |
| 13 | 72 | 0 | duodenum | 1 | 1 | 1 | G1 | <1% | 39 | |
| 14 | 76 | 0 | small intestine | 1 | 0 | 1 | G1 | NM | 192 | |
| 15 | 65 | 0 | small intestine | 1 | 1 | 1 | G1 | <1% | 172 | |
| 16 | 92 | 0 | small intestine | 1 | 0 | 0 | NM | 58 | ||
| 17 | 49 | 1 | ampulla | 0 | 0 | NF1 | 1 | G1 | ND | 243 |
| 18 | 74 | 0 | small intestine | 1 | 1 | 1 | G1 | 0–1% | 168 | |
| 19 | 74 | 0 | small intestine | 0 | 1 | 0 | NM | 101 | ||
| 20 | 59 | 0 | pancreas | 1 | 0 | 1 | G2 | 15% | 35 | |
| 21 | 57 | 1 | pancreas | 1 | 0 | 1 | 10% | 10 | ||
| 22 | 37 | 1 | pancreas | 0 | 0 | 1 | G2 | 3–4% | 119 | |
| 23 | 64 | 0 | small intestine | 1 | 0 | 1 | G1 | <2% | 100 | |
| 24 | 62 | 0 | œsophagus | 1 | 0 | 1 | CNE | 100% | 135 | |
| 25 | 43 | 0 | small intestine | 1 | 0 | 1 | G2 | 5% | 73 | |
| 26 | 49 | 1 | pancreas | 0 | 1 | 1 | G1 | 1% | 53 | |
| 27 | 77 | 0 | pancreas | 1 | 0 | 1 | G3 | 30–40% | 7 | |
| 8 | 31 | 0 | pancreas | 1 | 0 | 1 | G1 | <1% | 40 | |
| 29 | 48 | 0 | stomach | 0 | 0 | 1 | G2 | 2.10% | 20 | |
| 30 | 63 | 1 | pancreas | 0 | 0 | 1 | G1 | <1% | 14 | |
| 31 | 64 | 1 | pancreas | 1 | 0 | 1 | G2 | 10–15% | 56 | |
| 32 | 59 | 1 | pancreas | 1 | 0 | 1 | G1 | <2% | 18 | |
| 33 | 72 | 0 | stomach | 0 | 0 | 1 | G1 | 1% | 9 | |
| 34 | 47 | 0 | pancreas | 1 | 0 | NF1 | 1 | G2 | 15% | 149 |
| 35 | 80 | 1 | pancreas | 1 | 0 | 1 | G2 | 4–5% | 84 | |
| 36 | 49 | 1 | small intestine | 0 | 0 | 1 | G2 | 16% | 23 | |
| 37 | 52 | 1 | small intestine | 1 | 0 | 1 | G1 | <2% | 63 | |
| 38 | 71 | 1 | pancreas | 1 | 0 | 1 | G1 | <1% | 50 | |
| 39 | 65 | 1 | pancreas | 1 | 1 | 1 | G2 | 3% | 14 | |
| 40 | 49 | 0 | stomach | 1 | 0 | 1 | G1 | 2% | 158 | |
| 41 | 37 | 0 | pancreas | 1 | 0 | 1 | G2 | 15% | 16 | |
| 42 | 44 | 1 | duodenum | 1 | 0 | 1 | G1 | 1% | 122 | |
| 43 | 51 | 1 | small intestine | 1 | 0 | NF1 | 1 | G2 | 2–3% | 44 |
| 44 | 44 | 1 | pancreas | 0 | 1 | MEN1 | 1 | G1 | <1% | 51 |
| 45 | 59 | 0 | small intestine | 1 | 0 | 1 | G1 | 1% | 59 | |
| 46 | 75 | 0 | pancreas | 0 | 0 | 1 | G3 | >20% | 155 | |
| 47 | 57 | 1 | pancreas | 0 | 0 | 1 | G2 | 10% | 8 | |
| 48 | 65 | 0 | pancreas | 0 | 0 | 1 | G1 | <2% | 52 | |
| 49 | 54 | 1 | pancreas | 1 | 1 | 1 | CNE | 75% | 13 | |
| 50 | 80 | 1 | pancreas + small intestine | 1 | 0 | 1 | G1 | <2% | 148 | |
| 51 | 29 | 1 | pancreas | 0 | 0 | 0 | ND | ND | 12 | |
| 52 | 55 | 0 | small intestine | 1 | 1 | 1 | G2 | 5–8% | 97 | |
| 53 | 61 | 1 | pancreas | 1 | 0 | 1 | G1 | ND | 167 | |
| 54 | 49 | 0 | pancreas | 1 | 0 | NF1 | 1 | G1 | 2% | 193 |
| 55 | 69 | 1 | pancreas | 1 | 0 | 1 | G2 | 8% | 52 | |
| 56 | 56 | 1 | pancreas | 1 | 0 | 1 | G2 | 18% | 15 | |
| 57 | 71 | 1 | rectum | 0 | 0 | 1 | G1 | <1% | 9 | |
| 58 | 68 | 0 | small intetsine | 1 | 1 | 1 | G2 | <10% et <3% | 166 | |
| 59 | 82 | 1 | small intestine | 1 | 1 | 1 | G1 | ND | 38 | |
| 60 | 69 | 0 | pancreas | 1 | 1 | 1 | CNE | 80% | 63 | |
| 61 | 65 | 0 | small intestine | 1 | 0 | 1 | G1 | <2% | 112 | |
| 62 | 67 | 1 | pancreas | 1 | 0 | 1 | G2 | 15 | 197 | |
| 63 | 43 | 1 | rectum | 1 | 0 | 1 | G2 | 15% | 50 | |
| 64 | 76 | 0 | pancreas | 0 | 0 | 1 | G1 | ND | 143 | |
| 65 | 56 | 1 | pancreas | 1 | 0 | 1 | G2 | 15 | 77 | |
| 66 | 61 | 0 | pancreas + small intestine | 1 | 1 | 1 | G1 | <2% ET <1% | 75 | |
| 67 | 51 | 0 | pancreas | 0 | 0 | MEN1 | 1 | G1 | <2% | 24 |
| 68 | 88 | 0 | pancreas | 1 | 1 | 1 | G2 | 3–5% | 110 | |
| 69 | 71 | 0 | small intestine | 1 | 0 | 1 | G2 | 5–10% | 68 | |
| 70 | 64 | 1 | duodeno-pancreas | 0 | 1 | 1 | G1 | NM | 381 | |
| 71 | 85 | 0 | small intestine | 1 | 0 | 1 | G2 | 4–5% | 10 |
References
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal Neuroendocrine Neoplasms (GI-NENs): Hot Topics in Morphological, Functional, and Prognostic Imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, C.; Jiang, L.; Zhou, X.; Dai, X.; Song, Y.; Li, Y. Incidence and Risk Factors of Gastrointestinal Neuroendocrine Neoplasm Metastasis in Liver, Lung, Bone, and Brain: A Population-Based Study. Cancer Med. 2019, 8, 7288–7298. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Choi, J.-H.; Seo, D.-W.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasound for the Guidance and Monitoring of Endoscopic Radiofrequency Ablation. Gut Liver 2020, 14, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on Molecular Imaging and Theranostics in Neuroendocrine Neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Galgano, S.J.; Sharbidre, K.; Morgan, D.E. Multimodality Imaging of Neuroendocrine Tumors. Radiol. Clin. N. Am. 2020, 58, 1147–1159. [Google Scholar] [CrossRef]
- Kirchner, J.; Deuschl, C.; Grueneisen, J.; Herrmann, K.; Forsting, M.; Heusch, P.; Antoch, G.; Umutlu, L. 18F-FDG PET/MRI in Patients Suffering from Lymphoma: How Much MRI Information Is Really Needed? Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Kitajima, K.; Toriihara, A.; Nakajo, M.; Hirata, K. Recent Topics of the Clinical Utility of PET/MRI in Oncology and Neuroscience. Ann. Nucl. Med. 2022, 36, 798–803. [Google Scholar] [CrossRef] [PubMed]
- de Mestier, L.; Lepage, C.; Baudin, E.; Coriat, R.; Courbon, F.; Couvelard, A.; Do Cao, C.; Frampas, E.; Gaujoux, S.; Gincul, R.; et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 2020, 52, 473–492. [Google Scholar] [CrossRef]
- Toulouse, E.; Masseguin, C.; Lafont, B.; McGurk, G.; Harbonn, A.; Roberts, J.A.; Granier, S.; Dupeyron, A.; Bazin, J.E. French Legal Approach to Clinical Research. Anaesth. Crit. Care Pain. Med. 2018, 37, 607–614. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. WHO Classification of Tumours Editorial Board The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, P.; Shi, X.; Zhao, A.; Zhang, L.; Zhou, L. Clinicopathological Features and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms in a Chinese Population: A Large, Retrospective Single-Centre Study. BMC Endocr. Disord. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, Y.; Wang, Y.; Lin, H.; Lin, F.; Zhuang, Q.; Lin, X.; Wu, J. Prognostic Nomogram Based on the Metastatic Lymph Node Ratio for Gastric Neuroendocrine Tumour: SEER Database Analysis. ESMO Open 2020, 5, e000632. [Google Scholar] [CrossRef] [PubMed]
- Beiderwellen, K.; Geraldo, L.; Ruhlmann, V.; Heusch, P.; Gomez, B.; Nensa, F.; Umutlu, L.; Lauenstein, T.C. Accuracy of [18F]FDG PET/MRI for the Detection of Liver Metastases. PLoS ONE 2015, 10, e0137285. [Google Scholar] [CrossRef] [PubMed]
- Schreiter, N.F.; Nogami, M.; Steffen, I.; Pape, U.-F.; Hamm, B.; Brenner, W.; Röttgen, R. Evaluation of the Potential of PET-MRI Fusion for Detection of Liver Metastases in Patients with Neuroendocrine Tumours. Eur. Radiol. 2012, 22, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-L.; Kim, J.S.; Lee, J.H.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Utility of Combined (18)F-Fluorodeoxyglucose-Positron Emission Tomography and Computed Tomography in Patients with Cervical Metastases from Unknown Primary Tumors. Oral. Oncol. 2009, 45, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Becker, A.S.; Do, R.K.G.; Schöder, H.; Hricak, H.; Alberto Vargas, H. Impact of 18F-Fluorodeoxyglucose Positron Emission Tomography on Management of Cancer of Unknown Primary: Systematic Review and Meta-Analysis. Eur. J. Cancer 2021, 159, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, L.M.; Deuschl, C.; Beiderwellen, K.; Ruhlmann, V.; Poeppel, T.D.; Heusch, P.; Lahner, H.; Führer, D.; Bockisch, A.; Herrmann, K.; et al. Evaluation of 68Ga-DOTATOC PET/MRI for Whole-Body Staging of Neuroendocrine Tumours in Comparison with 68Ga-DOTATOC PET/CT. Eur. Radiol. 2017, 27, 4091–4099. [Google Scholar] [CrossRef]
- Beiderwellen, K.J.; Poeppel, T.D.; Hartung-Knemeyer, V.; Buchbender, C.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Simultaneous 68Ga-DOTATOC PET/MRI in Patients with Gastroenteropancreatic Neuroendocrine Tumors: Initial Results. Investig. Radiol. 2013, 48, 273–279. [Google Scholar] [CrossRef]
- Erlic, Z.; Ploeckinger, U.; Cascon, A.; Hoffmann, M.M.; von Duecker, L.; Winter, A.; Kammel, G.; Bacher, J.; Sullivan, M.; Isermann, B.; et al. Systematic Comparison of Sporadic and Syndromic Pancreatic Islet Cell Tumors. Endocr. Relat. Cancer 2010, 17, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.; White, B.E.; Rous, B.; Wong, K.; Bouvier Ellis, C.; Srirajaskanthan, R.; Van Hemelrijck, M.; Ramage, J.K. Second Primary Malignancies in Patients with a Neuroendocrine Neoplasm in England. Neuroendocrinology 2023, 113, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Ci, X.; Choi, S.Y.C.; Crea, F.; Lin, D.; Wang, Y. Molecular Events in Neuroendocrine Prostate Cancer Development. Nat. Rev. Urol. 2021, 18, 581–596. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N = 71 |
|---|---|
| Age, years (Extremes) | 61 (31–92) |
| Female gender, n (%) | 35 (49.3) |
| Primary location, n (%) | |
| Pancreas | 42 (59.15) |
| Small intestine | 20 (28.16) |
| Double location | 2 (2.81) |
| Others | 7 (9.85) |
| Metastasis, n (%) | |
| Yes | 50 (70) |
| No | 21 (30) |
| Histology grade, n (%) | |
| G1 | 39 (54.92) |
| G2 | 23 (32.39) |
| G3 | 3 (4.22) |
| NEC | 3 (4.22) |
| unknown | 3 (4.22) |
| Functional syndrome, n (%) | |
| Yes | 10 (14.08) |
| - Carcinoid syndrome | 5 (7.04) |
| - Gastrinoma | 3 (4.22) |
| - Insulinoma | 2 (2.81) |
| No | 61 (85.91) |
| Genetic syndrome association, n (%) | |
| Yes | 9 (12.67) |
| - MEN1 | 6 (8.45) |
| - NF1 | 5 (7.04) |
| No | 62 (87.32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abid, C.; Tannoury, J.; Uzzan, M.; Reizine, E.; Mulé, S.; Chalaye, J.; Luciani, A.; Itti, E.; Sobhani, I. Interest of Integrated Whole-Body PET/MR Imaging in Gastroenteropancreatic Neuroendocrine Neoplasms: A Retro-Prospective Study. Cancers 2024, 16, 2372. https://doi.org/10.3390/cancers16132372
Abid C, Tannoury J, Uzzan M, Reizine E, Mulé S, Chalaye J, Luciani A, Itti E, Sobhani I. Interest of Integrated Whole-Body PET/MR Imaging in Gastroenteropancreatic Neuroendocrine Neoplasms: A Retro-Prospective Study. Cancers. 2024; 16(13):2372. https://doi.org/10.3390/cancers16132372
Chicago/Turabian StyleAbid, Camelia, Jenny Tannoury, Mathieu Uzzan, Edouard Reizine, Sébastien Mulé, Julia Chalaye, Alain Luciani, Emmanuel Itti, and Iradj Sobhani. 2024. "Interest of Integrated Whole-Body PET/MR Imaging in Gastroenteropancreatic Neuroendocrine Neoplasms: A Retro-Prospective Study" Cancers 16, no. 13: 2372. https://doi.org/10.3390/cancers16132372
APA StyleAbid, C., Tannoury, J., Uzzan, M., Reizine, E., Mulé, S., Chalaye, J., Luciani, A., Itti, E., & Sobhani, I. (2024). Interest of Integrated Whole-Body PET/MR Imaging in Gastroenteropancreatic Neuroendocrine Neoplasms: A Retro-Prospective Study. Cancers, 16(13), 2372. https://doi.org/10.3390/cancers16132372







