Primary Tumor Characteristics as Biomarkers of Immunotherapy Response in Advanced Melanoma: A Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Overview
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Patient and Primary Tumor Characteristics
3.2. Univariate Analysis
3.2.1. Response to ICI
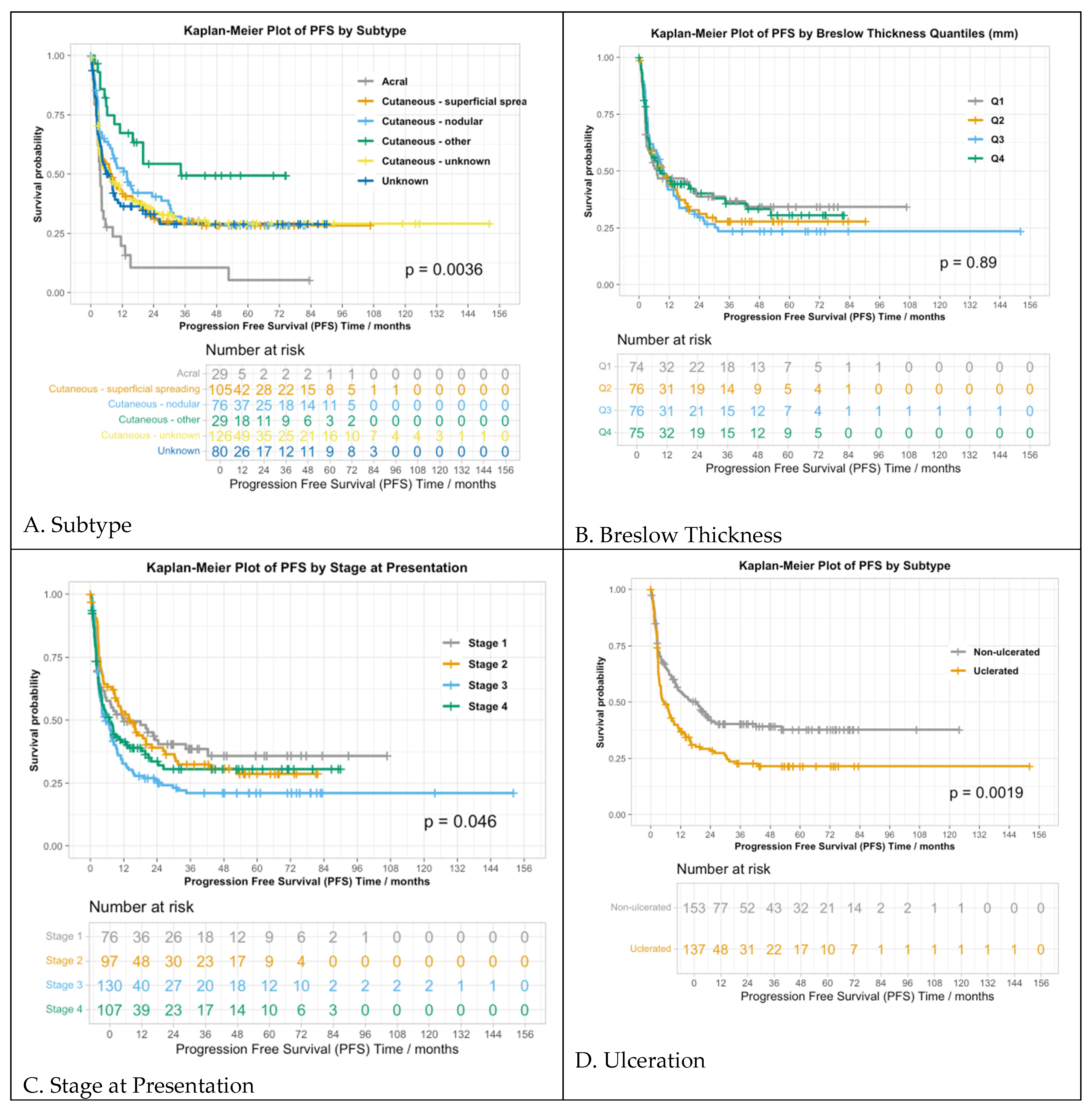
3.2.2. Progression-Free Survival
3.2.3. Overall Survival
3.3. Multivariable Analysis
3.3.1. Response to ICI
3.3.2. Progression-Free Survival
3.3.3. Overall Survival
| Response to Anti-PD-1 | |||
|---|---|---|---|
| Variable | Odds ratio | 95% CI | p-value |
| SSM subtype | 6.50 | 1.8–23.45 | 0.004 |
| Nodular type | 10.48 | 2.850–38.50 | <0.001 |
| Other cutaneous type | 8.97 | 2.13–37.71 | 0.003 |
| Unknown subtype | 7.36 | 2.06–26.32 | 0.002 |
| Unknown primary | 7.07 | 1.90–26.33 | 0.004 |
| 1–2 positive sentinel nodes | 0.50 | 0.30–0.83 | 0.008 |
| 3+ positive sentinel nodes | 0.68 | 0.32–1.41 | 0.300 |
| BRAF mutation | 0.38 | 0.24–0.61 | 0.001 |
| PFS | |||
| Variable | Odds ratio | 95% CI | p-value |
| SSM subtype | 0.58 | 0.36–0.94 | 0.026 |
| Nodular type | 0.53 | 0.33–0.86 | 0.010 |
| Other cutaneous type | 0.31 | 0.15–0.61 | <0.001 |
| Unknown subtype | 0.55 | 0.34–0.88 | 0.013 |
| Unknown primary | 0.50 | 0.28–0.88 | 0.016 |
| Ulceration | 1.24 | 0.92–1.66 | 0.162 |
| Mitoses | 0.99 | 0.97–1.01 | 0.202 |
| Stage II at presentation | 1.14 | 0.73–1.78 | 0.561 |
| Stage III at presentation | 1.42 | 0.93–2.16 | 0.103 |
| Stage IV M1a/b at presentation | 0.94 | 0.53–1.68 | 0.842 |
| Stage IV M1c/d at presentation | 1.79 | 1.07–3.00 | 0.027 |
| Male gender | 0.75 | 0.58–0.96 | 0.022 |
| Prior treatment | 1.04 | 0.91–1.20 | 0.542 |
| LDH > ULN | 0.92 | 0.79–1.06 | 0.236 |
| BRAF mutation | 1.64 | 1.27–2.11 | <0.001 |
| OS | |||
| Variable | Odds ratio | 95% CI | p-value |
| SSM subtype | 0.58 | 0.35–0.95 | 0.029 |
| Nodular type | 0.65 | 0.39–1.09 | 0.105 |
| Other cutaneous type | 0.46 | 0.24–0.90 | 0.023 |
| Unknown subtype | 0.50 | 0.31–0.83 | 0.008 |
| Unknown primary | 0.71 | 0.42–1.19 | 0.196 |
| Age | 1.01 | 1.00–1.02 | <0.001 |
| Prior treatment | 1.14 | 1.02–1.27 | 0.015 |
| LDH > ULN | 1.04 | 0.93–1.16 | 0.474 |
| BRAF mutation | 1.38 | 1.04–1.84 | 0.028 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mangin, M.-A.; Boespflug, A.; Maucort Boulch, D.; Vacheron, C.-H.; Carpentier, I.; Thomas, L.; Dalle, S. Decreased Survival in Patients Treated by Chemotherapy after Targeted Therapy Compared to Immunotherapy in Metastatic Melanoma. Cancer Med. 2021, 10, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Giubellino, A. The Current State of Treatment and Future Directions in Cutaneous Malignant Melanoma. Biomedicines 2022, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Patrinely, J.R.; Baker, L.X.; Davis, E.J.; Song, H.; Ye, F.; Johnson, D.B. Outcomes after Progression of Disease with Anti-PD-1/PD-L1 Therapy for Patients with Advanced Melanoma. Cancer 2020, 126, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Eroglu, Z.; Ozgun, A.; Leger, P.D.; Zhao, S.; Ye, F.; Luke, J.J.; Joseph, R.W.; Haq, R.; Ott, P.A.; et al. Clinical Features of Acquired Resistance to Anti-PD-1 Therapy in Advanced Melanoma. Cancer Immunol. Res. 2017, 5, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive Biomarkers for Cancer Immunotherapy with Immune Checkpoint Inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Zappasodi, R. A Decade of Checkpoint Blockade Immunotherapy in Melanoma: Understanding the Molecular Basis for Immune Sensitivity and Resistance. Nat. Immunol. 2022, 23, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.C.; Luke, J.J. Rapidly Evolving Pre- and Post-Surgical Systemic Treatment of Melanoma. Am. J. Clin. Dermatol. 2024, 25, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Kött, J.; Hoehne, I.L.; Heidrich, I.; Zimmermann, N.; Reese, K.-L.; Zell, T.; Geidel, G.; Rünger, A.; Schneider, S.W.; Pantel, K.; et al. High Serum Levels of CCL20 Are Associated with Recurrence and Unfavorable Overall Survival in Advanced Melanoma Patients Receiving Immunotherapy. Cancers 2024, 16, 1737. [Google Scholar] [CrossRef] [PubMed]
- Leek, L.V.M.; Notohardjo, J.C.L.; de Joode, K.; Velker, E.L.; Haanen, J.B.A.G.; Suijkerbuijk, K.P.M.; Aarts, M.J.B.; de Groot, J.W.B.; Kapiteijn, E.; van den Berkmortel, F.W.P.J.; et al. Multi-Omic Analysis Identifies Hypoalbuminemia as Independent Biomarker of Poor Outcome upon PD-1 Blockade in Metastatic Melanoma. Sci. Rep. 2024, 14, 11244. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.L.; Hunger, R.E.; Seyed Jafari, S.M. Eosinophilic Granulocytes as a Potential Prognostic Marker for Cancer Progression and Therapeutic Response in Malignant Melanoma. Front. Oncol. 2024, 14, 1366081. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chen, B.; Jiang, J.; Chen, Y.; Chen, Y.; Wang, H. Identification of a Pyroptosis-based Model for Predicting Clinical Outcomes from Immunotherapy in Patients with Metastatic Melanoma. Cancer Med. 2022, 12, 4921–4937. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Ma, M.W.; Fleming, N.H.; Lackaye, D.J.; Hernando, E.; Osman, I.; Shao, Y. Clinicopathological Characteristics at Primary Melanoma Diagnosis as Risk Factors for Brain Metastasis. Melanoma Res. 2013, 23, 461–467. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russo, D.; Dalle, S.; Dereure, O.; Mortier, L.; Dalac-Rat, S.; Dutriaux, C.; Leccia, M.-T.; Legoupil, D.; Montaudié, H.; Maubec, E.; et al. Differential Gradients of Immunotherapy vs Targeted Therapy Efficacy According to the Sun-Exposure Pattern of the Site of Occurrence of Primary Melanoma: A Multicenter Prospective Cohort Study (MelBase). Front. Oncol. 2023, 13, 1250026. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.G.; Navrazhina, K.; Ding, F.; Bhatia, R.; Tsai, K.; Abbate, K.; Durden, B.; Eroglu, Z.; Bhatia, S.; Park, S.; et al. Ipilimumab plus Nivolumab for Patients with Metastatic Uveal Melanoma: A Multicenter, Retrospective Study. J. Immunother. Cancer 2020, 8, e000331. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Munhoz, R.R.; Kuk, D.; Ott, P.A.; Johnson, D.B.; Tsai, K.K.; Rapisuwon, S.; Eroglu, Z.; Sullivan, R.J.; Luke, J.J.; et al. The Efficacy of Anti-PD-1 Agents in Acral and Mucosal Melanoma. Cancer 2016, 122, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical Outcomes in Metastatic Uveal Melanoma Treated with PD-1 and PD-L1 Antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Gershenwald, J.E. The Eighth Edition American Joint Committee on Cancer (AJCC) Melanoma Staging System: Implications for Melanoma Treatment and Care. Expert. Rev. Anticancer Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Kaunitz, G.J.; Cottrell, T.R.; Lilo, M.; Muthappan, V.; Esandrio, J.; Berry, S.; Xu, H.; Ogurtsova, A.; Anders, R.A.; Fischer, A.H.; et al. Melanoma Subtypes Demonstrate Distinct PD-L1 Expression Profiles. Lab. Investig. 2017, 97, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Rodig, S.J.; Gusenleitner, D.; Jackson, D.G.; Gjini, E.; Giobbie-Hurder, A.; Jin, C.; Chang, H.; Lovitch, S.B.; Horak, C.; Weber, J.S.; et al. MHC Proteins Confer Differential Sensitivity to CTLA-4 and PD-1 Blockade in Untreated Metastatic Melanoma. Sci. Transl. Med. 2018, 10, eaar3342. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Bordeaux, J.; Kim, J.Y.; Vaupel, C.; Rimm, D.L.; Ho, T.H.; Joseph, R.W.; Daud, A.I.; Conry, R.M.; Gaughan, E.M.; et al. Quantitative Spatial Profiling of PD-1/PD-L1 Interaction and HLA-DR/IDO-1 Predicts Improved Outcomes of Anti-PD-1 Therapies in Metastatic Melanoma. Clin. Cancer Res. 2018, 24, 5250–5260. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-Specific MHC-II Expression Represents a Tumour-Autonomous Phenotype and Predicts Response to Anti-PD-1/PD-L1 Therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Patel, V.G. The Role of PD-L1 Expression as a Predictive Biomarker: An Analysis of All US Food and Drug Administration (FDA) Approvals of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-Analysis. JAMA Oncol. 2019, 5, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Liu, Y.; Wang, M.; Li, Y.; Xu, T.; Wei, Y. The Predictive Value of Tumor Mutation Burden on Clinical Efficacy of Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 748674. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Frampton, G.M.; Rioth, M.J.; Yusko, E.; Xu, Y.; Guo, X.; Ennis, R.C.; Fabrizio, D.; Chalmers, Z.R.; Greenbowe, J.; et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol. Res. 2016, 4, 959–967. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Tsoi, J.; Onyshchenko, M.; Abril-Rodriguez, G.; Ross-Macdonald, P.; Wind-Rotolo, M.; Champhekar, A.; Medina, E.; Torrejon, D.Y.; Shin, D.S.; et al. Conserved Interferon-γ Signaling Drives Clinical Response to Immune Checkpoint Blockade Therapy in Melanoma. Cancer Cell 2021, 39, 122. [Google Scholar] [CrossRef] [PubMed]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.L.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.W.; Elassaiss-Schaap, J.; Kefford, R.; Hwu, W.-J.; Wolchok, J.D.; Joshua, A.M.; Ribas, A.; Hodi, F.S.; Hamid, O.; Robert, C.; et al. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin. Cancer Res. 2018, 24, 4960–4967. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, Z.; Zaretsky, J.M.; Hu-Lieskovan, S.; Kim, D.W.; Algazi, A.; Johnson, D.B.; Liniker, E.; Kong, B.; Munhoz, R.; Rapisuwon, S.; et al. High Response Rate to PD-1 Blockade in Desmoplastic Melanomas. Nature 2018, 553, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Blum, S.M.; Sun, X.; Da Silva, I.P.; Zubiri, L.; Ye, F.; Bai, K.; Zhang, K.; Ugurel, S.; Zimmer, L.; et al. Efficacy and Safety of Immune Checkpoint Inhibitors in Young Adults with Metastatic Melanoma. Eur. J. Cancer 2023, 181, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Kugel, C.H.; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.-G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
- Loo, K.; Gauvin, G.; Soliman, I.; Renzetti, M.; Deng, M.; Ross, E.; Luo, B.; Wu, H.; Reddy, S.; Olszanski, A.J.; et al. Primary Tumor Characteristics and Next Generation Sequencing Mutations as Biomarkers for Melanoma Immunotherapy Response. Pigment Cell Melanoma Res. 2020, 33, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Pires da Silva, I.; Ahmed, T.; McQuade, J.L.; Nebhan, C.A.; Park, J.J.; Versluis, J.M.; Serra-Bellver, P.; Khan, Y.; Slattery, T.; Oberoi, H.K.; et al. Clinical Models to Define Response and Survival with Anti-PD-1 Antibodies Alone or Combined with Ipilimumab in Metastatic Melanoma. J. Clin. Oncol. 2022, 40, 1068–1080. [Google Scholar] [CrossRef] [PubMed]

| PD-1 Monotherapy (N = 300) | Ipi/Nivo (N = 147) | Overall (N = 447) | |
|---|---|---|---|
| Subtype | |||
| Acral | 22 (7.3%) | 7 (4.8%) | 29 (6.5%) |
| Cutaneous–superficial spreading | 68 (22.7%) | 39 (26.5%) | 107 (23.9%) |
| Cutaneous–nodular | 53 (17.7%) | 23 (15.6%) | 76 (17.0%) |
| Cutaneous–other | 24 (8.0%) | 5 (3.4%) | 29 (6.5%) |
| Cutaneous–unknown | 94 (31.3%) | 32 (21.8%) | 126 (28.2%) |
| Unknown primary | 39 (13.0%) | 41 (27.9%) | 80 (17.9%) |
| Breslow thickness (mm) | |||
| Mean (SD) | 4.1 (4.7) | 4.3 (6.4) | 4.2 (5.3) |
| Median [Q1, Q3] | 2.8 [1.5, 4.5] | 2.1 [1.2, 5.8] | 2.7 [1.4, 5.0] |
| n/a | 86 (28.7%) | 58 (39.5%) | 144 (32.2%) |
| Ulceration | |||
| 0 | 96 (32.0%) | 59 (40.1%) | 155 (34.7%) |
| 1 | 105 (35.0%) | 32 (21.8%) | 137 (30.6%) |
| n/a | 99 (33.0%) | 56 (38.1%) | 155 (34.7%) |
| Mitoses (mm2) | |||
| Mean (SD) | 6.9 (7.4) | 5.4 (4.6) | 6.5 (6.7) |
| Median [Q1, Q3] | 5.0 [2.0, 10.0] | 4.0 [1.0, 8.0] | 4.0 [2.0, 10.0] |
| n/a | 143 (47.7%) | 78 (53.1%) | 221 (49.4%) |
| Lymphovascular invasion | |||
| 0 | 24 (8.0%) | 10 (6.8%) | 34 (7.6%) |
| 1 | 118 (39.3%) | 51 (34.7%) | 169 (37.8%) |
| n/a | 158 (52.7%) | 86 (58.5%) | 244 (54.6%) |
| Tumor-infiltrating lymphocytes | |||
| 0 | 46 (15.3%) | 20 (13.6%) | 66 (14.8%) |
| 1 | 89 (29.7%) | 39 (26.5%) | 128 (28.6%) |
| n/a | 165 (55.0%) | 88 (59.9%) | 253 (56.6%) |
| Number of positive sentinel nodes | |||
| 0 | 98 (32.7%) | 56 (38.1%) | 154 (34.5%) |
| 1–2 | 76 (25.3%) | 19 (12.9%) | 95 (21.3%) |
| 3+ | 21 (7.0%) | 10 (6.8%) | 31 (6.9%) |
| No SLN performed | 105 (35.0%) | 62 (42.2%) | 167 (37.4%) |
| Stage at presentation | |||
| 1 | 46 (15.3%) | 32 (21.8%) | 78 (17.4%) |
| 2 | 66 (22.0%) | 31 (21.1%) | 97 (21.7%) |
| 3 | 125 (41.6%) | 40 (27.2%) | 165 (36.9%) |
| 4 | 63 (21.0%) | 44 (29.9%) | 107 (23.9%) |
| M status at presentation | |||
| M0 | 237 (79.0%) | 102 (69.4%) | 339 (75.8%) |
| M1a | 18 (6.0%) | 8 (5.4%) | 26 (5.8%) |
| M1b | 9 (3.0%) | 2 (1.4%) | 11 (2.5%) |
| M1c | 26 (8.7%) | 18 (12.2%) | 44 (9.8%) |
| M1d | 10 (3.3%) | 17 (11.6%) | 27 (6.0%) |
| Primary presentation | |||
| Localized | 124 (41.3%) | 66 (44.9%) | 190 (42.5%) |
| Locoregional | 13 (4.3%) | 9 (6.1%) | 22 (4.9%) |
| Regional/Stage III | 99 (33.0%) | 26 (17.7%) | 125 (27.9%) |
| Metastatic | 64 (21.3%) | 46 (31.3%) | 110 (24.6%) |
| Missing | 1 (0.3%) | 1 (0.7%) | 2 (0.4%) |
| PD-1 Monotherapy (N = 300) | Ipi/Nivo (N = 147) | Overall (N = 447) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 62.6 (14.2) | 57.2 (14.9) | 60.9 (14.6) |
| Median [Q1, Q3] | 64.0 [54.0, 73.0] | 61.0 [47.0, 68.5] | 63.0 [51.0, 72.0] |
| Gender | |||
| Female | 94 (31.3%) | 43 (29.3%) | 137 (30.6%) |
| Male | 206 (68.7%) | 104 (70.7%) | 310 (69.4%) |
| Pre-immunotherapy treatment (metastatic) | |||
| No | 119 (39.7%) | 87 (59.2%) | 206 (46.1%) |
| Yes | 181 (60.3%) | 59 (40.1%) | 240 (53.7%) |
| Missing | 0 (0%) | 1 (0.7%) | 1 (0.2%) |
| LDH > ULN | |||
| 0 | 178 (59.3%) | 64 (43.5%) | 242 (54.1%) |
| 1 | 107 (35.7%) | 70 (47.6%) | 177 (39.6%) |
| Missing | 15 (5.0%) | 13 (8.8%) | 28 (6.3%) |
| BRAF V600E | |||
| No | 215 (71.7%) | 91 (61.9%) | 306 (68.5%) |
| Yes | 84 (28.0%) | 56 (38.1%) | 140 (31.3%) |
| Missing | 1 (0.3%) | 0 (0%) | 1 (0.2%) |
| Response to anti-PD-1 | |||
| PD/SD | 172 (57.3%) | 78 (53.1%) | 250 (55.9%) |
| CR/PR | 128 (42.7%) | 69 (46.9%) | 197 (44.1%) |
| Missing with PD/SD | 3 (1.0%) | 10 (6.8%) | 13 (2.9%) |
| Progressed | |||
| 0 | 93 (31.0%) | 52 (35.4%) | 145 (32.4%) |
| 1 | 207 (69.0%) | 95 (64.6%) | 302 (67.6%) |
| PFS (months) | |||
| Mean (SD) | 19.3 (26.5) | 20.2 (25.0) | 19.6 (26.0) |
| Median [Q1, Q3] | 7.4 [2.6, 23.0] | 7.2 [2.4, 30.5] | 7.4 [2.5, 26.2] |
| Died | |||
| 0 | 125 (41.7%) | 75 (51.0%) | 200 (44.7%) |
| 1 | 175 (58.3%) | 72 (49.0%) | 247 (55.3%) |
| OS (months) | |||
| Mean (SD) | 31.1 (30.9) | 28.7 (25.1) | 30.3 (29.1) |
| Median [Q1, Q3] | 19.5 [8.3, 48.7] | 22.2 [7.7, 44.8] | 19.8 [8.0, 48.2] |
| Response to Anti-PD-1 | ||
|---|---|---|
| Variable | p-value | FDR adjusted p-value |
| Subtype | 0.001 | 0.003 |
| Breslow thickness (mm) | 0.327 | 0.545 |
| Ulceration | 0.009 | 0.023 |
| Mitoses (mm2) | 0.527 | 0.589 |
| Lymphovascular invasion | 0.476 | 0.589 |
| Tumor-infiltrating lymphocytes | 1 | 1 |
| Number of positive sentinel nodes | 0 | 0.003 |
| Stage at presentation | 0.008 | 0.023 |
| M status at presentation | 0.53 | 0.589 |
| Primary presentation | 0.03 | 0.06 |
| BRAF mutation | <0.001 | <0.001 |
| PFS * | ||
| Variable | p-value | FDR adjusted p-value |
| Subtype | 0.004 | 0.012 |
| Breslow thickness (mm) | 0.889 | 0.889 |
| Ulceration | 0.002 | 0.01 |
| Mitoses (mm2) | 0.07 | 0.116 |
| Lymphovascular invasion | 0.093 | 0.133 |
| Tumor-infiltrating lymphocytes | 0.316 | 0.351 |
| Number of positive sentinel nodes | 0.001 | 0.008 |
| Stage at presentation | 0.046 | 0.114 |
| M status at presentation | 0.31 | 0.351 |
| Primary presentation | 0.059 | 0.116 |
| BRAF mutation | <0.001 | <0.001 |
| OS * | ||
| Variable | p-value | FDR adjusted p-value |
| Subtype | 0.21 | 0.262 |
| Breslow thickness (mm) | 0.641 | 0.641 |
| Ulceration | 0.039 | 0.118 |
| Mitoses (mm2) | 0.059 | 0.118 |
| Lymphovascular invasion | 0.566 | 0.629 |
| Tumor-infiltrating lymphocytes | 0.098 | 0.141 |
| Number of positive sentinel nodes | 0.004 | 0.04 |
| Stage at presentation | 0.095 | 0.141 |
| M status at presentation | 0.024 | 0.118 |
| Primary presentation | 0.059 | 0.118 |
| BRAF mutation | 0.19 | 0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodman, R.S.; Jung, S.; Fletcher, K.; Burnette, H.; Mohyuddin, I.; Irlmeier, R.; Ye, F.; Johnson, D.B. Primary Tumor Characteristics as Biomarkers of Immunotherapy Response in Advanced Melanoma: A Retrospective Cohort Study. Cancers 2024, 16, 2355. https://doi.org/10.3390/cancers16132355
Goodman RS, Jung S, Fletcher K, Burnette H, Mohyuddin I, Irlmeier R, Ye F, Johnson DB. Primary Tumor Characteristics as Biomarkers of Immunotherapy Response in Advanced Melanoma: A Retrospective Cohort Study. Cancers. 2024; 16(13):2355. https://doi.org/10.3390/cancers16132355
Chicago/Turabian StyleGoodman, Rachel S., Seungyeon Jung, Kylie Fletcher, Hannah Burnette, Ismail Mohyuddin, Rebecca Irlmeier, Fei Ye, and Douglas B. Johnson. 2024. "Primary Tumor Characteristics as Biomarkers of Immunotherapy Response in Advanced Melanoma: A Retrospective Cohort Study" Cancers 16, no. 13: 2355. https://doi.org/10.3390/cancers16132355
APA StyleGoodman, R. S., Jung, S., Fletcher, K., Burnette, H., Mohyuddin, I., Irlmeier, R., Ye, F., & Johnson, D. B. (2024). Primary Tumor Characteristics as Biomarkers of Immunotherapy Response in Advanced Melanoma: A Retrospective Cohort Study. Cancers, 16(13), 2355. https://doi.org/10.3390/cancers16132355






