Simple Summary
Uniportal video-assisted thoracoscopic segmentectomy is one of the most minimally invasive surgeries for early-stage lung cancer. However, mastering this technique requires high levels of skill and experience, especially in complex cases. This article reviews previous research on uniportal video-assisted thoracoscopic segmentectomy, including indications, instrument selection, tumor marking, intersegmental plane identification, lymph node dissection, and surgical videos.
Abstract
Twenty years have passed since uniportal video-assisted thoracoscopic surgery (VATS) was first reported. Several reports have already proven the minimal invasiveness of uniportal VATS. In addition, two large clinical trials recently demonstrated the benefits of segmentectomy for small peripheral early-stage non-small cell lung cancer. Uniportal VATS segmentectomy is considered the most beneficial minimally invasive surgery for patients with early-stage lung cancer. However, a high level of skill and experience are required to achieve this goal. Only a few reports have discussed specific techniques, particularly for complex segmentectomies. In this Special Issue, we reviewed previous reports on uniportal VATS segmentectomy regarding the indications, instrument selection, marking of the tumor location, methods of intersegmental plane identification, and lymph node dissection, including our own techniques with video content.
1. Introduction
In 2004, the first lung resection using uniportal video-assisted thoracoscopic surgery (U-VATS) was reported [1]. U-VATS lobectomy was first reported in 2012 [2]. In the years that followed the first report, this approach has spread worldwide [3,4]. Uniport surgeons have continued to develop their techniques [5,6]. The indications for their surgeries have been expanded to include highly difficult surgeries, such as segmentectomy [7], inflammatory lymph node cases [8], bronchoplasty [9], and angioplasty [10]. U-VATS has many advantages over conventional multiportal VATS (M-VATS), which uses 3–5 ports, including less postoperative pain and complications, shorter operative time, and shorter hospital stay [11,12,13,14,15,16,17,18,19,20,21].
The benefit of segmentectomy for early-stage peripheral non-small cell lung cancer (NSCLC) has recently been demonstrated in large clinical trials [22,23]. Against this background, segmentectomy with U-VATS is considered the most beneficial minimally invasive surgery for patients with early-stage NSCLC. However, a high level of skill and experience are required to perform the operation [24,25,26,27]. In particular, few reports have discussed specific techniques, including U-VATS complex segmentectomy.
In this Special Issue, we review previous reports on U-VATS segmentectomy, including studies discussing the indications, instrument selection, marking of the tumor location, methods of intersegmental plane identification, and lymph node dissection. We also discuss our own techniques.
2. About Uniportal VATS
2.1. Definition of Uniportal VATS
At the consensus meeting of the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS), U-VATS was defined as “VATS performed from a single incision of 4 cm or less” [24]. In this article, U-VATS is described based on this definition.
2.2. Comparison with Multiportal VATS
Several studies comparing perioperative outcomes between U-VATS and M-VATS are shown in Table 1. Each report demonstrated the superiority or equivalence of U-VATS for almost all of these parameters, even in patients of ≥70 years of age [12]. Three systematic reviews and meta-analyses have concluded that U-VATS is superior to traditional thoracotomy in terms of postoperative hospital stay, postoperative drainage duration, complication rate, conversion rate to thoracotomy, postoperative pain, and need for analgesics [28,29,30]. There are also reports of reduced medical costs due to faster postoperative recovery [31] and a reduced incidence of long-term post-thoracotomy pain syndrome [14]. In terms of prognosis, the 3-year overall survival (OS) rates of U-VATS and M-VATS in lobectomy were 74% and 76%, respectively, the 3-year disease-free survival (DFS) rates were 69% and 67% (including advanced lung cancer) [12], the 5-year OS rates were 89.2% and 86.5%, and the 5-year DFS rates were 89.5% and 89.6% [11]. In segmentectomy, the 3-year OS rates of U-VATS and M-VATS were 100% and 90.4%, respectively, the 3-year DFS rates were 99.7% and 99.0%, the 5-year OS rates were 97.7% and 99.4%, and the 5-year DFS rates were 99.7% and 98.2% [18]. There were no significant differences between the two groups [11,12,18]. Although these are mostly retrospective studies, almost all reports have demonstrated the superiority of U-VATS over M-VATS, and more than half (58%) of the participants at the UVIG meeting of the ESTS agreed that a randomized controlled trial comparing U-VATS and M-VATS is unnecessary [24].

Table 1.
Studies comparing uniportal VATS and multiportal VATS.
3. Before Performing Uniportal VATS Segmentectomy
3.1. Required Skills and Experience
U-VATS is more difficult than M-VATS because it is prone to surgical instrument interference. In addition, U-VATS requires the ability to control and perform surgeries independently because it is generally difficult to obtain help from assistants. In the UVIG report of ESTS, 84% of the members agreed that more than 50 cases were needed to reach the learning curve for lobectomy in U-VATS [24]. A study analyzing 1063 cases and examining the learning curve reported that the operative time plateaued at 40 U-VATS cases [27]. A study limited to U-VATS segmentectomy reported that it took 70 cases to reach the learning curve and 100 cases to achieve proficiency [26]. In contrast, U-VATS segmentectomy can be safely performed by surgeons with extensive experience in U-VATS [25], and U-VATS complex segmentectomy can be performed without any learning curve by surgeons who are experienced in U-VATS lobectomy or simple segmentectomy techniques [32]. With this background, it can be assumed that U-VATS simple or complex segmentectomy can be performed at a certain level with basic techniques and experience in U-VATS lobectomy.
3.2. Indications for Uniportal VATS Segmentectomy
Based on the results of JCOG0802/WJOG4607L [22] and CALGB 140503 [23], early-stage peripheral NSCLC of less than 2 cm in size is a good indication for segmentectomy. The number of cases is expected to increase with the spread of lung cancer screening using computed tomography (CT) [33,34,35]. However, palpation is often difficult with U-VATS, and in cases where tumor localization cannot be confirmed from the pleura, preoperative marking, as described below, should be used. We do not consider U-VATS to be a good approach for cases that are somewhat deeper in the lung parenchyma that should be resected by the palpation of the tumor, especially common in metastatic lung tumors. U-VATS segmentectomy is a good approach for minimizing the impact on the systemic condition in elderly patients with a poor performance status and patients with a low pulmonary function who cannot undergo wedge resection. At present, there is no evidence to support segmentectomy for NSCLC with lymph node metastasis, and in principle, it should only be performed in patients with clinical N0 disease, except for the passive indications mentioned above.
3.3. Preoperative Planning
In segmentectomy, it is important to (1) localize the tumor, (2) ensure surgical margins, and (3) understand the detailed anatomy.
3.3.1. Locating the Tumor
Since palpation is often difficult in U-VATS, it is important to visualize the localization of the tumor by preoperative marking. These methods include CT-guided hook wire [36], spiral wire [37], butterfly needle [38], microcoil [39], dye [40], lipiodol [41], electromagnetic navigation bronchoscopy [42], and intraoperative ultrasonography [43]. A novel application of the radiofrequency identification marking system was recently reported [44,45,46,47]. It has also been reported that the combined use of mechanical and chemical markings reduces the rate of conversion to thoracotomy in VATS. The success rate of the hook-wire has been reported to be 98.3% [48], and many institutions have made it their first choice [36,48,49,50,51]. However, several cases of air embolization, including fatal cases, have been reported [52,53,54,55], and hook wire marking is not recommended in Japan. A report of preoperative dye marking in U-VATS showed a 99.5% success rate of implementation and a 100% success rate of tumor resection without conversion to thoracotomy [56]. Similar to this report, our institution uses CT-guided dye marking and has had no experience of fatal complications. We perform preoperative marking in cases where the tumor may not be visible from the pleura and where the lesion mainly demonstrates ground-glass opacity. In addition, we consider that cases in which the tumor is located somewhat deep in the lung and thus may move in the parenchyma are not good indications for U-VATS segmentectomy, and such cases should instead be dissected between the intersegmental planes with palpation under thoracotomy.
3.3.2. Ensuring Tumor Margins
For solid-predominant lesions, the tumor margin should be 2 cm or larger than the tumor diameter [22]. For ground-glass opacity-predominant lesions, the tumor margin should be 5 mm or more [57,58]. The post hoc analysis of JCOG0802 showed the benefit of segmentectomy over lobectomy, even in pure-solid tumors [59], and it would be acceptable to ensure the above-mentioned tumor margin. In addition, because complex segmentectomy has been reported to have equivalent outcomes to simple segmentectomy [60], performing a combined resection of adjacent segments to ensure the tumor margin should also be considered.
3.3.3. Anatomical Variations
The 3D reconstruction of pulmonary arteries (PAs), pulmonary veins (PVs), and bronchi using an imaging analysis software program is highly recommended for segmentectomy [61,62,63]. At our institution, all patients underwent 3D reconstruction using Synapse Vincent (ver. 6.7, Fujifilm, Tokyo, Japan). Contrast-enhanced CT is the standard. However, plain CT can also be used to obtain a rough image of the vascular branching. Based on this information, the segments and vessels and bronchi to be resected can be thoroughly reviewed before surgery. For example, it has been reported that the anatomy of the PVs in the right upper lobe is extremely variable [64], and changes in the surgical procedure due to anatomical abnormalities should also be considered.
4. Surgical Techniques of Uniportal VATS
4.1. Patient Position
A patient is placed in the lateral recumbent position, and the surgical bed is tilted approximately 15 degrees at the top of the intercostal space (ICS) to be used for a uniport (Figure 1). This allows the ICS to open and is useful in cases of conversion to open thoracotomy. The hand on the affected side is placed on a handstand. In the event of emergency conversion to thoracotomy, a new axillary incision should be added at the 4th ICS to provide a proper view of the pulmonary hilum. If a uniport is not created at the 4th ICS, the first uniport should be used as an assist or camera port.
4.2. Approach and Incision
While many uniport surgeons use the anterior approach, standing on the ventral side of the patient [49,51,61,65,66], the first author always stands on the right side of the patient (Figure 2). This maximizes the use of the dominant hand for the manipulation of energy devices and staplers. The first assistant and scopist stand on the opposite side of the main surgeon. A uniport is created in the mid-axillary line for right-sided surgery and in the anterior axillary line for left-sided surgery. The strength of this approach is that it provides a bird’s-eye view of the thoracic cavity. It is, therefore, possible to secure a view of not only the hilum but also the dorsal side, thus making it suitable for dorsal pleural dissection and lymph node dissection in the upper and lower mediastinal regions. However, the ICS is slightly narrower at the mid-axillary line than at the anterior axillary line, which may be a disadvantage in small patients with a narrow ICS.
The ICS for uniport placement is determined by visualizing the location of the pulmonary hilum and interlobe in the thoracic cavity, without counting the ribs from the body surface. If there is any doubt regarding the ICS between the upper and lower ribs, it is better to select the lower ICS because manipulation is extremely difficult if the uniport is even slightly cephalad to the target pulmonary hilum.
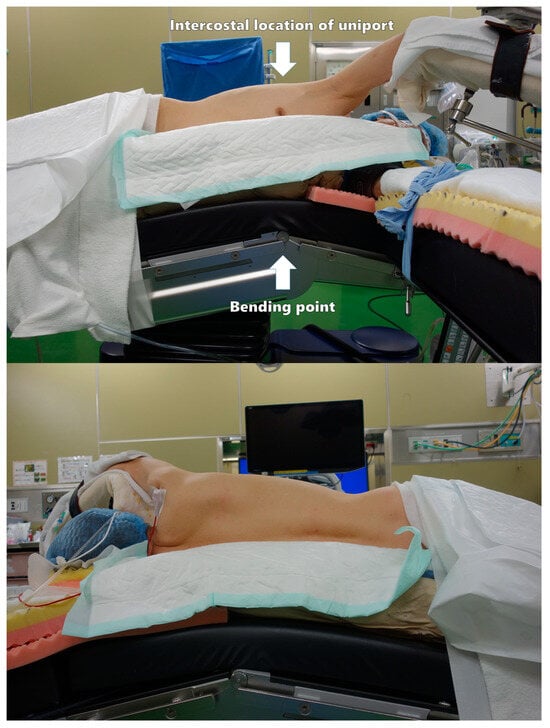
Figure 1.
Patient position.
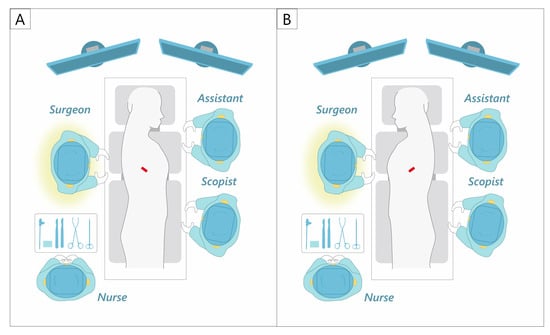
Figure 2.
Our uniportal VATS approach and setting. (A) Right side. (B) Left side.
A polyurethane retractor is placed on the wound to protect the intercostal space. A 14-Fr suction catheter is inserted through the edge of the retractor as a smoke evacuation device (Figure 3). This method has been reported to significantly reduce intraoperative camera clearing [67]. As the suction device does not need to be placed in the thoracic cavity, the left hand can be tubeless.
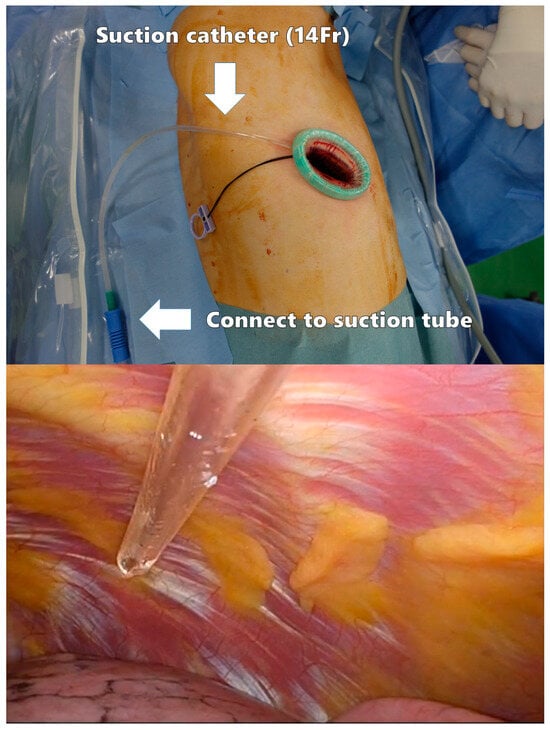
Figure 3.
Smoke evacuation device in the surgical field.
4.3. Surgical Instruments of Uniportal VATS
Several curved forceps need to be prepared, including suction tools, lung forceps, lymph node forceps, right-angle forceps, Kelly forceps, snake forceps, and curved forceps (Figure 4A). The thoracoscope is ENDOEYE (Olympus, Tokyo, Japan) with a 5 mm 30-degree rigid integrated scope. Energy devices are essential in U-VATS, although surgeons can choose their own devices according to their preferences. The first author frequently uses a 1 mm wide hook-type electrocautery scalpel, AdTec® mini (B.BRAUN AESCULAP, Melsungen, Germany) (Figure 4B) for the incision of vessels and bronchial sheaths. A 23 cm curved suction soft coagulator (AMCO, Tokyo, Japan) (Figure 4C) is also useful for dissection and hemostasis. The left hand is mainly used with the CS Two-Way HandleTM (Unimedic, Osaka, Japan) (Figure 4D). It is also useful for compression hemostasis in cases with oozing [68]. They are characterized by greater friction than suction instruments, making it easier to adjust the tension. Techniques using these instruments are presented with surgical videos in the segmentectomy case series section.
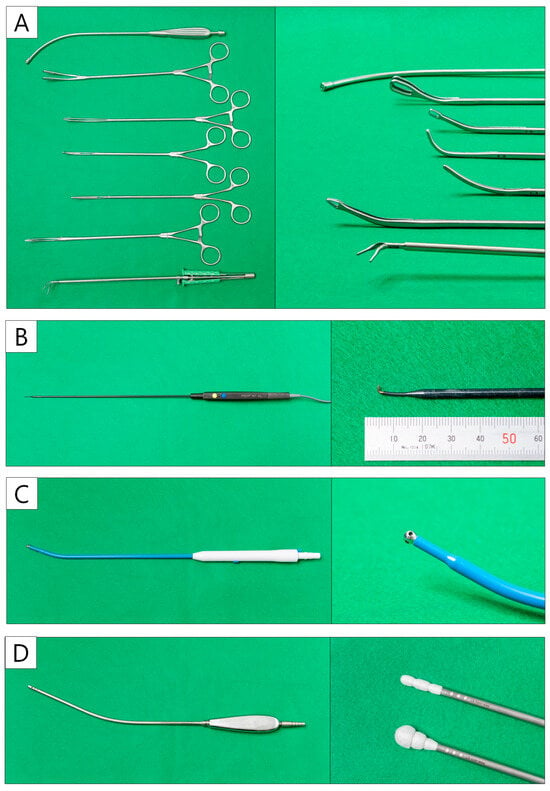
Figure 4.
Uniportal VATS instruments. (A) The curved forceps and suction device. (B) Hook-type electrocautery. (C) Curved suction soft coagulator. (D) CS two-way handle.
4.4. Vascular and Bronchial Dissection
In U-VATS, the main method of dissection is the opening of the energy device; however, the first author mainly uses hook-type electrocautery (Figure 4B). A sharp incision of the membrane facilitates anatomical understanding and a dry surgical field. The PA, PV, and bronchi should be cut with reference to preoperative 3D reconstruction after confirming that the orientation is correct. When dissecting the PA or PV, the stapler is inserted by sliding it over the Penrose drain. Two rows of staplers can also be used for (sub) segmental bronchi in U-VATS segmentectomy.
4.5. Lymph Node Dissection
Owing to the limited number of tools available for U-VATS, lymph node dissection (LND) needs to be designed. In a sub-analysis of JCOG0802, the frequency of upstaging (N2) by LND was 0.5% in part-solid tumors, whereas N2 was identified in 5.4% of pure-solid tumors. Therefore, selective LND is recommended [69,70]. There are also reports of a good prognosis in cases where more than 10 lymph nodes were dissected in segmentectomy [71], and in U-VATS segmentectomy, LND should be performed appropriately according to the case. For the dissection of the N1 lymph nodes, a sharp incision should be made through the vascular or bronchial sheath, and the lymph nodes should be dissected under the surrounding capsule; hook electrocautery is useful for sharp incisions in U-VATS (Video 1). The careful dissection of the N1 lymph nodes without crushing is particularly important to ensure curability in U-VATS segmentectomy for early-stage NSCLC. For superior mediastinal LND, dissection around the azygos vein before dissecting the superior trunk (A3) eliminates the need for an assistant to protect the A3 stump. The azygos vein can be taped and towed by an assistant to provide a good surgical field for LND (Figure 5A). For inferior mediastinal LND, a good surgical field around the tracheal bifurcation can be obtained by passing tape between the superior and inferior PVs and towing the entire lower lobe forward (Figure 5B). For this reason, inferior mediastinal LND is performed before cutting the inferior PV and inferior lobe bronchus during lower lobectomy.
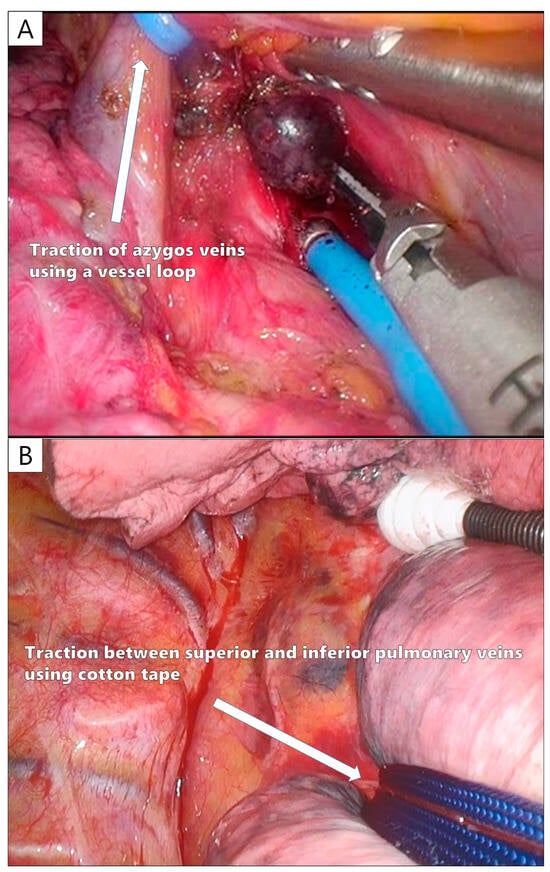
Figure 5.
The tips of a uniportal VATS lymph node dissection. (A) Taping and control of the azygos vein during upper mediastinal lymph node dissection. (B) Taping between the superior and inferior pulmonary veins during lower mediastinal lymph node dissection.
4.6. Identification of the Intersegmental Plane
Various methods have been used to identify intersegmental planes, including the inflation–deflation technique [72], jet ventilation [73], and intravenous indocyanine green (ICG) injection [74]. ICG injection is considered useful because of the limitations of the surgical field and the use of instruments in U-VATS [75]. Our institution uses ICG. It has also been reported that ICG injection using a syringe pump significantly clarifies the contrast between the circulation area and the ischemic area in comparison to bolus injection [76]. Specifically, 25 mg of ICG is dissolved in 10 mL of saline, with a maximum dose of 0.3 mg/kg, and administered intravenously at a rate of 300 mL/h. The contrast is visualized in approximately 30 s, and if the maximum dose is not reached, the administration is stopped. After 30 min, the ICG is washed out and the procedure can be repeated. The intersegmental plane should be adequately marked with dye.
4.7. Stapling between Intersegmental Planes
This is the most important step in U-VATS segmentectomies. Specifically, in cases where the tumor is not visible from the pleura, it is necessary to depend on preoperative markings to determine the appropriate staple line to ensure the tumor margin. All peripheral stumps of the dissected intersegmental PV or (sub) segmental bronchus are ligated and towed. The control of these threads facilitates stapling between the intersegmental planes (Figure 6). After stapling the intersegmental planes, the specimen should be thoroughly palpated. The staples should be removed and bled off to facilitate palpation. If no tumor is palpable in the specimen, the surgeon should not hesitate to convert to thoracotomy and palpate the lung again, even if the surgery is almost complete.
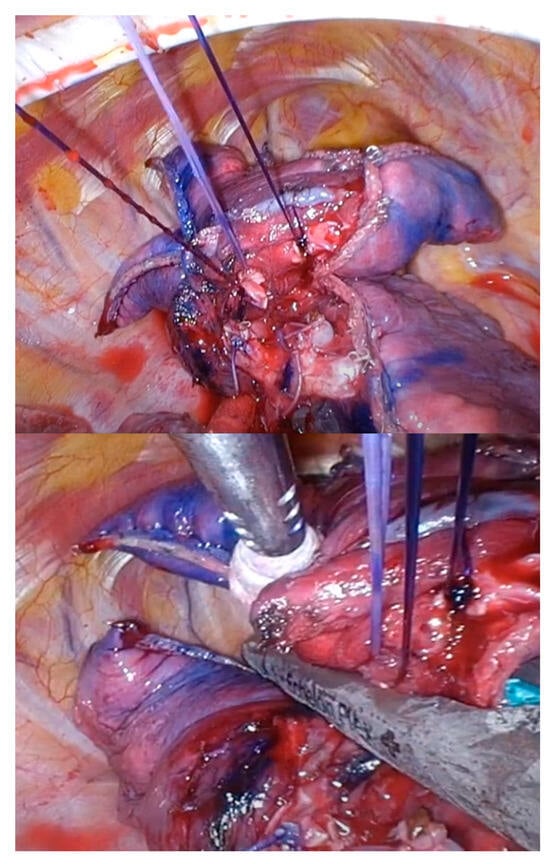
Figure 6.
Thread control during intersegmental plane stapling.
4.8. Before the End of Surgery
A sealing test is performed to confirm the absence of bronchial and pulmonary fistula. If an air leak is identified, it should be carefully repaired because it significantly affects the postoperative course. If the air leak is mild, it can be controlled with soft coagulation, and the use of fibrin glue or polyglycolic acid is effective. In cases of massive air leak, pericardial fat and subcutaneous fat are often reported to be effective in covering the leak point [77,78,79,80], and the technique can also be performed under U-VATS. Although there are some limitations in the use of instruments and the surgical view in U-VATS, the control of the needle is easy because the thoracic cavity space is larger than before lung resection. We used to perform intercostal nerve block to the ICS via a transthoracic approach [66]. However, it was reported that a percutaneous approach is more effective than a transthoracic approach in anesthesia [81]. Therefore, we now administer 20 mL of levobupivacaine percutaneously for three intercostal spaces. After the intercostal nerve block, a thoracic drain is placed and the uniport is closed.
5. Uniportal VATS Segmentectomy
Our experience with U-VATS segmentectomy is shown in Figure 7. All procedures, including complex segmentectomies, can be performed using U-VATS. Five of these cases are presented as surgical videos.
Figure 7.
Our experience with uniportal VATS segmentectomy.
5.1. Right Lower Lobe Apical Segmentectomy (S6)
In this case, the tumor was located caudal to S6 and proximal to V6bc. Preoperative CT-guided dye marking was performed to ensure the tumor margin in the intersegmental plane with S10 (Supplementary Video S1: 4 min, 6 s).
- (I)
- The uniport is placed on the lateral side of the 6th ICS.
- (II)
- The PA sheath is dissected, and #11s LND is performed.
- (III)
- A6 is cut.
- (IV)
- V6 is dissected from the dorsal side.
- (V)
- #12L LND is performed, and B6 is cut.
- (VI)
- A6 and B6 stumps are ligated together.
- (VII)
- The intersegmental planes are identified using ICG.
- (VIII)
- Referring to the preoperative marking, stapling is performed between S6 and the basal segment with a sufficient tumor margin.
5.2. Left Upper Lobe Tri-Segmentectomy (S1+2+3) with Fissureless Technique
A fissureless technique was required because this patient had an incomplete fissure (Supplementary Video S2: 4 min, 25 s).
- (I)
- The uniport is placed on the anterior side of the 5th ICS.
- (II)
- The pleura is incised from the hilum to expose V1-3 and cut.
- (III)
- The distal stump of the PV is pulled posteriorly, and the branches of A3 are dissected. The PA branches are cut.
- (IV)
- The mediastinal lingual PA is exposed to the periphery, and the stump of the PV and lung is dissected.
- (V)
- A1 + 2ab and A1 + 2c are cut.
- (VI)
- #12U LND is performed, and B1-3 are exposed. B1-3 are cut with attention to the posterior PA.
- (VII)
- The PV stump is ligated.
- (VIII)
- The intersegmental planes are identified using ICG.
- (IX)
- Stapling is performed between the superior and lingual segments in a straight line from the anterior to the posterior.
5.3. Right Upper Lobe Horizontal and Lateral Sub-Segmentectomy (S2b+S3a)
This case represents a complex subsegmentectomy for an early-stage NSCLC of less than 2 cm in size (Supplementary Video S3: 4 min, 18 s).
- (I)
- The uniport is placed on the lateral side of the 6th ICS.
- (II)
- Wedge resection is performed. The tumor was diagnosed as adenocarcinoma.
- (III)
- The upper-lower fissure is separated, and LN#11s LND is performed (no metastasis).
- (IV)
- The anterior type II PV (V2t + V2c + V2b) is dissected and cut.
- (V)
- The distal stump of the PV is pulled cranially, and B2b and B3a are exposed.
- (VI)
- B2b and B3a are cut.
- (VII)
- A3a and A2b are dissected and cut on the posterior side of the bronchial stumps.
- (VIII)
- The intersegmental planes are identified using ICG.
- (IX)
- All stumps are ligated, and stapling between the intersubsegmental planes is performed under thread control.
5.4. Left Lower Lobe Dorsobasal Segmentectomy (S10) with Fissure-Based Approach
This case involved left S10 segmentectomy using the interlobar approach (Supplementary Video S4: 7 min, 40 s).
- (I)
- The uniport is placed on the anterior side of the 6th ICS.
- (II)
- The fissure is separated, and the basal PA is identified.
- (III)
- V6 is dissected.
- (IV)
- This case has a narrow space between V6bc and A10. Therefore, A10 is cut first.
- (V)
- Tape is used to tunnel between S6 and S10. The intersegments are then separated.
- (VI)
- B10 is dissected and cut.
- (VII)
- V10 is identified and cut.
- (VIII)
- The intersegmental planes are identified using ICG.
- (IX)
- All stumps are ligated, and stapling between the intersegmental planes is performed under thread control.
5.5. Left Upper Lobe Apicodorsal Segmentectomy (S1+2) and Lower Lobe Ventro-Laterobasal Segmentectomy (S8+9)
In this case, two complex segmentectomies were performed simultaneously in U-VATS (Supplementary Video S5: 7 min 16 s).
- (I)
- The uniport is placed on the anterior side of the 6th ICS.
- (II)
- The fissure is separated, and the interlobar PAs are widely exposed.
- (III)
- The branches of A1+2 are cut.
- (IV)
- B1 + 2c and B1 + 2 ab are exposed and cut.
- (V)
- V1 + 2b behind B1 + 2 is identified and cut.
- (VI)
- The intersegmental planes are identified using ICG, stapling between S1 + 2 and S3 is performed, and the left upper lobe apicodorsal segmentectomy is completed.
- (VII)
- Next, the interlobar PA is exposed to the periphery, and A5, A8a, and A8b are identified.
- (VIII)
- This case had a common trunk of the PV; therefore, the anterior interlobar space was narrow.
- (IX)
- A8a is cut first, and the interlobe is separated next.
- (X)
- A8b and A9 are cut.
- (XI)
- B8+9 is dissected and cut.
- (XII)
- V8+9 is dissected and cut.
- (XIII)
- The identification of the intersegmental plane using ICG is performed again.
- (XIV)
- All stumps are ligated and stapling between the intersegmental planes is performed under thread control.
6. Limitations
This study is associated with several limitations. First of all, this article is a narrative review rather than a systematic review. It describes basic intercostal U-VATS segmentectomy and does not mention the subxiphoid approach U-VATS or robot-assisted reduced port surgery.
7. Conclusions
The potential of U-VATS has been developed and proven to be beneficial through the efforts of various uniport surgeons. We believe that further development will continue worldwide and that it will be widely recognized as one of the best minimally invasive procedures for early-stage NSCLC. Videos of other surgeries not featured in this special issue are available on the authors’ YouTube channel (Nabetaku Channel: https://www.youtube.com/@taku527 (accessed on 15 May 2024). We hope that these findings will be helpful in learning surgical techniques for U-VATS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16132343/s1, Video S1: Right Lower Lobe Apical Segmentectomy (S6); Video S2: Left Upper Lobe Tri-Segmentectomy (S1+2+3) with Fissureless Technique; Video S3: Right Upper Lobe Horizontal and Lateral Sub-Segmentectomy (S2b+S3a); Video S4: Left Lower Lobe Dorsobasal Segmentectomy (S10) with Fissure-Based Approach; Video S5: Left Upper Lobe Apicodorsal Segmentectomy (S1+2) and Lower Lobe Ventro-Laterobasal Segmentectomy (S8+9).
Author Contributions
Conceptualization: T.W.; Methodology: T.W.; Software: T.W. and N.Y.; Validation: T.W., T.K. and M.T.; Investigation: T.W.; Resources: T.W., T.K. and E.S.; Data Curation: T.W.; Writing—Original Draft Preparation: T.W.; Writing—Review and Editing, T.W. and M.T.; Visualization, T.W.; Supervision, M.T.; Project Administration, T.W; Resources: T.W., E.S., T.K., K.I. and T.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by Institutional Review Board of Seirei Mikatahara General Hospital (approval number: 24-05, 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rocco, G.; Martin-Ucar, A.; Passera, E. Uniportal VATS wedge pulmonary resections. Ann. Thorac. Surg. 2004, 77, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, D.; Paradela, M.; Fieira, E.; Velasco, C. Single-incision video-assisted thoracoscopic lobectomy: Initial results. J. Thorac. Cardiovasc. Surg. 2012, 143, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S. Uniportal VATS in Asia. J. Thorac. Dis. 2013, 5, S221–S225. [Google Scholar] [PubMed]
- Igai, H.; Kamiyoshihara, M. Overview of uniportal video-assisted thoracic surgery pulmonary segmentectomy—The movement of minimally invasive surgery. Video-Assist. Thorac. Surg. 2020, 5, 3. [Google Scholar] [CrossRef]
- Ojanguren, A.; Gonzalez, M. What is the optimal way to succeed in uniportal VATS? J. Thorac. Dis. 2020, 12, 3018–3021. [Google Scholar] [CrossRef] [PubMed]
- Anile, M.; Diso, D.; Giacomo, T.D.; Rendina, E.A.; Venuta, F. Uniportal Thoracoscopic Lobectomy. Ann. Thorac. Surg. 2013, 96, 740–745. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Fieira, E.; Mendez, L.; Garcia, J. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur. J. Cardiothorac. Surg. 2012, 42, e169–e171. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Suzuki, E.; Yoshii, N.; Kohama, T.; Iguchi, K.; Nakamura, M.; Endo, T.; Tanahashi, M. A uniportal video-assisted thoracic surgery approach for lung cancer with silicosis and highly inflammatory lymph nodes. Journal of Clinical and Medical Images. Case Reports 2023, 3, 3. [Google Scholar]
- Gonzalez-Rivas, D.; Fernandez, R.; Fieira, E.; Rellan, L. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: First report. J. Thorac. Cardiovasc. Surg. 2013, 145, 1676–1677. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Delgado, M.; Fieira, E.; Mendez, L. Single-port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 889–891. [Google Scholar] [CrossRef][Green Version]
- Ruan, Y.; Cao, W.; Xue, H.; You, M.; Zhao, Z. Long-term outcome of uniport vs. multiport video-assisted thoracoscopic lobectomy for lung cancer. Sci Rep. 2024, 14, 5316. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Lin, Q.; Zhang, J.; Liu, Y.; Zhan, Z. Short- and medium-term outcomes after uniportal and multiportal video-assisted thoracic surgery lobectomy in elderly patients with non-small cell lung cancer. J. BUON 2021, 26, 1453–1459. [Google Scholar] [PubMed]
- Bourdages-Pageau, E.; Vieira, A.; Lacasse, Y.; Figueroa, P.U. Outcomes of Uniportal vs. Multiportal Video-Assisted Thoracoscopic Lobectomy. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Usuda, J. Uniportal video-assisted thoracic surgery reduced the occurrence of post-thoracotomy pain syndrome after lobectomy for lung cancer. J. Thorac. Dis. 2019, 11, 3896–3902. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.R.; Liu, C.Q.; Xu, M.Q.; Xu, M.Q.; Xiong, R.; Li, C.W.; Xie, M.R. Systematic mediastinal lymph node dissection outcomes and conversion rates of uniportal video-assisted thoracoscopic lobectomy for lung cancer. ANZ J. Surg. 2019, 89, 1056–1060. [Google Scholar] [CrossRef]
- Wang, B.Y.; Liu, C.Y.; Hsu, P.K.; Shih, C.S.; Liu, C.C. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: A propensity-matched analysis. Ann. Surg. 2015, 261, 793–799. [Google Scholar] [CrossRef]
- Liu, C.C.; Shih, C.S.; Pennarun, N.; Cheng, C.T. Transition from a multiport technique to a single-port technique for lung cancer surgery: Is lymph node dissection inferior using the single-port technique? Eur. J. Cardiothorac. Surg. 2016, 49, i64–i72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zheng, Q.; Pu, Q.; Mei, J.; Ma, L.; Lin, F.; Liu, C.; Guo, C.; Liao, F.; Liu, Z.; et al. Perioperative and oncological outcomes of uniportal versus three-port thoracoscopic segmentectomy for lung cancer: A propensity score matching analysis. Transl. Lung Cancer Res. 2023, 12, 446–459. [Google Scholar] [CrossRef]
- Numajiri, K.; Matsuura, N.; Igai, H.; Ohsawa, F.; Kamiyoshihara, M. Uniportal thoracoscopic pulmonary segmentectomy provides good perioperative results and early postoperative recovery. J. Thorac. Dis. 2022, 14, 2908–2916. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, L.; Li, R.; Wang, Z.; Zhao, X.; Zhou, D.; Yu, Q.; Yang, X. The Application of Uniportal Video-Assisted Thoracoscopic Anatomical Segmentectomy for Lung Resection: A Retrospective Clinical Study. World J. Surg. 2021, 45, 331–338. [Google Scholar] [CrossRef]
- Hernandez-Arenas, L.A.; Lin, L.; Yang, Y.; Liu, M.; Guido, W.; Gonzalez-Rivas, D.; Jiang, G.; Jiang, L. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur. J. Cardiothorac. Surg. 2016, 50, 1060–1066. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, L.; Batirel, H.; Brunelli, A.; Gonzalez-Rivas, D.; Ismail, M.; Ucar, A.M.; Ng, C.S.H.; Scarci, M.; Sihoe, A.D.L.; Ugalde, P.A.; et al. Uniportal video-assisted thoracic surgery lobectomy: A consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2019, 56, 224–229. [Google Scholar] [CrossRef] [PubMed]
- van Roozendaal, L.M.; Daemen, J.H.T.; Franssen, A.J.P.M.; Hulsewé, K.W.E.; Vissers, Y.L.J.; de Loos, E.R. Uniportal versus multiportal VATS segmentectomy: Less is more? Transl. Lung Cancer Res. 2023, 12, 1140–1142. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.; Wan, Z.; Chen, Y.; She, Y.; Xie, D.; Hu, X.; Zhao, D.; Chen, C. The learning curve for uniportal video-assisted thoracoscopic anatomical segmentectomy. J. Surg. Oncol. 2021, 124, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Wang, H.; Fei, K.; Chen, C.; Zhao, D.; Zhou, X.; Xie, B.; Jiang, J.; Chen, Q.; Song, N.; et al. Single-port video-assisted thoracic surgery in 1063 cases: A single-institution experience. Eur. J. Cardiothorac. Surg. 2016, 49, i31–i36. [Google Scholar] [CrossRef]
- Ng, C.S.H.; MacDonald, J.K.; Gilbert, S.; Khan, A.Z.; Kim, Y.T.; Louie, B.E.; Marshall, M.B.; Santos, R.S.; Scarci, M.; Shargal, Y.; et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innovations 2019, 14, 90–116. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Q.; Lv, D. The single-incision versus multiple-incision video-assisted thoracoscopic surgery in the treatment of lung cancer: A systematic review and meta-analysis. Indian J. Cancer 2017, 54, 291–300. [Google Scholar]
- Harris, C.G.; James, R.S.; Tian, D.H.; Yan, T.D.; Doyle, M.P.; Gonzalez-Rivas, D.; Cao, C. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann. Cardiothorac. Surg. 2016, 5, 76–84. [Google Scholar] [CrossRef]
- Gonzalez, M.; Abdelnour-Berchtold, E.; Perentes, J.Y.; Doucet, V.; Zellweger, M.; Marcucci, C.; Ris, H.B.; Krueger, T.; Gronchi, F. An enhanced recovery after surgery program for video-assisted thoracoscopic surgery anatomical lung resections is cost-effective. J. Thorac. Dis. 2018, 10, 5879–5888. [Google Scholar] [CrossRef]
- Ahn, S.; Moon, Y. Learning curve for complex segmentectomy via uniportal video-assisted thoracoscopic surgery for the treatment of early-stage lung cancer. J. Thorac. Dis. 2024, 16, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Yamada, K.; Saito, H.; Oshita, F.; Ito, H.; Kameda, Y.; Noda, K. Sublobar Resection for Patients with Peripheral Small Adenocarcinomas of the Lung: Surgical Outcome is Associated with Features on Computed Tomographic Imaging. Ann. Thorac. Surg. 2007, 84, 1675–1679. [Google Scholar] [CrossRef]
- Suzuki, K.; Kusumoto, M.; Watanabe, S.; Tsuchiya, R.; Asamura, H. Radiologic classification of small adenocarcinoma of the lung: Radiologic-pathologic correlation and its prognostic impact. Ann. Thorac. Surg. 2006, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Hisao, A. Minimally invasive approach to early, peripheral adenocarcinoma with ground-glass opacity appearance. Ann. Thorac. Surg. 2008, 85, S701–S704. [Google Scholar]
- Chen, S.; Zhou, J.; Zhang, J.; Hu, H.; Luo, X.; Zhang, Y.; Chen, H. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg. Endosc. 2011, 25, 1723–1729. [Google Scholar] [CrossRef]
- Eichfeld, U.; Dietrich, A.; Ott, R.; Kloeppel, R. Video-assisted thoracoscopic surgery for pulmonary nodules after computed tomography-guided marking with a spiral wire. Ann. Thorac. Surg. 2005, 79, 313–316. [Google Scholar] [CrossRef]
- Kamiyoshihara, M.; Kakegawa, S.; Morishita, Y. Convenient and improved method to distinguish the intersegmental plane in pulmonary segmentectomy using a butterfly needle. Ann. Thorac. Surg. 2007, 83, 1913–1914. [Google Scholar] [CrossRef]
- Lempel, J.K.; Raymond, D.P.; Ahmad, U.; O’Malley, S.; Bolen, M.A.; Graham, R.; Azok, J.T.; Bullen, J.; Raja, S.; Murthy, S. Video-Assisted Thoracic Surgery Resection without Intraoperative Fluoroscopy after CT-Guided Microcoil Localization of Peripheral Pulmonary Nodules. J. Vasc. Interv. Radiol. 2018, 29, 1423–1428. [Google Scholar] [CrossRef]
- Findik, G.; Demiröz, M.; Apaydın, S.M.K.; Ertürk, H.; Biri, S.; Incekara, F.; Aydogdu, K.; Kaya, S. Computed Tomography-Guided Methylene Blue Labeling Prior to Thoracoscopic Resection of Small Deeply Placed Pulmonary Nodules. Do We Really Need Palpation? Thorac. Cardiovasc. Surg. 2017, 65, 387–391. [Google Scholar]
- Mogi, A.; Yajima, T.; Tomizawa, K.; Onozato, R.; Tanaka, S.; Kuwano, H. Video-Assisted Thoracoscopic Surgery after Preoperative CT-guided Lipiodol Marking of Small or Impalpable Pulmonary Nodules. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Lin, Y.; Lin, X.; Yu, X.; Wen, J.; Xi, K.; Lin, P.; Zhang, L. Localization of peripheral pulmonary lesions to aid surgical resection: A novel approach for electromagnetic navigation bronchoscopic dye marking. Eur. J. Cardiothorac. Surg. 2017, 52, 516–521. [Google Scholar] [CrossRef]
- Wada, H.; Anayama, T.; Hirohashi, K.; Nakajima, T.; Kato, T.; Waddell, T.K.; Keshavjee, S.; Yoshino, I.; Yasufuku, K. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules. Eur. J. Cardiothorac. Surg. 2016, 49, 690–697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kojima, F.; Sato, T.; Takahata, H.; Okada, M.; Sugiura, T.; Oshiro, O.; Date, H.; Nakamura, T. A novel surgical marking system for small peripheral lung nodules based on radio frequency identification technology: Feasibility study in a canine model. J. Thorac. Cardiovasc. Surg. 2014, 147, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yutaka, Y.; Nakamura, T.; Date, H. First clinical application of radiofrequency identification (RFID) marking system-Precise localization of a small lung nodule. JTCVS Tech. 2020, 24, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, S.; Waseda, R.; Ueda, Y.; Yutaka, Y.; Date, H.; Suzuki, J.; Oizumi, H.; Goto, M.; Nakagawa, T.; Kojima, F.; et al. Evaluation of the radiofrequency identification lung marking system: A multicenter study in Japan. Surg. Endosc. 2023, 37, 3619–3626. [Google Scholar] [CrossRef]
- Zaman, M.; Bilal, H.; Woo, C.Y.; Tang, A. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact. Cardiovasc. Thorac. Surg. 2012, 15, 266–272. [Google Scholar] [CrossRef]
- Hanauer, M.; Perentes, J.Y.; Krueger, T.; Ris, H.B.; Bize, P.; Schmidt, S.; Gonzalez, M. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: Report of 181 patients. J. Cardiothorac. Surg. 2016, 11, 5. [Google Scholar] [CrossRef]
- Gonzalez, M.; Federici, S.; Perentes, J. How I do VATS segmentectomy: The uniportal approach. J. Vis. Surg. 2022, 8, 22. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Yimin, N.; He, Z.; Chen, X. CT-guided hook-wire localization of malignant pulmonary nodules for video assisted thoracoscopic surgery. J. Cardiothorac. Surg. 2020, 15, 307. [Google Scholar] [CrossRef]
- Maqueda, L.B.; Jiang, L. Tips for uniportal video assisted thoracic surgery S1 segmentectomy. Video-Assist. Thorac. Surg. 2020, 5, 8. [Google Scholar] [CrossRef]
- Sakiyama, S.; Kondo, K.; Matsuoka, H.; Yoshida, M.; Miyoshi, T.; Yoshida, S.; Monden, Y. Fatal air embolism during computed tomography–guided pulmonary marking with a hook-type marker. J. Thorac. Cardiovasc. Surg. 2003, 126, 1207–1209. [Google Scholar] [CrossRef]
- Kamiyoshihara, M.; Sakata, K.; Ishikawa, S.; Morishita, Y. Cerebral arterial air embolism following CT-guided lung needle marking. Report of a case. J. Cardiovasc. Surg. 2001, 42, 699–700. [Google Scholar]
- Ohi, S.; Itoh, Y.; Neyatani, H.; Suzuki, K.; Kazui, T. Air embolism following computed tomography-guided lung needle marking; report of a case. Kyobu Geka 2004, 57, 421–423. [Google Scholar]
- Sun, C.; Bian, J.; Lai, S.; Li, X. Systemic air embolism as a complication of CT-guided percutaneous core needle lung biopsy: A case report and review of the literature. Exp. Ther. Med. 2015, 10, 1157–1160. [Google Scholar] [CrossRef]
- Lin, M.W.; Tseng, Y.H.; Lee, Y.F.; Hsieh, M.S.; Ko, W.C.; Chen, J.Y.; Hsu, H.H.; Chang, Y.C.; Chen, J.S. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J. Thorac. Cardiovasc. Surg. 2016, 152, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Koike, T.; Asakawa, T.; Kusumoto, M.; Asamura, H.; Nagai, K.; Tada, H.; Mitsudomi, T.; Tsuboi, M.; Shibata, T.; et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J. Thorac. Oncol. 2011, 6, 751–756. [Google Scholar] [CrossRef]
- Suzuki, K.; Watanabe, S.I.; Wakabayashi, M.; Saji, H.; Aokage, K.; Moriya, Y.; Yoshino, I.; Tsuboi, M.; Nakamura, S.; Nakamura, K.; et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J. Thorac. Cardiovasc. Surg. 2022, 163, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Suzuki, K.; Takamochi, K.; Wakabayashi, M.; Sekino, Y.; Tsutani, Y.; Nakajima, R.; Aokage, K.; Saji, H.; Tsuboi, M.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer with radiologically pure-solid appearance in Japan (JCOG0802/WJOG4607L): A post-hoc supplemental analysis of a multicentre, open-label, phase 3 trial. Lancet Respir. Med. 2024, 12, 105–116. [Google Scholar] [CrossRef]
- Handa, Y.; Tsutani, Y.; Mimae, T.; Tasaki, T.; Miyata, Y.; Okada, M. Surgical Outcomes of Complex Versus Simple Segmentectomy for Stage I Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2019, 107, 1032–1039. [Google Scholar] [CrossRef]
- Mahendran, K.; Hernandez-Arenas, L.A.; Gonzalez-Rivas, D. Uniportal VATS segmentectomy. Video-Assist. Thorac. Surg. 2020, 5, 36. [Google Scholar] [CrossRef]
- Chan, E.G.; Landreneau, J.R.; Schuchert, M.J.; Odell, D.D.; Gu, S.; Pu, J.; Luketich, J.D.; Landreneau, R.J. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2015, 150, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, k.; Nakazawa, S.; Nagashima, T.; Kuwano, H.; Mogi, A. 3D-CT anatomy for VATS segmentectomy. J. Vis. Surg. 2017, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, S.; Shimizu, K.; Kawatani, N.; Obayashi, K.; Ohtaki, Y.; Nagashima, T.; Eguchi, T.; Yajima, T.; Shirabe, K. Right upper lobe segmentectomy guided by simplified anatomic models. JTCVS Tech. 2020, 4, 288–297. [Google Scholar] [CrossRef]
- Hernandez-Arenas, L.A.; Purmessur, R.D.; Gonzalez-Rivas, D. Uniportal video-assisted thoracoscopic segmentectomy. J. Thorac. Dis. 2018, 10, S1205–S1214. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Swierzy, M.; Nachira, D.; Rückert, J.C.; Gonzalez-Rivas, D. Uniportal video-assisted thoracic surgery for major lung resections: Pitfalls, tips and tricks. J. Thorac. Dis. 2017, 9, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Maki, R.; Adachi, H. Simple smoke ventilation method for uniportal video-assisted thoracoscopic surgery. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac061. [Google Scholar] [CrossRef]
- Homma, T.; Saji, H.; Shimada, Y.; Tanabe, K. Experiences of novel cotton device for uniportal video-assisted thoracoscopic surgery: CS Two-Way HandleTM. J. Thorac. Dis. 2023, 15, 2800–2805. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, T.; Okami, J.; Miyoshi, T.; Wakabayashi, M.; Yoshioka, H.; Mimae, T.; Endo, M.; Hattori, A.; Nakagawa, K.; Isaka, T.; et al. Lymph node dissection in small peripheral lung cancer: Supplemental analysis of JCOG0802/WJOG4607L. J. Thorac. Cardiovasc. Surg. 2023. ahead of print. [Google Scholar] [CrossRef]
- Woo, W.; Shin, J.I.; Kipkorir, V.; Yang, Y.H.; Lee, S.; Lee, C.Y. Clinical Benefits of Lobe-Specific Lymph Node Dissection in Surgery for NSCLC: A Systematic Review and Meta-Analysis. JTO Clin. Res. Rep. 2023, 10, 100516. [Google Scholar] [CrossRef]
- Takamori, S.; Komiya, T.; Shimokawa, M.; Powell, E. Lymph node dissections and survival in sublobar resection of non-small cell lung cancer ≤ 20 mm. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 189–197. [Google Scholar]
- Tsubota, N. An improved method for distinguishing the intersegmental plane of the lung. Surg. Today 2000, 30, 963–964. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Mimura, T.; Ikegaki, J.; Katoh, H.; Itoh, H.; Tsubota, N. A novel video-assisted anatomic segmentectomy technique: Selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J. Thorac. Cardiovasc. Surg. 2007, 133, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Misaki, N.; Chang, S.S.; Gotoh, M.; Yamamoto, Y.; Satoh, K.; Yokomise, H. A novel method for determining adjacent lung segments with infrared thoracoscopy. J. Thorac. Cardiovasc. Surg. 2009, 138, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Igai, H.; Kamiyoshihara, M. How to identify and divide an intersegmental plane in uniportal VATS segmentectomy. Video-Assist. Thorac. Surg. 2020, 5, 1. [Google Scholar] [CrossRef]
- Misaki, N.; Tatakawa, K.; Chang, S.S.; Go, T.; Yokomise, H. Constant-rate intravenous infusion of indocyanine green leading to high fluorescence intensity in infrared thoracoscopic segmentectomy. JTCVS Tech. 2020, 3, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Ueshima, Y.; Ikebe, S.; Nakazono, C.; Urata, Y.; Okada, S.; Inoue, M. Usefulness of free pericardial fat pads as pledgets for air leaks in pulmonary resection. Surg. Today 2023, 53, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Kobayashi, N.; Miyazawa, M. Intraoperative control of air leak using a sutureless free pericardial fat pad covering method in lung cancer resection. Thorac. Cancer 2023, 14, 2627–2630. [Google Scholar] [CrossRef]
- Shintani, Y.; Inoue, M.; Funaki, S.; Kawamura, T.; Minami, M.; Okumura, M. Clinical usefulness of free subcutaneous fat pad for reduction of intraoperative air leakage during thoracoscopic pulmonary resection in lung cancer cases. Surg. Endosc. 2015, 29, 2910–2913. [Google Scholar] [CrossRef]
- Ikeda, T.; Sasaki, M.; Yamada, N.; Takamori, A.; Tanabe, S.; Okada, A.; Sakon, K.; Mizunaga, T.; Koshiji, T. Controlling Air Leaks Using Free Pericardial Fat Pads as Surgical Sealant in Pulmonary Resection. Ann. Thorac. Surg. 2015, 99, 1170–1175. [Google Scholar] [CrossRef]
- Hui, H.; Miao, H.; Qiu, F.; Li, H.; Lin, Y.; Jiang, B.; Zhang, Y. Comparison of analgesic effects of percutaneous and transthoracic intercostal nerve block in video-assisted thoracic surgery: A propensity score-matched study. J. Cardiothorac. Surg. 2024, 19, 33. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).