Exploring Extravasation in Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Extravasation and Its Significance in Cancer Care
3. Review Scope and Objectives
- Elucidating the pathophysiology of extravasation: This includes examining the mechanisms by which chemotherapy agents and radiotherapy cause tissue damage upon extravasation and identifying factors that influence the severity of extravasation reactions.
- Evaluating the incidence and risk factors associated with extravasation: This involves analyzing the available data on the prevalence of extravasation in cancer patients and identifying patient-related and treatment-related factors that predispose individuals to extravasation events.
- Clinical presentation and diagnosis of extravasation: This includes describing the typical signs and symptoms of extravasation, as well as reviewing the diagnostic tools and techniques used in confirming extravasation.
- Reviewing preventive strategies for extravasation: This part outlines the measures aimed at reducing the risk of extravasation during chemotherapy and radiotherapy administration, such as proper vascular access device selection, administration techniques, and patient education.
- Exploring management approaches for extravasation events: This involves presenting current guidelines and protocols for managing extravasation injuries, including pharmacological and non-pharmacological interventions.
- Complications and long-term effects of extravasation: This includes exploring potential complications arising from extravasation, as well as the impact of extravasation on patients’ quality of life and treatment outcomes.
- Patient education and support: This involves highlighting the importance of patient education regarding extravasation risks and early symptom recognition, as well as strategies for providing psychological support to the affected individuals.
- Identifying future research directions and opportunities: This includes identifying gaps in the current knowledge, areas for further investigation, and emerging technologies or therapies that may improve extravasation prevention and management.
4. Pathophysiology of Extravasation
4.1. Extravasation in Chemotherapy
4.2. Extravasation in Radiotherapy
4.3. Mechanisms of Tissue Damage Induced by Chemotherapy and Radiation Extravasation
4.4. Factors Influencing the Severity of Extravasation Reactions in Both Treatment Modalities
5. Incidence and Risk Factors
6. Clinical Presentation and Diagnosis
6.1. Typical Signs and Symptoms of Extravasation in Patients Receiving Chemotherapy and Radiotherapy
6.2. Challenges in Diagnosing Extravasation and Distinguishing It from Other Complications
6.3. Diagnostic Tools and Techniques Used in Confirming Extravasation for Both Treatment Modalities
7. Prevention Strategies
7.1. Preventive Measures Aimed at Reducing the Risk of Extravasation during Chemotherapy and Radiotherapy
7.2. Importance of Proper Vascular Access Device Selection, Administration Techniques, and Patient Education for Both Modalities
7.3. Management of Extravasation of Vesicants
- Stop the infusion: Immediately stop the infusion and leave the IV cannula or catheter in place.
- Aspirate the vesicant: Use a syringe to gently aspirate as much of the extravasated drug as possible through the existing IV cannula.
- Disconnect IV tubing: Disconnect the IV tubing while keeping the needle or cannula in place.
- Mark the area: Gently outline the affected area with a skin marker to monitor the changes.
- Administer the antidote if available: Refer to Table 2 for the specific antidote and administer it according to the guidelines.
- Elevate the affected limb: Elevate the affected limb to reduce swelling and promote reabsorption.
- Pain management: Administer the appropriate analgesics for pain relief.
- Monitor and document: Regularly assess the affected area for signs of improvement or worsening. Document all observations, interventions, and patient responses in the medical record.
- Follow-up care: Consult a specialist if significant tissue damage or necrosis is suspected. Educate the patient on the signs of infection or worsening condition and when to seek further medical attention. Schedule follow-up visits to monitor healing and manage complications.
- Review and improve practice: Conduct a review of the incident to identify areas for improvement. Provide additional training for staff on the proper administration of vesicants and the management of extravasation.
8. Complications and Long-Term Effects
9. Radiopharmaceutical Extravasation
10. Future Directions and Research Opportunities
10.1. Gaps in Current Knowledge and Areas for Future Research in Extravasation Prevention and Management for Both Chemotherapy and Radiotherapy
- Risk Factors and Predictive Models: Further research is needed to identify additional risk factors for extravasation, including patient-specific factors such as age, comorbidities, and vascular status. Developing predictive models that incorporate these factors could help stratify patients based on their risk of extravasation and guide personalized prevention strategies.
- Novel Prevention Strategies: Current prevention strategies primarily focus on proper vascular access device selection and administration techniques. Future research could explore the efficacy of novel preventive interventions, such as vein mapping technologies, protective dressings, or pharmacological agents, in reducing the incidence of extravasation.
- Early Detection Methods: Research into non-invasive or minimally invasive methods for the early detection of extravasation is warranted. Developing innovative imaging techniques or biomarkers that can accurately detect extravasation at its earliest stages could facilitate timely intervention and prevent tissue damage.
- Optimal Management Protocols: There is a need for standardized management protocols for extravasation events, including clear guidelines on the selection and administration of antidotes, wound care techniques, and follow-up strategies. Comparative studies evaluating the effectiveness of different management approaches and interventions are essential for establishing evidence-based protocols.
- Patient Education and Support: Research focusing on the effectiveness of patient education programs and supportive interventions in enhancing patient awareness, self-management, and coping strategies following extravasation events is needed. Understanding patients’ perspectives, experiences, and information needs can inform the development of tailored educational resources and support services.
- Long-Term Outcomes and Quality of Life: Limited research has examined the long-term consequences of extravasation on patients’ quality of life, functional outcomes, and psychological well-being. Longitudinal studies investigating the impact of extravasation-related complications, such as scarring, neuropathy, or chronic pain, on patients’ long-term health outcomes are necessary.
- Healthcare Provider Training and Competency: Research into effective strategies for training healthcare providers in extravasation prevention, recognition, and management is essential. Assessing the impact of educational interventions, simulation training, and competency assessments on healthcare providers’ knowledge, skills, and confidence in managing extravasation events can help improve patient safety and outcomes.
- Health Economics and Resource Utilization: Evaluating the economic burden of extravasation-related complications, including healthcare resource utilization, hospitalization costs, and productivity losses, is crucial for healthcare decision-making and resource allocation. Cost-effectiveness analyses of different prevention and management strategies can inform policy and practice guidelines.
10.2. Emerging Technologies or Therapies That May Improve Extravasation Prevention and Management in Both Treatment Modalities
- Vein Visualization Technologies: Advanced vein visualization devices, such as near-infrared imaging and augmented reality systems, offer real-time visualization of the peripheral veins, enhancing the accuracy of vascular access device placement and reducing the risk of extravasation. These technologies provide healthcare providers with improved guidance for catheter insertion, especially in patients with difficult-to-access veins.
- Smart Catheters and Infusion Systems: The development of smart catheters and infusion systems equipped with sensors and feedback mechanisms enables the real-time monitoring of infusion parameters, including flow rates, pressure, and drug compatibility. These systems can alert healthcare providers to potential extravasation events and automatically adjust the infusion parameters to minimize the risk of tissue damage.
- Targeted Drug Delivery Systems: Targeted drug delivery systems, such as liposomes, nanoparticles, or drug-eluting implants, offer a localized and controlled release of chemotherapy or radiotherapy agents, reducing systemic toxicity and the risk of extravasation-related complications. These technologies allow for the precise delivery of therapeutic agents to tumor tissues while minimizing exposure to healthy tissues.
- Topical Treatments and Wound Care Products: Novel topical treatments and wound care products specifically designed for extravasation injuries offer promising avenues for improving tissue healing and minimizing scarring—advanced wound dressings incorporating growth factors, antimicrobial agents, or tissue-engineered scaffolds to promote tissue regeneration and accelerate wound closure are some of these avenues.
- AI for Image Analysis: AI-based image analysis algorithms can enhance the detection and diagnosis of extravasation events in imaging studies, such as ultrasound, MRI, and CT. These algorithms can automatically identify subtle signs of extravasation, assist healthcare providers in interpreting the imaging findings, and facilitate a timely intervention. This suggestion is subsequently extended as a separate discussion.
11. AI for Image Analysis in Extravasation Detection
- RetinaNet [126]: This CNN is a single-stage object detection model known for its effectiveness in detecting small and transient objects. It addresses the challenge of class imbalance in dense detection tasks by employing a focal loss function. This enables RetinaNet to assign higher weights to hard-to-detect objects, making it particularly suitable for detecting subtle abnormalities like extravasation.
- Mask R-CNN [127]: This model extends Faster R-CNN by adding a mask prediction branch, enabling pixel-level segmentation in addition to object detection. This makes it well-suited for tasks requiring precise localization of small and transient objects. Mask R-CNN has been successfully applied to various medical imaging tasks and can be adapted for detecting extravasation with high accuracy.
- YOLOv4 [128]: It is an advanced version of the YOLO (you only look once) object detection algorithm known for its speed and accuracy. It utilizes a single neural network to predict bounding boxes and class probabilities for small and transient objects in real time. YOLOv4’s efficiency and effectiveness make it suitable for detecting extravasation events in medical images.
- EfficientDet [129]: This pretrained CNN is a family of efficient and accurate object detection models based on the EfficientNet backbone architecture. These models achieve state-of-the-art performance with significantly fewer parameters compared to traditional models, making them well-suited for resource-constrained environments like medical imaging applications. EfficientDet’s lightweight design and high accuracy make it suitable for detecting small and transient objects like extravasation.
- CenterNet [130]: This net is a simple and efficient object detection model that directly predicts object centers and regresses bounding boxes from them. This approach makes CenterNet particularly effective for detecting small and transient objects with high accuracy. CenterNet’s simplicity and effectiveness make it a viable option for detecting extravasation events in medical images.
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, M.; Borders, L.; Wesolow, J.T.; Greene, J. Chemotherapy extravasation causing soft-tissue necrosis mimicking infection: A longitudinal case study. Cureus 2024, 16, e55333. [Google Scholar] [CrossRef] [PubMed]
- Kreidieh, F.Y.; Moukadem, H.A.; El Saghir, N.S. Overview, prevention and management of chemotherapy extravasation. World J. Clin. Oncol. 2016, 7, 87–97. [Google Scholar] [CrossRef]
- Pluschnig, U.; Haslik, W.; Bartsch, R.; Mader, R.M. Extravasation emergencies: State-of-the-art management and progress in clinical research. Mag. Eur. Med. Oncol. 2016, 9, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Perez Fidalgo, J.A.; Garcia Fabregat, L.; Cervantes, A.; Margulies, A.; Vidall, C.; Roila, F.; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann. Oncol. 2012, 23 (Suppl. S7), vii167–vii173. [Google Scholar] [CrossRef] [PubMed]
- Al-Benna, S.; O’Boyle, C.; Holley, J. Extravasation injuries in adults. ISRN Dermatol. 2013, 2013, 856541. [Google Scholar] [CrossRef] [PubMed]
- Apisarnthanarax, N.; Duvic, M.M. Extravasation Reactions. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Jr., Gansler, T.S., Holland, J.F., Frei, E., III, Eds.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Advice Sheet following Extravasation of Radiographic Contrast Material. NHS Hull University Teaching Hospitals. Last Updated: 22 April 2021. Available online: https://www.hey.nhs.uk/patient-leaflet/advice-sheet-following-extravasation-radiographic-contrast-material-2/ (accessed on 5 June 2024).
- Liu, W.; Wang, P.; Zhu, H.; Tang, H.; Guan, H.; Wang, X.; Wang, C.; Qiu, Y.; He, L. Contrast media extravasation injury: A prospective observational cohort study. Eur. J. Med. Res. 2023, 28, 458. [Google Scholar] [CrossRef] [PubMed]
- Smolders, E.J.; Benoist, G.E.; Smit, C.C.H.; Ter Horst, P. An update on extravasation: Basic knowledge for clinical pharmacists. Eur. J. Hosp. Pharm. 2020, 28, 165–167. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, J.; Voo, S.; Bucerius, J.; Mottaghy, F.M. Consequences of radiopharmaceutical extravasation and therapeutic interventions: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L. Extravasation injuries: A trivial injury often overlooked with disastrous consequences. World J. Plast. Surg. 2020, 9, 326–330. [Google Scholar] [CrossRef]
- Kim, J.T.; Park, J.Y.; Lee, H.J.; Cheon, Y.J. Guidelines for the management of extravasation. J. Educ. Eval. Health Prof. 2020, 17, 21. [Google Scholar] [CrossRef]
- Ener, R.A.; Meglathery, S.B.; Styler, M. Extravasation of systemic hemato-oncological therapies. Ann. Oncol. 2004, 15, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Keritam, O.; Juhasz, V.; Schofer, C.; Thallinger, C.; Aretin, M.B.; Schabbauer, G.; Breuss, J.; Unseld, M.; Uhrin, P. Determination of extravasation effects of nal-iri and trabectedin and evaluation of treatment options for trabectedin extravasation in a preclinical animal model. Front. Pharmacol. 2022, 13, 875695. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls (Internet); StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564367/ (accessed on 5 June 2024).
- O’Malley, D.M.; Alfano, C.M.; Doose, M.; Kinney, A.Y.; Lee, S.J.C.; Nekhlyudov, L.; Duberstein, P.; Hudson, S.V. Cancer prevention, risk reduction, and control: Opportunities for the next decade of health care delivery research. Transl. Behav. Med. 2021, 11, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, R.; Larson, D.L. Etiology and treatment of chemotherapeutic agent extravasation injuries: A review. J. Clin. Oncol. 1987, 5, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.Y.; Mammadova, A.; Benkirane-Jessel, N.; Desaubry, L.; Nebigil, C.G. Updates on anticancer therapy-mediated vascular toxicity and new horizons in therapeutic strategies. Front. Cardiovasc. Med. 2021, 8, 694711. [Google Scholar] [CrossRef]
- Terwoord, J.D.; Beyer, A.M.; Gutterman, D.D. Endothelial dysfunction as a complication of anti-cancer therapy. Pharmacol. Ther. 2022, 237, 108116. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.C.; Touyz, R.M.; Lang, N.N. Vascular complications of cancer chemotherapy. Can. J. Cardiol. 2016, 32, 852–862. [Google Scholar] [CrossRef]
- Barton, A. Extravasation and infiltration: Under-recognised complications of intravenous therapy. Br. J. Nurs. 2024, 33, S18–S26. [Google Scholar] [CrossRef]
- Armenteros-Yeguas, V.; Garate-Echenique, L.; Tomas-Lopez, M.A.; Cristobal-Domínguez, E.; Moreno-de Gusmao, B.; Miranda-Serrano, E.; Moraza-Dulanto, M.I. Prevalence of difficult venous access and associated risk factors in highly complex hospitalised patients. J. Clin. Nurs. 2017, 26, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Billingham, M.J.; Mittal, R. Peripheral venous extravasation injury. BJA Educ. 2023, 23, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Nepon, H.; Safran, T.; Reece, E.M.; Murphy, A.M.; Vorstenbosch, J.; Davison, P.G. Radiation-Induced Tissue Damage: Clinical Consequences and Current Treatment Options. Semin. Plast. Surg. 2021, 35, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, A.; Sharma, N.; Sarin, A.; Bhatnagar, S.; Chakravarty, N.; Mukundan, H.; Suhag, V.; Singh, S. Radiation fibrosis syndrome: The evergreen menace of radiation therapy. Asia Pac. J. Oncol. Nurs. 2019, 6, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Wynn, T.A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol. 2009, 2, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Sodji, Q.H.; Oyelere, A.K. Inflammation, fibrosis and cancer: Mechanisms, therapeutic options and challenges. Cancers 2022, 14, 552. [Google Scholar] [CrossRef]
- Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Yang, X.; Bodd, M.H.; Singh, P.K.; Yusuf, S.W.; Abe, J.I.; Krishnan, S. Radiation-induced endothelial vascular injury: A review of possible mechanisms. JACC Basic Transl. Sci. 2018, 3, 563–572. [Google Scholar] [CrossRef]
- Yang, E.H.; Marmagkiolis, K.; Balanescu, D.V.; Hakeem, A.; Donisan, T.; Finch, W.; Virmani, R.; Herrman, J.; Cilingiroglu, M.; Grines, C.L.; et al. Radiation-induced vascular disease-A state-of-the-art review. Front. Cardiovasc. Med. 2021, 8, 652761. [Google Scholar] [CrossRef]
- LeBlanc, A.J.; Krishnan, L.; Sullivan, C.J.; Williams, S.K.; Hoying, J.B. Microvascular repair: Post-angiogenesis vascular dynamics. Microcirculation 2012, 19, 676–695. [Google Scholar] [CrossRef]
- Fernandes, G.N.C. Immunotherapy as a turning point in the treatment of melanoma brain metastases. Discoveries 2023, 11, e169. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Guipaud, O.; Jaillet, C.; Clement-Colmou, K.; François, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef]
- Kirthi Koushik, A.S.; Harish, K.; Avinash, H.U. Principles of radiation oncology: A beams eye view for a surgeon. Indian J. Surg. Oncol. 2013, 4, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Chaput, G.; Regnier, L. Radiotherapy: Clinical pearls for primary care. Can. Fam. Physician 2021, 67, 753–757. [Google Scholar] [CrossRef]
- Suzuki, G.; Yamazaki, H.; Aibe, N.; Masui, K.; Shimizu, D.; Kimoto, T.; Nishimura, T.; Kawabata, K.; Nagasawa, S.; Machida, K.; et al. Comparison of three fractionation schedules in radiotherapy for early glottic squamous cell carcinoma. In Vivo 2020, 34, 2769–2774. [Google Scholar] [CrossRef]
- Brand, D.H.; Kirby, A.M.; Yarnold, J.R.; Somaiah, N. How low can you go? The radiobiology of hypofractionation. Clin. Oncol. 2022, 34, 280–287. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Liu, Y.P.; Xie, Y.L.; Lin, C.; Duan, C.Y.; Chen, D.P.; Pan, Y.; Qi, B.; Zou, X.; Guo, L.; et al. Hyperfractionation compared with standard fractionation in intensity-modulated radiotherapy for patients with locally advanced recurrent nasopharyngeal carcinoma: A multicentre, randomised, open-label, phase 3 trial. Lancet 2023, 401, 917–927. [Google Scholar] [CrossRef]
- Paoletti, L.; Ceccarelli, C.; Menichelli, C.; Aristei, C.; Borghesi, S.; Tucci, E.; Bastiani, P.; Cozzi, S. Special stereotactic radiotherapy techniques: Procedures and equipment for treatment simulation and dose delivery. Rep. Pract. Oncol. Radiother. 2022, 27, 1–9. [Google Scholar] [CrossRef]
- Tsang, M.W. Stereotactic body radiotherapy: Current strategies and future development. J. Thorac. Dis. 2016, 8 (Suppl. S6), S517–S527. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.C.; van der Kogel, A.J. Basic Clinical Radiobiology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Pajonk, F.; Vlashi, E.; McBride, W.H. Radiation resistance of cancer stem cells: The 4 R’s of radiobiology revisited. Stem Cells 2010, 28, 639–648. [Google Scholar] [CrossRef]
- Brand, D.H.; Yarnold, J.R. The linear-quadratic model and implications for fractionation. Clin. Oncol. 2019, 31, 673–677. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy side-effects: Not all DNA damage is equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Borrego-Soto, G.; Ortiz-Lopez, R.; Rojas-Martínez, A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, Z.; Ma, S.; Liu, X. Radiotherapy and Cytokine Storm: Risk and Mechanism. Front. Oncol. 2021, 11, 670464. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, C.; Song, B.; Zhang, S.; Chen, C.; Li, C.; Zhang, S. Tissue fibrosis induced by radiotherapy: Current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J. Transl. Med. 2023, 21, 708. [Google Scholar] [CrossRef]
- Corbett, M.; Marshall, D.; Harden, M.; Oddie, S.; Phillips, R.; McGuire, W. Treatment of extravasation injuries in infants and young children: A scoping review and survey. Health Technol. Assess. 2018, 22, 1–112. [Google Scholar] [CrossRef]
- Liu, W.; Wang, P.; Zhu, H.; Tang, H.; Wang, X.; Guan, H.; Wang, C.; Qiu, Y.; Peng, A.; He, L. Risk factors for contrast media extravasation in intravenous contrast-enhanced computed tomography: An observational cohort study. Acad. Radiol. 2024, 31, 1792–1798. [Google Scholar] [CrossRef]
- Chiu, K.; Tindholdt, T.T.; Tonseth, K.A. Extravasation injuries. Tidsskr. Nor. Laegeforen. 2016, 136, 233–235. (In Norwegian) [Google Scholar] [CrossRef]
- Russ, E.; Davis, C.M.; Slaven, J.E.; Bradfield, D.T.; Selwyn, R.G.; Day, R.M. Comparison of the medical uses and cellular effects of high and low linear energy transfer radiation. Toxics 2022, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Behlendorf, T.; Mueller, F.; Schmoll, H.J. Anthracycline extravasation injuries: Management with dexrazoxane. Ther. Clin. Risk Manag. 2009, 5, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.C.S.; Bitencourt, A.G.V.; Chojniak, R. Extravasation of iodinated contrast medium in cancer patients undergoing computed tomography. Radiol. Bras. 2018, 51, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Nicola, R.; Shaqdan, K.W.; Aran, S.; Prabhakar, A.M.; Singh, A.K.; Abujudeh, H.H. Contrast media extravasation of computed tomography and magnetic resonance imaging: Management guidelines for the radiologist. Curr. Probl. Diagn. Radiol. 2016, 45, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Meng, L.; Hou, X.; Qu, C.; Wang, B.; Xin, Y.; Jiang, X. Radiation-induced skin reactions: Mechanism and treatment. Cancer Manag. Res. 2018, 11, 167–177. [Google Scholar] [CrossRef]
- Dormand, E.L.; Banwell, P.E.; Goodacre, T.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Roditi, G.; Khan, N.; van der Molen, A.J.; Bellin, M.F.; Bertolotto, M.; Brismar, T.; Correas, J.M.; Dekkers, I.A.; Geenen, R.W.F.; Heinz-Peer, G.; et al. Intravenous contrast medium extravasation: Systematic review and updated ESUR Contrast Media Safety Committee Guidelines. Eur. Radiol. 2022, 32, 3056–3066. [Google Scholar] [CrossRef]
- West Midlands Expert Advisory Group for Chemotherapy. Guideline for the Management of Extravasation of a Systemic Anti-Cancer Therapy Including Cytotoxic Agents; West Midlands Expert Advisory Group for Systemic Anti-Cancer Therapy (SACT): London, UK, 2017; Available online: https://www.england.nhs.uk/midlands/wp-content/uploads/sites/46/2019/05/management-extravasation-of-a-systemic-anti-cancer-therapy-including-cytotoxic-agents.pdf (accessed on 5 June 2024).
- Belzunegui, T.; Louis, C.J.; Torrededia, L.; Oteiza, J. Extravasation of radiographic contrast material and compartment syndrome in the hand: A case report. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- IV Infiltrations and Extravasations: Causes, Signs, Side Effects, and Treatment. ivWatch. Available online: https://www.ivwatch.com/2020/05/27/iv-infiltrations-and-extravasations-causes-signs-side-effects-and-treatment/ (accessed on 6 May 2024).
- Bahrami, M.; Karimi, T.; Yadegarfar, G.; Norouzi, A. Assessing the quality of existing clinical practice guidelines for chemotherapy drug extravasation by appraisal of guidelines for research and evaluation II. Iran. J. Nurs. Midwifery Res. 2019, 24, 410–416. [Google Scholar] [CrossRef]
- Spinnato, P.; Patel, D.B.; Di Carlo, M.; Bartoloni, A.; Cevolani, L.; Matcuk, G.R.; Crombé, A. Imaging of musculoskeletal soft-tissue infections in clinical practice: A comprehensive updated review. Microorganisms 2022, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Yemane, P.T.; Aslund, A.K.O.; Snipstad, S.; Bjorkoy, A.; Grendstad, K.; Berg, S.; Morch, Y.; Torp, S.H.; Hansen, R.; Davies, C.L. Effect of ultrasound on the vasculature and extravasation of nanoscale particles imaged in real time. Ultrasound Med. Biol. 2019, 45, 3028–3041. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L.; Hossack, J.A. Ultrasound in radiology: From anatomic, functional, molecular imaging to drug delivery and image-guided therapy. Investig. Radiol. 2015, 50, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Okuda, H.; Masatsugu, A.; Sijimaya, T.; Arai, R. Skin necrosis due to the extravasation of irritant anticancer agents. Intern. Med. 2018, 57, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Jeraj, R.; Cao, Y.; Ten Haken, R.K.; Hahn, C.; Marks, L. Imaging for assessment of radiation-induced normal tissue effects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. S3), S140–S144. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.E.; Griffie, J.; Czaplewski, L.M. Eliminating extravasation events: A multidisciplinary approach. J. Infus. Nurs. 2015, 38 (Suppl. S6), S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.S.; Mohammad, Z.A.; Azer, S.Z.; Khallaf, S.M. Impact of in- service training program on nurses’ performance for minimizing chemotherapy extravasation. Asian Pac. J. Cancer Prev. 2023, 24, 3537–3542. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.F.; Jakobsen, J.A.; Tomassin, I.; Thomsen, H.S.; Morcos, S.K.; Thomsen, H.S.; Morcos, S.K.; Almen, T.; Aspelin, P.; Bellin, M.F.; et al. Contrast medium extravasation injury: Guidelines for prevention and management. Eur. Radiol. 2002, 12, 2807–2812. [Google Scholar] [CrossRef]
- Sugawara, S.; Sone, M.; Sakamoto, N.; Sofue, K.; Hashimoto, K.; Arai, Y.; Tokue, H.; Takigawa, M.; Mimura, H.; Yamanishi, T.; et al. Guidelines for central venous port placement and management (abridged translation of the Japanese version). Interv. Radiol. 2023, 8, 105–117. [Google Scholar] [CrossRef]
- Chan, K.M.; Chau, J.P.C.; Choi, K.C.; Fung, G.P.G.; Lui, W.W.; Chan, M.S.Y.; Lo, S.H.S. Clinical practice guideline on the prevention and management of neonatal extravasation injury: A before-and-after study design. BMC Pediatr. 2020, 20, 445. [Google Scholar] [CrossRef]
- Vyas, V.; Sharma, A.; Goyal, S.; Kothari, N. Infrared vein visualization devices for ease of intravenous access in children: Hope versus hype. Anaesthesiol. Intensive Ther. 2021, 53, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Chiao, F.B.; Resta-Flarer, F.; Lesser, J.; Ng, J.; Ganz, A.; Pino-Luey, D.; Bennett, H.; Perkins, C., Jr.; Witek, B. Vein visualization: Patient characteristic factors and efficacy of a new infrared vein finder technology. Br. J. Anaesth. 2013, 110, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Hirata, I.; Mazzotta, A.; Makvandi, P.; Cesini, I.; Brioschi, C.; Ferraris, A.; Mattoli, V. Sensing technologies for extravasation detection: A review. ACS Sens. 2023, 8, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Murayama, R.; Tanabe, H.; Oe, M.; Motoo, Y.; Wagatsuma, T.; Michibuchi, M.; Kinoshita, S.; Sakai, K.; Konya, C.; et al. Evaluation of the predictive validity of thermography in identifying extravasation with intravenous chemotherapy infusions. J. Infus. Nurs. 2017, 40, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, R.K.; Kabir, K.; Müller, A.; Heydweiller, A.; Burger, C.; Welle, K. extravasation injuries of the limbs in neonates and children—Development of a treatment algorithm. Dtsch. Arztebl. Int. 2021, 118, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Bahoush, G.; Salajegheh, P.; Anari, A.M.; Eshghi, A.; Aski, B.H. A review of peripherally inserted central catheters and various types of vascular access in very small children and pediatric patients and their potential complications. J. Med. Life 2021, 14, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Patel, A.R.; Singh, S.; Singh, S.; Khawaja, I. Central line catheters and associated complications: A review. Cureus 2019, 11, e4717. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Hermand, V.; Bonny, V.; Preda, G.; Urbina, T.; Gasperment, M.; Gabarre, P.; Missri, L.; Baudel, J.L.; Zafimahazo, D.; et al. Improvement of central vein ultrasound-guided puncture success using a homemade needle guide-a simulation study. Crit. Care 2023, 27, 379. [Google Scholar] [CrossRef]
- Goossens, G.A. Flushing and locking of venous catheters: Available evidence and evidence deficit. Nurs. Res. Pract. 2015, 2015, 985686. [Google Scholar] [CrossRef]
- Vahdat, S.; Hamzehgardeshi, L.; Hessam, S.; Hamzehgardeshi, Z. Patient involvement in health care decision making: A review. Iran. Red. Crescent Med. J. 2014, 16, e12454. [Google Scholar] [CrossRef]
- NHS Cambridge University Hospitals. Central Venous Line—Care of. Available online: https://www.cuh.nhs.uk/patient-information/central-venous-line-care-of/#:~:text=Caring%20for%20your%20central%20venous%20line,looks%20mucky%20or%20is%20bleeding (accessed on 17 May 2024).
- Management of Chemotherapy Extravasation: ESMO Clinical Practice Guidelines. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-supportive-and-palliative-care/chemotherapy-extravasation (accessed on 1 June 2024).
- Olsen, M.M.; LeFebvre, K.; Walker, S.L.; Dunphy, E.P. Chemotherapy and Immunotherapy Guidelines and Recommendations for Practice, 2nd ed.; Oncology Nursing Society: Pittsburgh, PA, USA, 2023. [Google Scholar]
- National Comprehensive Cancer Network (NCCN). Development and Update of Guidelines. Available online: https://www.nccn.org/guidelines/guidelines-process/development-and-update-of-guidelines (accessed on 1 June 2024).
- Schulmeister, L. Extravasation management: Clinical update. Semin. Oncol. Nurs. 2011, 27, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N. Anthracyclines in the treatment of cancer. An overview. Drugs 1997, 54 (Suppl. S4), 1–7. [Google Scholar] [CrossRef] [PubMed]
- MedicalNewsToday. What to Know about Anthracycline Chemotherapy. 29 June 2022. Available online: https://www.medicalnewstoday.com/articles/anthracycline-chemotherapy (accessed on 30 May 2024).
- Moudi, M.; Go, R.; Yien, C.Y.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updat. 2021, 54, 100742. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Racioppi, M.; D’Agostino, D.; Cappa, E.; Filianoti, A.; Bassi, P.F. Mitomycin C for the treatment of bladder cancer. Minerva Urol. Nefrol. 2010, 62, 133–144. [Google Scholar] [PubMed]
- Liu, X.F.; Xiang, L.; Zhou, Q.; Carralot, J.P.; Prunotto, M.; Niederfellner, G.; Pastan, I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 10666–10671. [Google Scholar] [CrossRef]
- Zhang, W.; Gou, P.; Dupret, J.M.; Chomienne, C.; Rodrigues-Lima, F. Etoposide, an anticancer drug involved in therapy-related secondary leukemia: Enzymes at play. Transl. Oncol. 2021, 14, 101169. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Meystre, N.R.; Campeanu, C.; Gullo, G. Contrast media extravasations in patients undergoing computerized tomography scanning: A systematic review and meta-analysis of risk factors and interventions. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 87–116. [Google Scholar] [CrossRef]
- Gundeşlioglu, A.O.; Ozen, E.C. Necrotizing fasciitis of the cervical region following extravasation injury. Case Rep. Med. 2012, 2012, 941578. [Google Scholar] [CrossRef]
- Ghanem, A.M.; Mansour, A.; Exton, R.; Powell, J.; Mashhadi, S.; Bulstrode, N.; Smith, G. Childhood extravasation injuries: Improved outcome following the introduction of hospital-wide guidelines. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Extravasation Injuries: Prevention and Management. MCN for Neonatology West of Scotland Neonatal Guideline; Scottish Perinatal Network: Glasgow, UK, 2019; 11p, Available online: https://perinatalnetwork.scot/wp-content/uploads/2022/06/Extravasation-Neonates_WoS.pdf (accessed on 7 May 2024).
- Albano, D.; Benenati, M.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Cozzi, D.; De Robertis, R.; Gentili, F.; Grazzini, I.; et al. Imaging side effects and complications of chemotherapy and radiation therapy: A pictorial review from head to toe. Insights Imaging 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Harding-Lister, S. Common Injuries in Iron Extravasation. Apex Health Associate. Available online: https://www.apexhealth.net/post/common-injuries-in-iron-extravasation#:~:text=Anxiety%20and%20Fear%20of%20Medical,a%20shadow%20over%20future%20treatments (accessed on 8 May 2024).
- Duarte, A.C.D.S.; Chicharo, S.C.R.; Silva, T.A.S.M.D.; Oliveira, A.B. Ethical dilemmas and illicit acts in nursing: Reflections on the legal (dis)order. Rev. Bras. Enferm. 2023, 76 (Suppl. S3), e20220558. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Salih, S.; Alkatheeri, A.; Alomaim, W.; Elliyanti, A. Radiopharmaceutical treatments for cancer therapy, radionuclides characteristics, applications, and challenges. Molecules 2022, 27, 5231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lei, H.; Chen, X.; Dou, Z.; Yu, B.; Su, W.; Wang, W.; Jin, X.; Katsube, T.; Wang, B.; et al. Carrier systems of radiopharmaceuticals and the application in cancer therapy. Cell Death Discov. 2024, 10, 16. [Google Scholar] [CrossRef]
- Hayes, R.B. Dose estimation for extravasation of 177Lu, 99mTc, and 18F. Health Phys. 2023, 124, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.R. Radiopharmaceutical extravasations: A twenty year mini-review. Front. Nucl. Med. 2023, 3, 1219202. [Google Scholar] [CrossRef]
- Wilson, S.; Osborne, D.; Long, M.; Knowland, J.; Fisher, D.R. Practical tools for patient-specific characterization and dosimetry of radiopharmaceutical extravasation. Health Phys. 2022, 123, 343–347. [Google Scholar] [CrossRef]
- Iori, M.; Grassi, E.; Piergallini, L.; Meglioli, G.; Botti, A.; Sceni, G.; Cucurachi, N.; Verzellesi, L.; Finocchiaro, D.; Versari, A.; et al. Safety injections of nuclear medicine radiotracers: Towards a new modality for a real-time detection of extravasation events and 18F-FDG SUV data correction. EJNMMI Phys. 2023, 10, 31. [Google Scholar] [CrossRef]
- Santos-Oliveira, R. Undesirable events with radiopharmaceuticals. Tohoku J. Exp. Med. 2009, 217, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.; Lattanze, R.; Knowland, J.; Bryant, T.E.; Barvi, I.; Fu, Y.; Kiser, J.W. The scientific and clinical case for reviewing diagnostic radiopharmaceutical extravasation long-standing assumptions. Front. Med. 2021, 8, 684157. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Crowley, J.R.; Warden, N. Radiopharmaceutical administration practices—Are they best practice? Front. Nucl. Med. 2023, 3, 1244660. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Borcoman, E.; Kamal, M. Molecular profiling in precision medicine oncology. Nat. Med. 2019, 25, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Mortellaro, G.; Trapani, G.; Valenti, V.; Ognibene, L.; De Gregorio, G.; Quartuccio, E.; Luca, N.; Tripoli, A.; Serretta, V.; et al. Acute and late toxicity and preliminary outcomes report of moderately hypofractionated helical tomotherapy for localized prostate cancer: A mono-institutional analysis. Radiol. Med. 2020, 125, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.; Jia, J.B.; Green, C.S.; Gulati, A.T.; Lall, C. Cancer therapy related complications in the liver, pancreas, and biliary system: An imaging perspective. Insights Imaging 2015, 6, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.; Hindocha, S.; Lee, R.W. The role of artificial intelligence in early cancer diagnosis. Cancers 2022, 14, 1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, H.; Wang, H. Machine learning and AI in cancer prognosis, prediction, and treatment selection: A critical approach. J. Multidiscip. Healthc. 2023, 16, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.M.; Papanikolaou, N.; Bick, U.; Illing, R.; Kahn, C.E., Jr.; Kalpathi-Cramer, J.; Matos, C.; Martí-Bonmatí, L.; Miles, A.; Mun, S.K.; et al. Artificial intelligence and machine learning in cancer imaging. Commun. Med. 2022, 2, 133. [Google Scholar] [CrossRef]
- Gala, D.; Behl, H.; Shah, M.; Makaryus, A.N. The role of artificial intelligence in improving patient outcomes and future of healthcare delivery in cardiology: A narrative review of the literature. Healthcare 2024, 12, 481. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Nakada, A.; Niikura, R.; Otani, K.; Kurose, Y.; Hayashi, Y.; Kitamura, K.; Nakanishi, H.; Kawano, S.; Honda, T.; Hasatani, K.; et al. Improved object detection artificial intelligence using the revised RetinaNet model for the automatic detection of ulcerations, vascular lesions, and tumors in wireless capsule endoscopy. Biomedicines 2023, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chu, J.; Leng, L.; Miao, J. Mask-refined R-CNN: A network for refining object details in instance segmentation. Sensors 2020, 20, 1010. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, J.; Wang, J.; Yuan, J. Lightweight YOLOv4 with multiple receptive fields for detection of pulmonary tuberculosis. Comput. Intell. Neurosci. 2022, 2022, 9465646. [Google Scholar] [CrossRef]
- Tan, M.; Pang, R.; Le, Q.V. EfficientDet: Scalable and efficient object detection. In Proceedings of the 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Seattle, WA, USA, 18–24 June 2020; pp. 10778–10787. [Google Scholar] [CrossRef]
- Duan, K.; Bai, S.; Xie, L.; Qi, H.; Huang, Q.; Tian, Q. CenterNet: Keypoint triplets for object detection. In Proceedings of the 2019 IEEE/CVF International Conference on Computer Vision (ICCV), Seoul, Republic of Korea, 27 October–2 November 2019; pp. 6568–6577. [Google Scholar] [CrossRef]
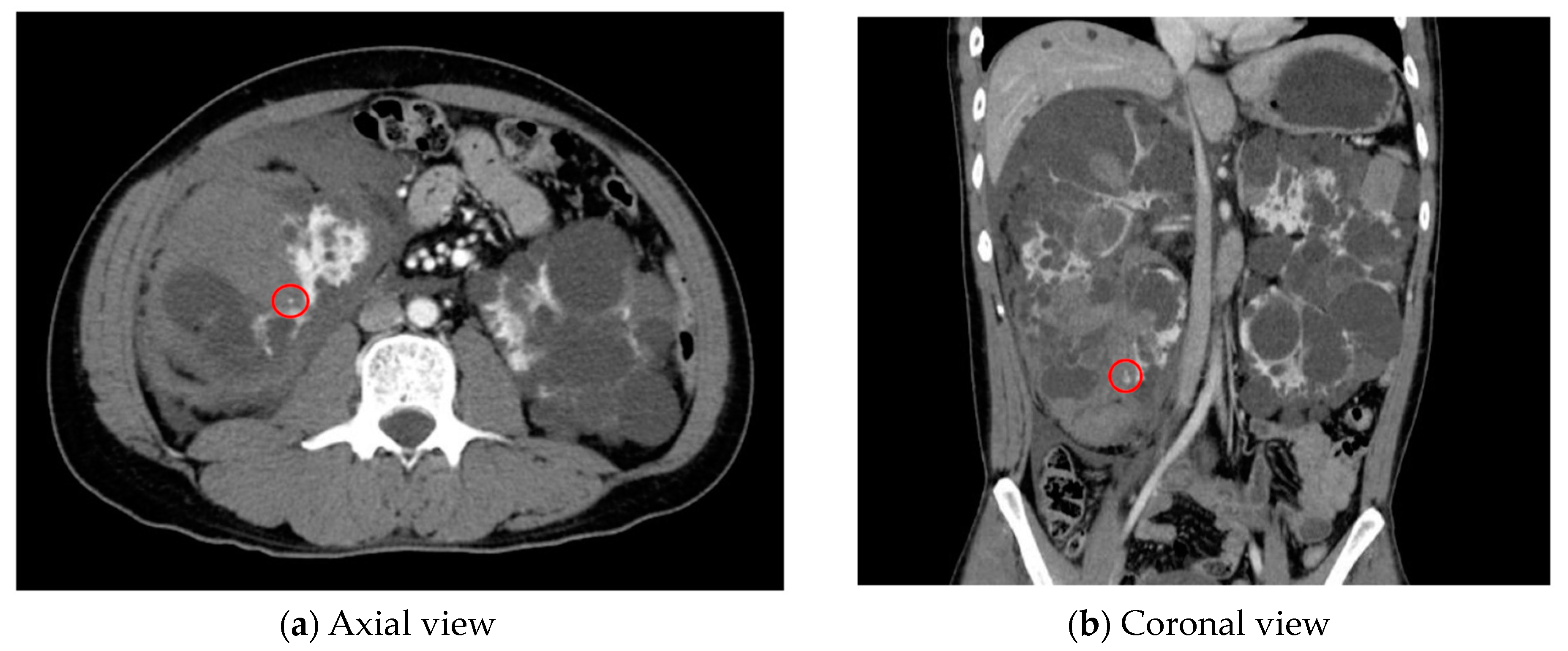
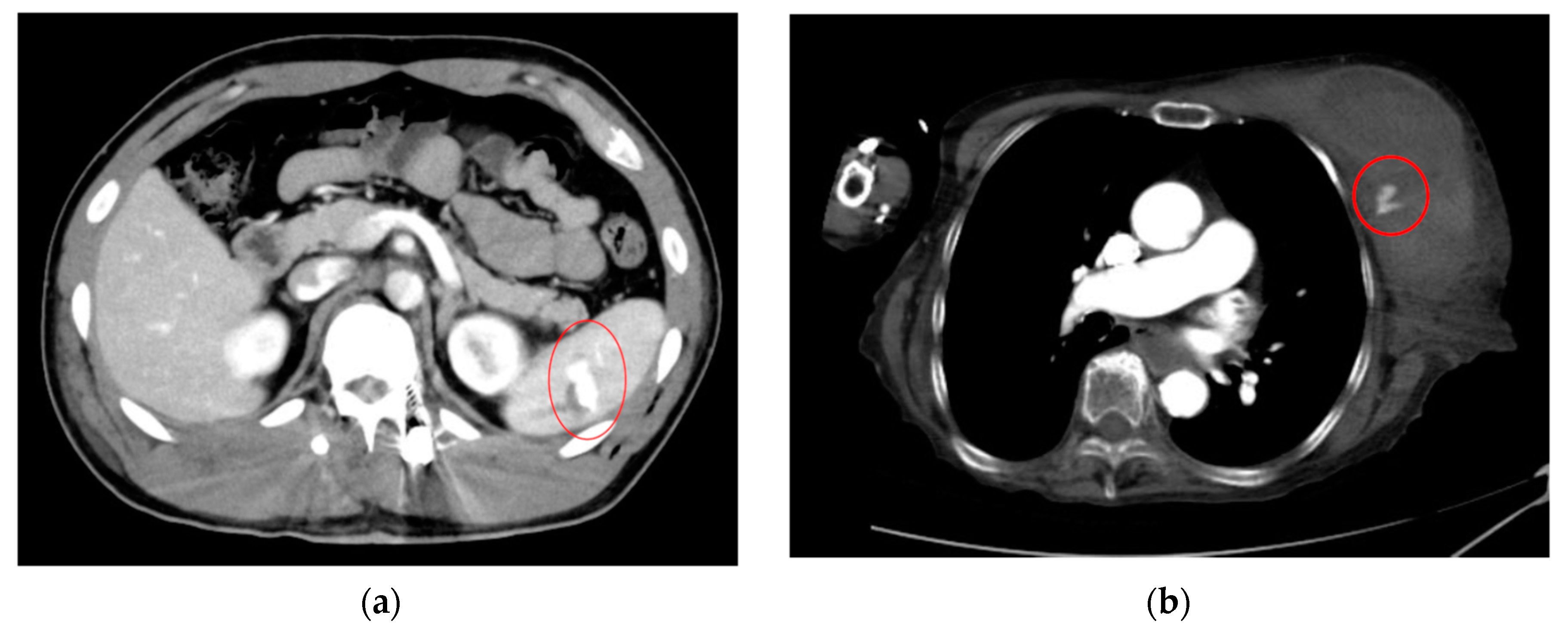
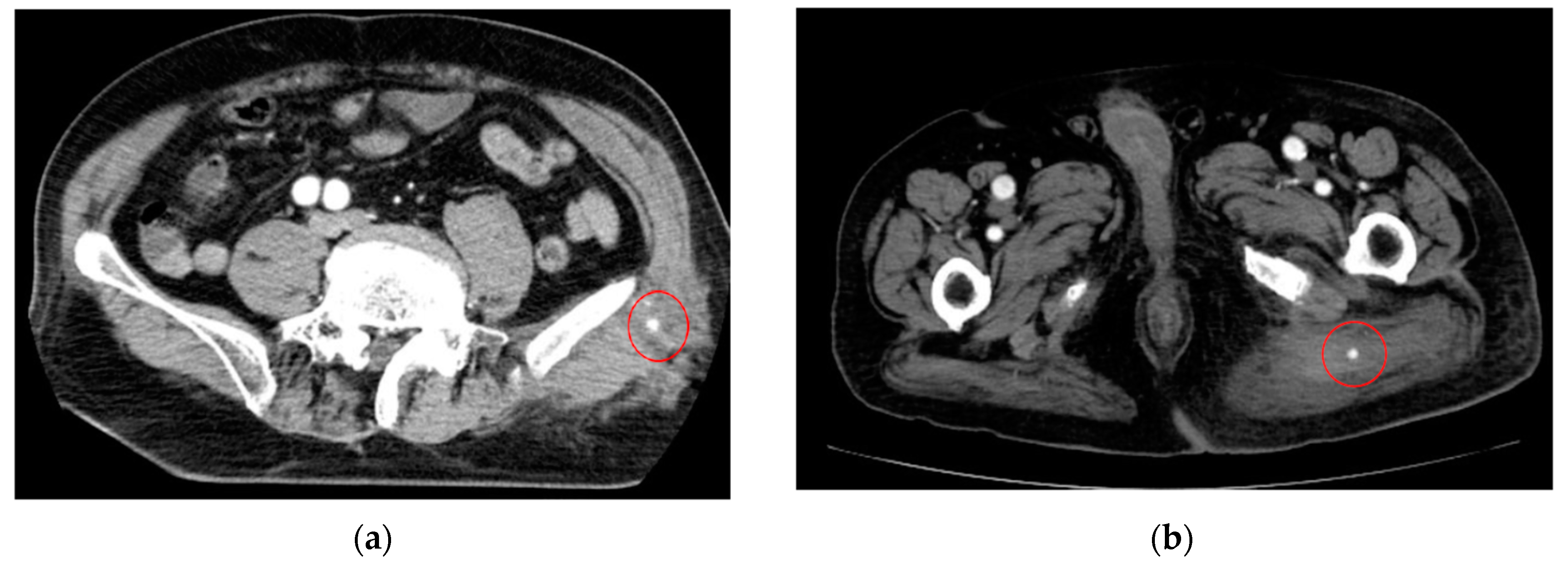
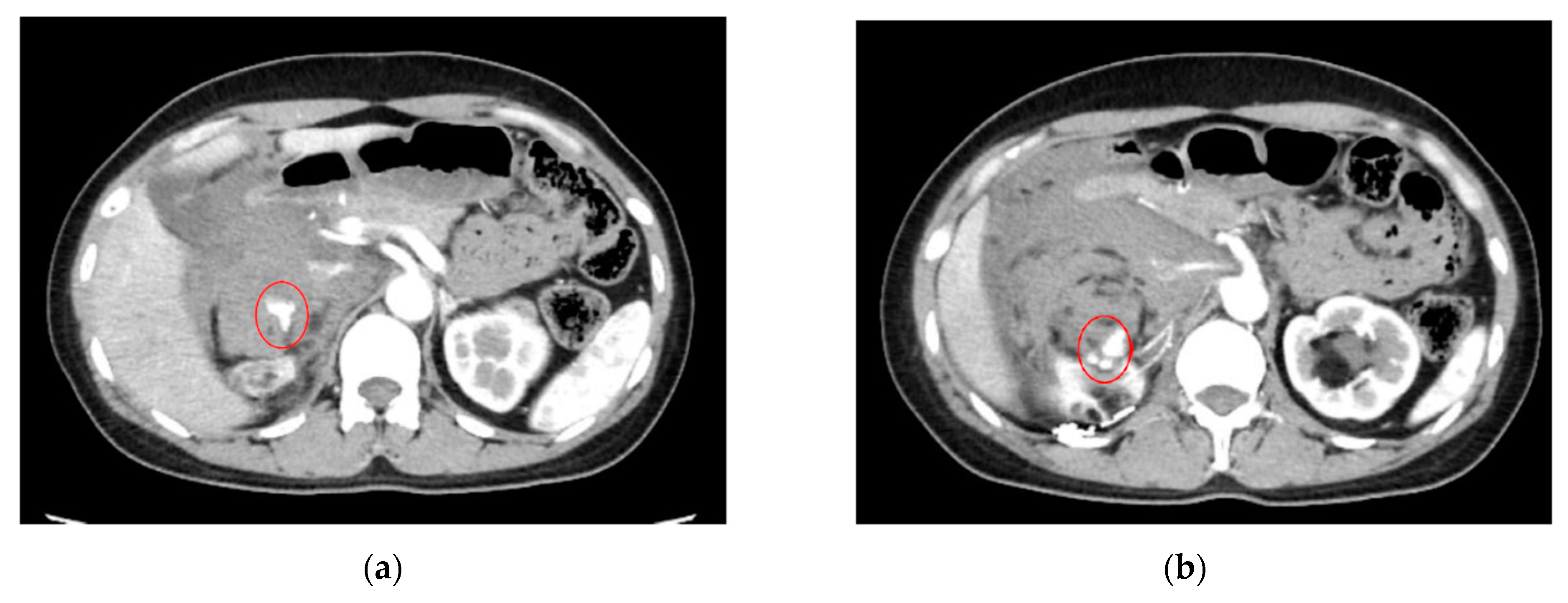



| Category | Example Drugs |
|---|---|
| Anthracyclines | Doxorubicin (Adriamycin) |
| Daunorubicin (Cerubidine) | |
| Epirubicin (Ellence) | |
| Idarubicin (Idamycin) | |
| Vinca alkaloids | Vincristine (Oncovin) |
| Vinblastine (Velban) | |
| Vinorelbine (Navelbine) | |
| Taxanes | Paclitaxel (Taxol) |
| Docetaxel (Taxotere) | |
| Nitrogen mustards | Mechlorethamine (Mustargen) |
| Other vesicants | Mitomycin-C |
| Dactinomycin (Actinomycin D) | |
| Etoposide (at high concentrations) |
| Vesicant Category | Example Drugs | Compress Type | Specific Antidote | Additional Notes |
|---|---|---|---|---|
| Anthracyclines | Doxorubicin, Daunorubicin | Cold | Dexrazoxane | Administer dexrazoxane IV within 6 h |
| Vinca alkaloids | Vincristine, Vinblastine | Warm | Hyaluronidase | Inject hyaluronidase subcutaneously |
| Taxanes | Paclitaxel, Docetaxel | Cold | None | Follow general extravasation protocols |
| Nitrogen mustards | Mechlorethamine | Cold | Sodium Thiosulfate | Inject sodium thiosulfate subcutaneously |
| Other vesicants | Mitomycin-C, Dactinomycin | Cold | None | Follow general extravasation protocols |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.D.; Tsunoyama, T. Exploring Extravasation in Cancer Patients. Cancers 2024, 16, 2308. https://doi.org/10.3390/cancers16132308
Pham TD, Tsunoyama T. Exploring Extravasation in Cancer Patients. Cancers. 2024; 16(13):2308. https://doi.org/10.3390/cancers16132308
Chicago/Turabian StylePham, Tuan D., and Taichiro Tsunoyama. 2024. "Exploring Extravasation in Cancer Patients" Cancers 16, no. 13: 2308. https://doi.org/10.3390/cancers16132308
APA StylePham, T. D., & Tsunoyama, T. (2024). Exploring Extravasation in Cancer Patients. Cancers, 16(13), 2308. https://doi.org/10.3390/cancers16132308







