Perioperative Immunosuppressive Factors during Cancer Surgery: An Updated Review
Abstract
Simple Summary
Abstract
1. Introduction
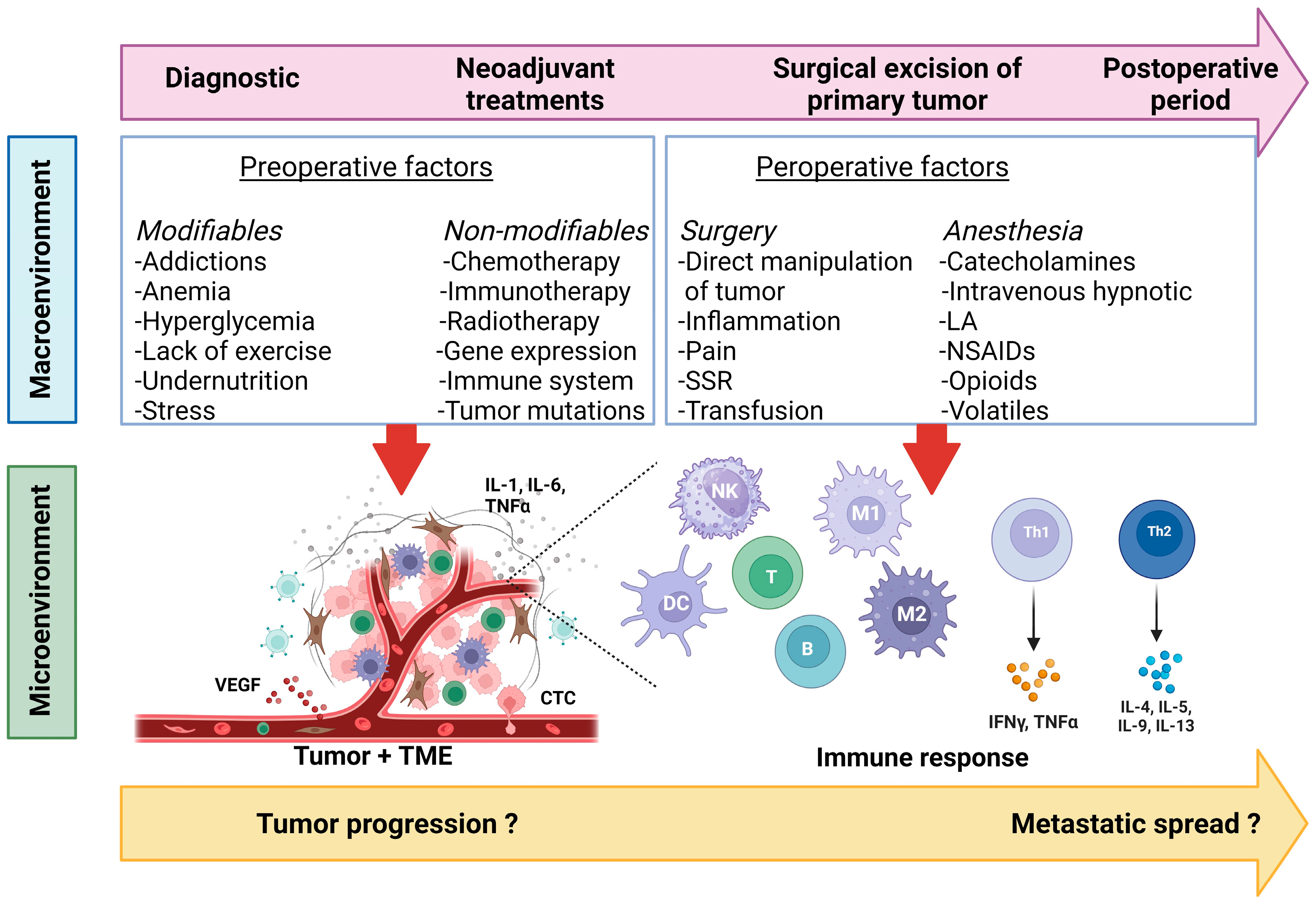
2. Preoperative Factors and Oncological Outcomes
2.1. Modifiable Factors
2.1.1. Value of Prehabilitation Programs in Oncological Care
2.1.2. Hyperglycemia and Insulin Resistance
2.1.3. Anemia and Allogeneic Blood Transfusion
2.1.4. Psychological Stress
2.2. Non-Modifiable Factors
2.2.1. Neoadjuvant Anticancer Therapies: Generalities
2.2.2. Neoadjuvant Chemotherapy
2.2.3. Neoadjuvant Immunotherapy
3. Perioperative Factors and Oncological Outcomes
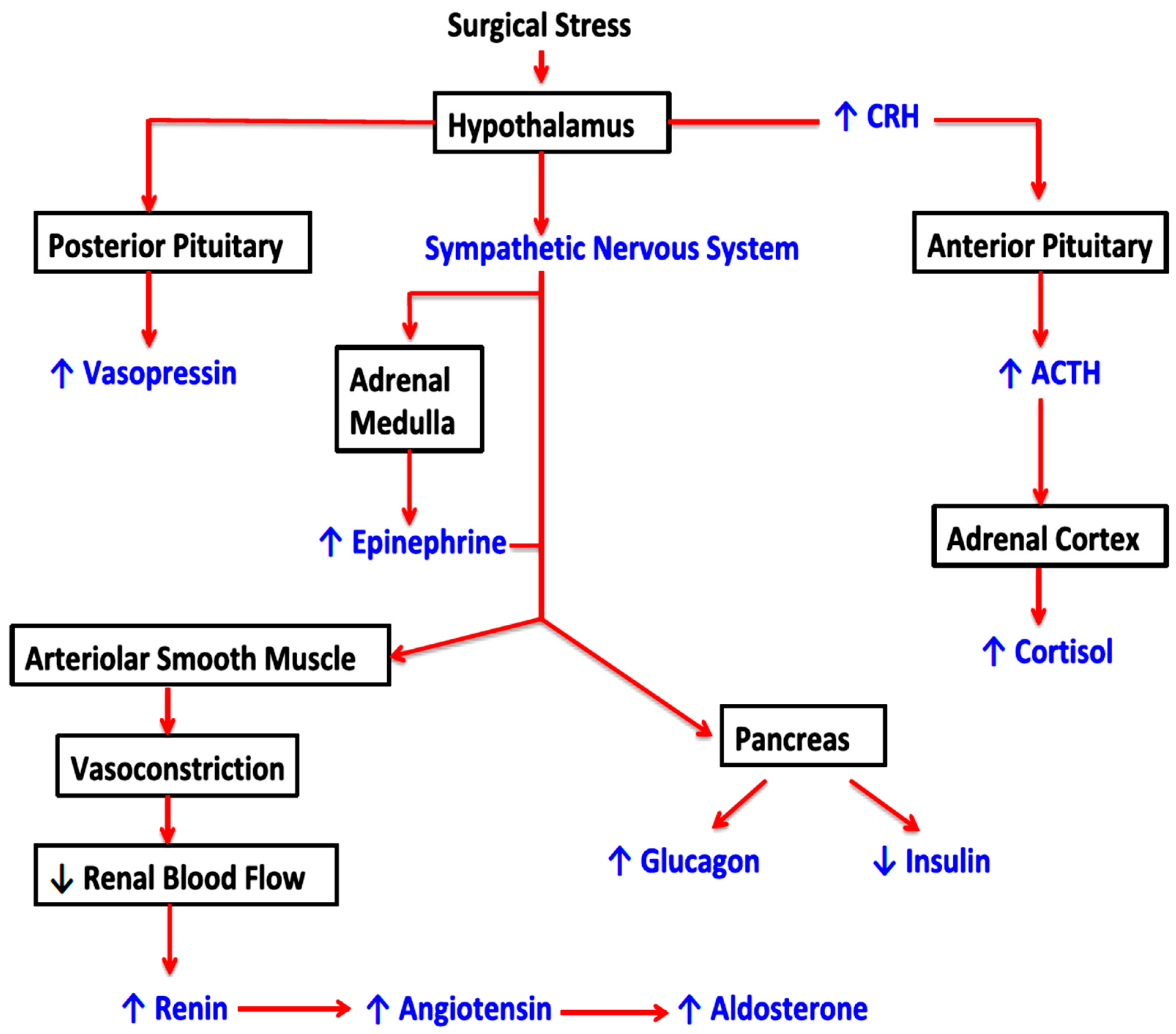
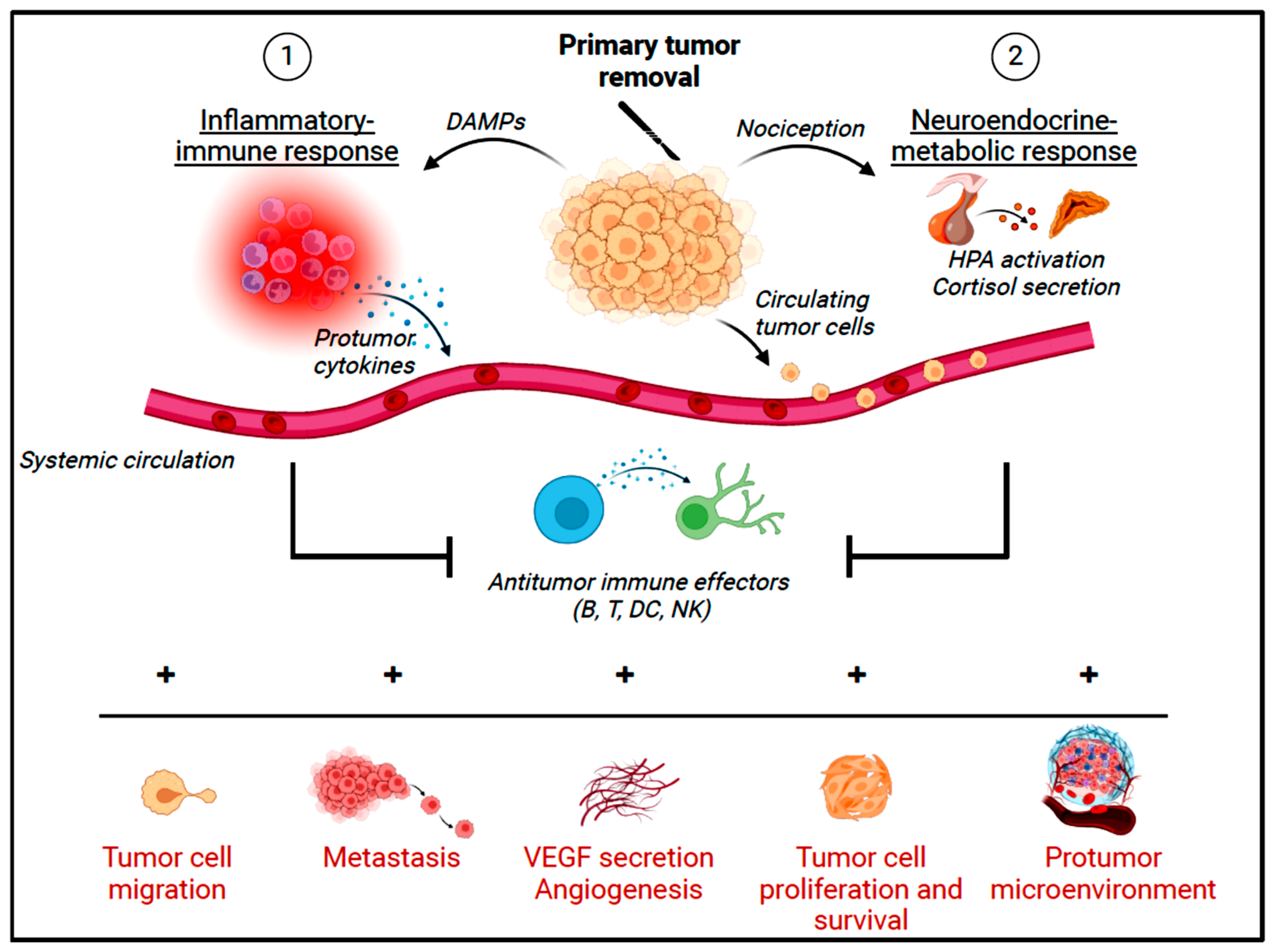
3.1. Surgical Stress Response and Oncological Outcomes
3.2. Could Anesthetics Alleviate Perioperative Immunosuppression and Prevent Recurrences?
3.2.1. Opioids
3.2.2. Local Anesthetics and Tumor Biology
3.2.3. Inhalational Anesthesia
3.2.4. Intravenous Anesthetic Agents
3.2.5. Choice of Anesthetics for Onco-Surgery: A Future Challenge?
4. The Postoperative Period: Specificities
4.1. Postoperative Acute Pain
4.2. Detection and Optimized Management of Immunosuppressive Complications
4.3. Expert Team in Oncological Surgery
4.4. Impact of the ERAS Program on Oncological Outcomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Pisarska, M.; Pędziwiatr, M.; Małczak, P.; Major, P.; Ochenduszko, S.; Zub-Pokrowiecka, A.; Kulawik, J.; Budzyński, A. Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. Int. J. Surg. 2016, 36, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Ripollés-Melchor, J.; Abad-Motos, A.; Zorrilla-Vaca, A. Enhanced Recovery After Surgery (ERAS) in Surgical Oncology. Curr. Oncol. Rep. 2022, 24, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.; Paul, M.; Phillips, B.E. Nutritional interventions in prehabilitation for cancer surgery. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Montemuiño, S.; Luna, J.; de Torres, M.V.; Amaya, E. The role of immunonutritional support in cancer treatment: Current evidence. Clin. Nutr. 2017, 36, 1457–1464. [Google Scholar] [CrossRef]
- Cerantola, Y.; Hübner, M.; Grass, F.; Demartines, N.; Schäfer, M. Immunonutrition in gastrointestinal surgery. Br. J. Surg. 2011, 98, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Zierath, J.R. The Limits of Exercise Physiology: From Performance to Health. Cell Metab. 2017, 25, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P.; et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goete, L.; et al. Position Statement Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Valenzuela, P.L.; Saco-Ledo, G.; Santos-Lozano, A.; Morales, J.S.; Castillo-García, A.; Simpson, R.J.; Lucia, A.; Fiuza-Luces, C. Exercise Training and Natural Killer Cells in Cancer Survivors: Current Evidence and Research Gaps Based on a Systematic Review and Meta-analysis. Sports Med. Open 2022, 8, 36. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer–natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- Lyu, D.-W. Immunomodulatory effects of exercise in cancer prevention and adjuvant therapy: A narrative review. Front. Physiol. 2023, 14, 1292580. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Tasdemir, N.; Banito, A.; Roe, J.S.; Alonso-Curbelo, D.; Camiolo, M.; Tschaharganeh, D.F.; Huang, C.H.; Aksoy, O.; Bolden, J.E.; Chen, C.C.; et al. BRD4 Connects Enhancer Remodeling to Senescence Immune Surveillance. Cancer Discov. 2016, 6, 612–629. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Pan, P.; Zhang, J.; Hou, X.; Wang, Y.; Chen, G.; Muhammad, P.; Reis, R.L.; Ding, L.; et al. Humanistic Health Management and Cancer: Associations of Psychology, Nutrition, and Exercise with Cancer Progression and Pathogenesis. Adv. Sci. 2024, 11, e2400665. [Google Scholar] [CrossRef] [PubMed]
- Cortiula, F.; Hendriks, L.E.; van de Worp, W.R.; Schols, A.M.; Vaes, R.D.; Langen, R.C.; De Ruysscher, D. Physical exercise at the crossroad between muscle wasting and the immune system: Implications for lung cancer cachexia. J. Cachex. Sarcopenia Muscle 2022, 13, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Angelin, A.; Gil-de-Gomez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J., 3rd; Kopinski, P.K.; Wang, L.; et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017, 25, 1282–1293.e7. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Pu, Y.; Jia, C.; Wu, M.; He, H.; Xia, Y. The Influence of Exercise on Cancer Risk, the Tumor Microenvironment and the Treatment of Cancer. Sports Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Esteve, E.; Ricart, W.; Fernandez-Real, J.M. Adipocytokines and insulin resistance: The possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009, 32 (Suppl. 2), S362–S367. [Google Scholar] [CrossRef]
- Klil-Drori, A.J.; Azoulay, L.; Pollak, M.N. Cancer, obesity, diabetes, and antidiabetic drugs: Is the fog clearing? Nat. Rev. Clin. Oncol. 2017, 14, 85–99. [Google Scholar] [CrossRef]
- Orgel, E.; Mittelman, S.D. The links between insulin resistance, diabetes, and cancer. Curr. Diab. Rep. 2013, 13, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.; Scanaill, P.; Hermanides, J.; Buggy, D.J. Current practice in the perioperative management of patients with diabetes mellitus: A narrative review. Br. J. Anaesth. 2023, 131, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Schootman, M.; Jeffe, D.B.; Ratnapradipa, K.L.; Eberth, J.M.; Davidson, N.O.M. Increased 30-Day Mortality Risk in Patients with Diabetes Mellitus After Colon Cancer Surgery: A Mediation Analysis. Dis. Colon Rectum 2020, 63, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kitayama, J.; Horie, H.; Koinuma, K.; Ohzawa, H.; Yamaguchi, H.; Kawahira, H.; Mimura, T.; Lefor, A.K.; Sata, N. Metformin changes the immune microenvironment of colorectal cancer in patients with type 2 diabetes mellitus. Cancer Sci. 2020, 111, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and cancer hallmarks: Shedding new lights on therapeutic repurposing. J. Transl. Med. 2023, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Pallister, C.J. The impact of anaemia on outcome in cancer. Int. J. Lab. Hematol. 2005, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Al-Mozain, N.; Arora, S.; Goel, R.; Pavenski, K.; So-Osman, C. Patient blood management in adults and children: What have we achieved, and what still needs to be addressed? Transfus. Clin. Biol. 2023, 30, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Zenda, S.; Hironaka, S.; Boku, N.; Yamazaki, K.; Yasui, H.; Fukutomi, A.; Yoshino, T.; Onozawa, Y.; Nishimura, T. Impact of hemoglobin level on survival in definitive chemoradiotherapy for T4/M1 lymph node esophageal cancer. Dis. Esophagus. 2008, 21, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kanda, M.; Kodera, Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J. Gastroenterol. 2019, 25, 2743–2751. [Google Scholar] [CrossRef]
- Caro, J.J.; Salas, M.; Ward, A.; Goss, G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer 2001, 91, 2214–2221. [Google Scholar] [CrossRef]
- Chandra, S.; Kulkarni, H.; Westphal, M. The bloody mess of red blood cell transfusion. Crit. Care 2017, 21, 310. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, J.E.; Hillyer, C.D. Noninfectious serious hazards of transfusion. Anesth. Analg. 2009, 108, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Towe, C.W.; Gulack, B.C.; Kim, S.; Ho, V.P.; Perry, Y.; Donahue, J.M.; Linden, P.A. Restrictive Transfusion Practices After Esophagectomy Are Associated with Improved Outcome: A Review of the Society of Thoracic Surgeons General Thoracic Database. Ann. Surg. 2018, 267, 886–891. [Google Scholar] [CrossRef]
- Amato, A.; Pescatori, M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst. Rev. 2006, 2006, CD005033. [Google Scholar] [CrossRef] [PubMed]
- Vamvakas, E.C.; Blajchman, M.A. Transfusion-related immunomodulation (TRIM): An update. Blood Rev. 2007, 21, 327–348. [Google Scholar] [CrossRef]
- Piegeler, T.; Beck-Schimmer, B. Anesthesia and colorectal cancer—The perioperative period as a window of opportunity? Eur. J. Surg. Oncol. 2016, 42, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.; Cerwenka, A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology 2017, 222, 11–20. [Google Scholar] [CrossRef]
- Ling, F.C.; Hoelscher, A.H.; Vallböhmer, D.; Schmidt, D.; Picker, S.; Gathof, B.S.; Bollschweiler, E.; Schneider, P.M. Leukocyte depletion in allogeneic blood transfusion does not change the negative influence on survival following transthoracic resection for esophageal cancer. J. Gastrointest. Surg. 2009, 13, 581–586. [Google Scholar] [CrossRef]
- Müller, S.D.; Both, C.P.; Sponholz, C.; Voelker, M.T.; Christiansen, H.; Niggli, F.; Schmitz, A.; Weiss, M.; Thomas, J.; Stehr, S.N.; et al. Association between Intraoperative Blood Transfusion, Regional Anesthesia and Outcome after Pediatric Tumor Surgery for Nephroblastoma. Cancers 2022, 14, 5585. [Google Scholar] [CrossRef]
- Boshier, P.R.; Ziff, C.; Adam, M.E.; Fehervari, M.; Markar, S.R.; Hanna, G.B. Effect of perioperative blood transfusion on the long-term survival of patients undergoing esophagectomy for esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2018, 31, 1–10. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Xu, X.; Chen, C.; Guo, Y.; Tan, Y. Risk factors of intraoperative blood loss and transfusion for pediatric patients undergoing brain tumor removal: A retrospective cohort study. J. Neurosurg. Pediatr. 2023, 31, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Althoff, F.C.; Neb, H.; Herrmann, E.; Trentino, K.M.; Vernich, L.; Fullenbach, C.; Freedman, J.; Waters, J.H.; Farmer, S.; Leahy, M.F.; et al. Multimodal Patient Blood Management Program Based on a Three-pillar Strategy: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 269, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.; Jericó, C.; Miranda, C.; Santamaría, M.; Artigau, E.; Galofré, G.; Garsot, E.; Luna, A.; Puértolas, N.; Aldeano, A.; et al. Improved postoperative outcomes and reduced transfusion rates after implementation of a Patient Blood Management program in gastric cancer surgery. Eur. J. Surg. Oncol. 2021, 47, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Meybohm, P.; Richards, T.; Isbister, J.; Hofmann, A.; Shander, A.; Goodnough, L.T.; Muñoz, M.; Gombotz, H.; Weber, C.F.; Choorapoikayil, S.; et al. Patient Blood Management Bundles to Facilitate Implementation. Transfus. Med. Rev. 2016, 31, 62–71. [Google Scholar] [CrossRef] [PubMed]
- UK Royal Colleges Tranexamic Acid in Surgery Implementation Group; Grocott, M.P.W.; Murphy, M.; Roberts, I.; Sayers, R.; Toh, C.H. Tranexamic acid for safer surgery: The time is now. Br. J. Surg. 2022, 109, 1182–1183. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascon, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. 4), iv96–iv110. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, P.; Viganò, M.; Rocco, G.; Della Pona, C.; Buscemi, A.; Rizzi, A. Effectiveness of leukocyte filters in reducing tumor cell contamination after intraoperative blood salvage in lung cancer patients. Vox Sang. 1997, 72, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lapierre, V.; Billard, V. Perioperative autotransfusion with salvage blood in cancer surgery. Ann. Fr. Anesth. Reanim. 2000, 19, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Loprinzi, C.L. Erythropoietin use in cancer patients: A matter of life and death? J. Clin. Oncol. 2005, 23, 5865–5868. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Sgroi, G.; Vavassori, I.; Petrò, D.; Cabiddu, M.; Aiolfi, A.; Bonitta, G.; Zaniboni, A.; et al. Red blood cell transfusions and the survival in patients with cancer undergoing curative surgery: A systematic review and meta-analysis. Surg. Today 2021, 51, 1535–1557. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Huang, B.; Xu, Y.; Huang, J. Association of perioperative allogeneic blood transfusions and long-term outcomes following radical surgery for gastric and colorectal cancers: Systematic review and meta-analysis of propensity-adjusted observational studies. BJS Open 2023, 7, zrad075. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Park, J.; Lee, M.; Lee, D.; Choi, H.; Gim, G.; Kim, L.; Kang, C.Y.; Oh, Y.; Viveiros, P.; et al. Blood transfusions may adversely affect survival outcomes of patients with lung cancer: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2021, 10, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, X.; Wen, Z.; Huang, J.; Wang, C.; Chen, C.; Yang, X. The effect of perioperative blood transfusion on survival after renal cell carcinoma nephrectomy: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1092734. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Robles, T.F.; Heffner, K.L.; Loving, T.J.; Glaser, R. Psycho-oncology and cancer: Psychoneuroimmunology and cancer. Ann. Oncol. 2002, 13, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Carpiniello, B.; Orrù, G.; Sitzia, R.; Piras, A.; Farci, G.; Del Giacco, S.; Piludu, G.; Miller, A.H. Chronic caregiving stress alters peripheral blood immune parameters: The role of age and severity of stress. Psychother. Psychosom. 1997, 66, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.J.; Liu, K.; Liang, D.F. Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2009, 38, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Wang, H.C.; Yuan, Z.; Huang, J.; Zheng, Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via beta-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepatogastroenterology 2012, 59, 889–893. [Google Scholar] [PubMed]
- Liu, X.; Wu, W.K.; Yu, L.; Sung, J.J.; Srivastava, G.; Zhang, S.T.; Cho, C.H. Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J. Cell. Biochem. 2008, 105, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Chun, K.J.; Kil, H.K.; Jung, N.; Shin, H.A.; Jang, J.Y.; Choi, H.G.; Oh, K.H.; Kim, M.S. beta2-adrenergic receptor expression and the effects of norepinephrine and propranolol on various head and neck cancer subtypes. Oncol. Lett. 2021, 22, 804. [Google Scholar] [CrossRef]
- İşeri, O.D.; Sahin, F.I.; Terzi, Y.K.; Yurtcu, E.; Erdem, S.R.; Sarialioglu, F. beta-Adrenoreceptor antagonists reduce cancer cell proliferation, invasion, and migration. Pharm. Biol. 2014, 52, 1374–1381. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Luo, Y.; Tian, P.; Peng, F.; Lu, J.; Yang, Y.; Su, Q.; Liu, B.; Yu, J.; Luo, X.; et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Investig. 2019, 129, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorf, S.K.; Sood, A.K.; Anderson, B.; McGinn, S.; Maiseri, H.; Dao, M.; Sorosky, J.I.; De Geest, K.; Ritchie, J.; Lubaroff, D.M. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005, 23, 7105–7113. [Google Scholar] [CrossRef] [PubMed]
- Carnet Le Provost, K.; Kepp, O.; Kroemer, G.; Bezu, L. Trial watch: Beta-blockers in cancer therapy. Oncoimmunology 2023, 12, 2284486. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-H.; Wu, T.-Y.; Chen, C.-H.; Kuo, Y.-H.; Hour, M.-J. Survival outcomes of beta-blocker usage in HER2-positive advanced breast cancer patients: A retrospective cohort study. Ther. Adv. Drug Saf. 2023, 14, 20420986231181338. [Google Scholar] [CrossRef] [PubMed]
- Ramondetta, L.M.; Hu, W.; Thaker, P.H.; Urbauer, D.L.; Chisholm, G.B.; Westin, S.N.; Sun, Y.; Ramirez, P.T.; Fleming, N.; Sahai, S.K.; et al. Prospective pilot trial with combination of propranolol with chemotherapy in patients with epithelial ovarian cancer and evaluation on circulating immune cell gene expression. Gynecol. Oncol. 2019, 154, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, P.; Xu, Y.; Yan, J.; Liu, Z.; Lau, W.B.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; et al. Surgical stress and cancer progression: The twisted tango. Mol. Cancer 2019, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, J.K.; Park, Y.; Huh, J.W.; Kim, H.C.; Yun, S.H.; Lee, W.Y.; Cho, Y.B. Bevacizumab increases the risk of anastomosis site leakage in metastatic colorectal cancer. Front. Oncol. 2022, 12, 1018458. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Bakos, O.; Lawson, C.; Rouleau, S.; Tai, L.-H. Combining surgery and immunotherapy: Turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Matzner, P.; Sandbank, E.; Neeman, E.; Zmora, O.; Gottumukkala, V.; Ben-Eliyahu, S. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat. Rev. Clin. Oncol. 2020, 17, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Sandbank, E.; Eckerling, A.; Margalit, A.; Sorski, L.; Ben-Eliyahu, S. Immunotherapy during the Immediate Perioperative Period: A Promising Approach against Metastatic Disease. Curr. Oncol. 2023, 30, 7450–7477. [Google Scholar] [CrossRef] [PubMed]
- Tagliamento, M.; Remon, J.; Planchard, D.; Besse, B. Does perioperative immunotherapy reduce the risk of second primary cancers? Eur. J. Cancer 2023, 194, 113355. [Google Scholar] [CrossRef] [PubMed]
- Cusack, B.; Buggy, D. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Manou-Stathopoulou, V.; Korbonits, M.; Ackland, G.L. Redefining the perioperative stress response: A narrative review. Br. J. Anaesth. 2019, 123, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Asavarut, P.; Zhao, H.; Gu, J.; Ma, D. The role of HMGB1 in inflammation-mediated organ injury. Acta Anaesthesiol. Taiwanica 2013, 51, 28–33. [Google Scholar] [CrossRef]
- Silk, E.; Zhao, H.; Weng, H.; Ma, D. The role of extracellular histone in organ injury. Cell Death Dis. 2017, 8, e2812. [Google Scholar] [CrossRef]
- Zhao, H.; Kilgas, S.; Alam, A.; Eguchi, S.; Ma, D. The Role of Extracellular Adenosine Triphosphate in Ischemic Organ Injury. Crit. Care Med. 2016, 44, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10, eaan3463. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Marone, G.; Mantovani, A. Cancer Inflammation and Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028662. [Google Scholar] [CrossRef] [PubMed]
- Desborough, J. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. OncoImmunology 2016, 5, e1093722. [Google Scholar]
- Ionescu, D.C.; Margarit, S.C.D.; Hadade, A.N.I.; Mocan, T.N.; Miron, N.A.; Sessler, D.I. Choice of anesthetic technique on plasma concentrations of interleukins and cell adhesion molecules. Perioper. Med. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Franzén, S.; Semenas, E.; Larsson, A.; Hultström, M.; Frithiof, R. Plasma cytokine levels in spinal surgery with sevoflurane or total intravenous propofol anesthesia—A post hoc analysis of a randomized controlled trial. Cytokine 2023, 169, 156290. [Google Scholar] [CrossRef]
- Alazawi, W.; Pirmadjid, N.; Lahiri, R.; Bhattacharya, S. Inflammatory and Immune Responses to Surgery and Their Clinical Impact. Ann. Surg. 2016, 264, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Salanti, A.; Gögenur, I. Premetastatic niches, exosomes and circulating tumor cells: Early mechanisms of tumor dissemination and the relation to surgery. Int. J. Cancer 2020, 146, 3244–3255. [Google Scholar] [CrossRef]
- Angka, L.; Khan, S.T.; Kilgour, M.K.; Xu, R.; Kennedy, M.A.; Auer, R.C. Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery. Int. J. Mol. Sci. 2017, 18, 1787. [Google Scholar] [CrossRef]
- Huang, W.-W.; Zhu, W.-Z.; Mu, D.-L.; Ji, X.-Q.; Nie, X.-L.; Li, X.-Y.; Wang, D.-X.; Ma, D. Perioperative Management May Improve Long-term Survival in Patients after Lung Cancer Surgery: A Retrospective Cohort Study. Anesth. Analg. 2018, 126, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, C.; Demicheli, R.; Fornili, M.; Bachir, I.; Duca, M.; Viglietti, G.; Berlière, M.; Piccart, M.; Sotiriou, C.; Sosnowski, M.; et al. Potential Benefit of Intra-operative Administration of Ketorolac on Breast Cancer Recurrence According to the Patient’s Body Mass Index. JNCI J. Natl. Cancer Inst. 2018, 110, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Vandenhende, J.; Berliere, M.; Machiels, J.-P.; Nussbaum, B.; Legrand, C.; De Kock, M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth. Analg. 2010, 110, 1630–1635. [Google Scholar] [CrossRef]
- Forget, P.; Bentin, C.; Machiels, J.-P.; Berliere, M.; Coulie, P.G.; De Kock, M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br. J. Anaesth. 2014, 113, i82–i87. [Google Scholar] [CrossRef]
- Sonawane, V.; Ghosalkar, J.; Achrekar, S.; Joshi, K. Ketorolac modulates Rac-1/HIF-1alpha/DDX3/beta-catenin signalling via a tumor suppressor prostate apoptosis response-4 (Par-4) in renal cell carcinoma. Sci. Rep. 2023, 13, 5659. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheng, S.; Fu, G.; Ji, F.; Wang, C.; Cao, M. Postoperative administration of ketorolac averts morphine-induced angiogenesis and metastasis in triple-negative breast cancer. Life Sci. 2020, 251, 117604. [Google Scholar] [CrossRef]
- Alam, A.; Hana, Z.; Jin, Z.; Suen, K.C.; Ma, D. Surgery, neuroinflammation and cognitive impairment. eBioMedicine 2018, 37, 547–556. [Google Scholar] [CrossRef]
- Coussons, M.E.; Dykstra, L.A.; Lysle, D.T. Pavlovian conditioning of morphine-induced alterations of immune status. J. Neuroimmunol. 1992, 39, 219–230. [Google Scholar] [CrossRef]
- Nair, M.P.; Schwartz, S.A.; Polasani, R.; Hou, J.; Sweet, A.; Chadha, K.C. Immunoregulatory effects of morphine on human lymphocytes. Clin. Diagn. Lab. Immunol. 1997, 4, 127–132. [Google Scholar] [CrossRef]
- Scroope, C.A.; Singleton, Z.; Hollmann, M.W.; Parat, M.-O. Opioid Receptor-Mediated and Non-Opioid Receptor-Mediated Roles of Opioids in Tumour Growth and Metastasis. Front. Oncol. 2021, 11, 792290. [Google Scholar] [CrossRef]
- Forget, P.; De Kock, M. Could anaesthesia, analgesia and sympathetic modulation affect neoplasic recurrence after surgery? A systematic review centred over the modulation of natural killer cells activity. Ann. Fr. Anesth. Reanim. 2009, 28, 751–768. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Properties and Overview of Immune responses. In Cellular and Molecular Immunology, 6th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Smith, L.; Cata, J.P.; Forget, P. Immunological Insights into Opioid-Free Anaesthesia in Oncological Surgery: A Scoping Review. Curr. Oncol. Rep. 2022, 24, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Rangel, F.P.; Auler, J.O.; Carmona, M.J.; Cordeiro, M.D.; Nahas, W.C.; Coelho, R.F.; Simões, C.M. Opioids and premature biochemical recurrence of prostate cancer: A randomised prospective clinical trial. Br. J. Anaesth. 2021, 126, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Aboalsoud, A.; El-Ghaiesh, S.H.; Elmonem, F.F.A.; Salem, M.L.; Rahman, M.N.A. The effect of low-dose naltrexone on solid Ehrlich carcinoma in mice: The role of OGFr, BCL2, and immune response. Int. Immunopharmacol. 2020, 78, 106068. [Google Scholar] [CrossRef] [PubMed]
- Titon, O.J.; Titon, J.P.; da Silva, J.C.; Ferreira, M.O.; Garbim, M.R.; Rech, D.; de Souza, J.A.; Panis, C. Influence of exogenous opioids on the acute inflammatory response in the perioperative period of oncological surgery: A clinical study. Braz. J. Anesthesiol. 2024, 74, 744290. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.; Yang, C.; Huang, X.; Han, M.; Kang, F.; Li, J. Morphine promotes the malignant biological behavior of non-small cell lung cancer cells through the MOR/Src/mTOR pathway. Cancer Cell Int. 2021, 21, 622. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Duran, T. Effects of Fentanyl on pancreatic cancer cell proliferation and cancer stem cell differentiation. Cell. Mol. Biol. 2019, 65, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, A.; Fichna, J.; Zielińska, M. Opioids in Cancer Development, Progression and Metastasis: Focus on Colorectal Cancer. Curr. Treat. Options Oncol. 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qin, Y.; Zhong, Y.; Qin, Y.; Wei, Y.; Li, L.; Xie, Y. Fentanyl inhibits the progression of gastric cancer through the suppression of MMP-9 via the PI3K/Akt signaling pathway. Ann. Transl. Med. 2020, 8, 118. [Google Scholar] [CrossRef]
- Lennon, F.E.; Mirzapoiazova, T.; Mambetsariev, B.; Salgia, R.; Moss, J.; Singleton, P.A. Overexpression of the mu-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology 2012, 116, 857–867. [Google Scholar] [CrossRef]
- Doornebal, C.W.; Vrijland, K.; Hau, C.-S.; Coffelt, S.B.; Ciampricotti, M.; Jonkers, J.; de Visser, K.E.; Hollmann, M.W. Morphine does not facilitate breast cancer progression in two preclinical mouse models for human invasive lobular and HER2+ breast cancer. Pain 2015, 156, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Khabbazi, S.; Hassanshahi, M.; Hassanshahi, A.; Peymanfar, Y.; Su, Y.-W.; Xian, C.J. Opioids and matrix metalloproteinases: The influence of morphine on MMP-9 production and cancer progression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.-H.; Wu, H.-L.; Chang, W.-K.; Tsou, M.-Y.; Chen, H.-H.; Chang, K.-Y. Intraoperative Fentanyl Consumption Does Not Impact Cancer Recurrence or Overall Survival after Curative Colorectal Cancer Resection. Sci. Rep. 2017, 7, 10816. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, J.F.; Strichartz, G.R. Molecular mechanisms of local anesthesia: A review. Anesthesiology 1990, 72, 711–734. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, M.W.; Durieux, M.E. Local anesthetics and the inflammatory response: A new therapeutic indication? Anesthesiology 2000, 93, 858–875. [Google Scholar] [CrossRef] [PubMed]
- Piegeler, T.; Dull, R.O.; Hu, G.; Castellon, M.; Chignalia, A.Z.; Koshy, R.G.; Votta-Velis, E.G.; Borgeat, A.; Schwartz, D.E.; Beck-Schimmer, B.; et al. Ropivacaine attenuates endotoxin plus hyperinflation-mediated acute lung injury via inhibition of early-onset Src-dependent signaling. BMC Anesthesiol. 2014, 14, 57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piegeler, T.; Votta-Velis, E.G.; Bakhshi, F.R.; Mao, M.; Carnegie, G.; Bonini, M.G.; Schwartz, D.E.; Borgeat, A.; Beck-Schimmer, B.; Minshall, R.D. Endothelial Barrier Protection by Local Anesthetics: Ropivacaine and Lidocaine Block Tumor Necrosis Factor-alpha-induced Endothelial Cell Src Activation. Anesthesiology 2014, 120, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Herroeder, S.; Pecher, S.; Schonherr, M.E.; Kaulitz, G.; Hahnenkamp, K.; Friess, H.; Bottiger, B.W.; Bauer, H.; Dijkgraaf, M.G.; Durieux, M.E.; et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: A double-blinded, randomized, placebo-controlled trial. Ann. Surg. 2007, 246, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Carvalho, P.; Vale, N.; Monjardino, T.; Mourão, J. Systemic Anti-Inflammatory Effects of Intravenous Lidocaine in Surgical Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3772. [Google Scholar] [CrossRef]
- Piegeler, T.; Hollmann, M.W.; Borgeat, A.; Lirk, P. Do Amide Local Anesthetics Play a Therapeutic Role in the Perioperative Management of Cancer Patients? Int. Anesthesiol. Clin. 2016, 54, e17–e32. [Google Scholar] [CrossRef]
- Müller, S.D.; Ziegler, J.S.H.; Piegeler, T. Local Anesthetics and Recurrence after Cancer Surgery-What’s New? A Narrative Review. J. Clin. Med. 2021, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Bezu, L.; Chuang, A.W.; Sauvat, A.; Humeau, J.; Xie, W.; Cerrato, G.; Liu, P.; Zhao, L.; Zhang, S.; Le Naour, J.; et al. Local anesthetics elicit immune-dependent anticancer effects. J. Immunother. Cancer 2022, 10, e004151. [Google Scholar] [CrossRef] [PubMed]
- Boavista Barros Heil, L.; Leme Silva, P.; Ferreira Cruz, F.; Pelosi, P.; Rieken Macedo Rocco, P. Immunomodulatory effects of anesthetic agents in perioperative medicine. Minerva Anestesiol. 2020, 86, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Di Russo, S.; Liberati, F.R.; Riva, A.; Di Fonzo, F.; Macone, A.; Giardina, G.; Arese, M.; Rinaldo, S.; Cutruzzolà, F.; Paone, A. Beyond the barrier: The immune-inspired pathways of tumor extravasation. Cell Commun. Signal. 2024, 22, 104. [Google Scholar] [CrossRef] [PubMed]
- García-Román, J.; Zentella-Dehesa, A. Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013, 335, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M. Src signaling in cancer invasion. J. Cell. Physiol. 2010, 223, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Minshall, R.D. Regulation of transendothelial permeability by Src Kinase. Microvasc. Res. 2009, 77, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Piegeler, T.; Schlapfer, M.; Dull, R.O.; Schwartz, D.E.; Borgeat, A.; Minshall, R.D.; Beck-Schimmer, B. Clinically relevant concentrations of lidocaine and ropivacaine inhibit TNFalpha-induced invasion of lung adenocarcinoma cells in vitro by blocking the activation of Akt and focal adhesion kinase. Br. J. Anaesth. 2015, 115, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Hon, D.; Robertson, F.; Robertson, G.; Owen, S.; Rogers, G.; Lydon, E.; Lee, N.; Hales, T. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br. J. Anaesth. 2014, 113, i39–i48. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Liu, C.-L.; Chen, M.-J.; Hsu, Y.-W.; Chen, S.-N.; Lin, C.-H.; Chen, C.-M.; Yang, F.-M.; Hu, M.-C. Local Anesthetics Induce Apoptosis in Human Breast Tumor Cells. Anesth. Analg. 2014, 118, 116–124. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Li, Y.; Li, Y.; Pang, T. Lidocaine hampers colorectal cancer process via circITFG2/miR-1204/SOCS2 axis. Anticancer Drugs 2022, 33, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhao, H.; Hankin, J.; Chen, L.; Yao, S.; Ma, D. Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci. Rep. 2016, 6, 26277. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, Y. Lidocaine inhibits proliferation and metastasis of lung cancer cell via regulation of miR-539/EGFR axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Chen, D.-T.; Pan, J.-H.; Chen, Y.-H.; Yan, Y.; Li, Q.; Xue, R.-F.; Yuan, Y.-F.; Zeng, W.-A. Lidocaine Induces Apoptosis and Suppresses Tumor Growth in Human Hepatocellular Carcinoma Cells In Vitro and in a Xenograft Model In Vivo. Anesthesiology 2017, 126, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Bezu, L.; Kepp, O.; Kroemer, G. Local anesthetics and immunotherapy: A novel combination to fight cancer. Semin. Immunopathol. 2023, 45, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; Meyer-Treschan, T.A.; Grady, M.; Tan, E.Y.; et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Z.; Li, H.J.; Li, M.H.; Huang, S.M.; Li, X.; Liu, Q.H.; Li, J.; Li, X.Y.; Wang, D.X.; Sessler, D.I. Epidural Anesthesia-Analgesia and Recurrence-free Survival after Lung Cancer Surgery: A Randomized Trial. Anesthesiology 2021, 135, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Mincer, J.S.; Buggy, D.J. Anaesthesia, analgesia, and cancer outcomes: Time to think like oncologists? Br. J. Anaesth. 2023, 131, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Badwe, R.A.; Parmar, V.; Nair, N.; Joshi, S.; Hawaldar, R.; Pawar, S.; Kadayaprath, G.; Borthakur, B.B.; Thammineedi, S.R.; Pandya, S.; et al. Effect of Peritumoral Infiltration of Local Anesthetic Before Surgery on Survival in Early Breast Cancer. J. Clin. Oncol. 2023, 41, 3318–3328. [Google Scholar] [CrossRef]
- Hayden, J.M.; Oras, J.; Block, L.; Thörn, S.-E.; Palmqvist, C.; Salehi, S.; Nordstrom, J.L.; Gupta, A. Intraperitoneal ropivacaine reduces time interval to initiation of chemotherapy after surgery for advanced ovarian cancer: Randomised controlled double-blind pilot study. Br. J. Anaesth. 2020, 124, 562–570. [Google Scholar] [CrossRef]
- Both, C.P.; Thomas, J.; Bühler, P.K.; Schmitz, A.; Weiss, M.; Piegeler, T. Factors associated with intravenous lidocaine in pediatric patients undergoing laparoscopic appendectomy—A retrospective, single-centre experience. BMC Anesthesiol. 2018, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, A.; Sharma, S.; Ford, J.; Durieux, M.E.; Tiouririne, M. Review article: The role of the perioperative period in recurrence after cancer surgery. Anesth. Analg. 2010, 110, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Stollings, L.M.; Jia, L.-J.; Tang, P.; Dou, H.; Lu, B.; Xu, Y. Immune Modulation by Volatile Anesthetics. Anesthesiology 2016, 125, 399–411. [Google Scholar] [CrossRef]
- Alexa, A.L.; Sargarovschi, S.; Ionescu, D. Neutrophils and Anesthetic Drugs: Implications in Onco-Anesthesia. Int. J. Mol. Sci. 2024, 25, 4033. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.; Skitzki, J.J. Impact of anesthesia choice in cutaneous melanoma surgery. Melanoma Res. 2024, 34, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, E.; Li, Y.; Liu, S. Attenuating sevoflurane-induced cellular injury of human peripheral lymphocytes by propofol in a concentration-dependent manner. Arch. Pharmacal Res. 2011, 34, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Loop, T.; Dovi-Akue, D.; Frick, M.; Roesslein, M.; Egger, L.; Humar, M.; Hoetzel, A.; Schmidt, R.; Borner, C.; Pahl, H.L.; et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology 2005, 102, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Kurosawa, S.; Horinouchi, T.; Kato, M.; Hashimoto, Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology 2001, 95, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Astrof, N.S.; Bracken, C.; Soriano, S.G.; Shimaoka, M. Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology 2010, 113, 600–609. [Google Scholar] [CrossRef]
- Itoh, T.; Hirota, K.; Hisano, T.; Namba, T.; Fukuda, K. The volatile anesthetics halothane and isoflurane differentially modulate proinflammatory cytokine-induced p38 mitogen-activated protein kinase activation. J. Anesth. 2004, 18, 203–209. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Lin, J. Distinct effects of general anesthetics on lung metastasis mediated by IL-6/JAK/STAT3 pathway in mouse models. Nat. Commun. 2020, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Dong, R.; Zhang, F.; Wang, W.; Lu, H.; Luo, Y.; Xue, Q.; Yu, B. Propofol Provides More Effective Protection for Circulating Lymphocytes Than Sevoflurane in Patients Undergoing Off-Pump Coronary Artery Bypass Graft Surgery. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, T.J.; Mohammed, K.; Jhanji, S. Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology 2016, 124, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Pan, X.; Wang, C.; Wang, L. The benefits of propofol on cancer treatment: Decipher its modulation code to immunocytes. Front. Pharmacol. 2022, 13, 919636. [Google Scholar] [CrossRef] [PubMed]
- Tavare, A.N.; Perry, N.J.; Benzonana, L.L.; Takata, M.; Ma, D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int. J. Cancer 2012, 130, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Kim, R. Anesthetic technique and cancer recurrence in oncologic surgery: Unraveling the puzzle. Cancer Metastasis Rev. 2016, 36, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Kubo, K.; Shingu, K. Possible link between cyclooxygenase-inhibiting and antitumor properties of propofol. J. Anesth. 2011, 25, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Sen, Y.; Xiyang, H.; Yu, H. Effect of thoracic paraspinal block-propofol intravenous general anesthesia on VEGF and TGF-beta in patients receiving radical resection of lung cancer. Medicine 2019, 98, e18088. [Google Scholar] [CrossRef] [PubMed]
- Salo, M.; Pirttikangas, C.O.; Pulkki, K. Effects of propofol emulsion and thiopentone on T helper cell type-1/type-2 balance in vitro. Anaesthesia 1997, 52, 341–344. [Google Scholar] [CrossRef]
- Enlund, M.; Berglund, A.; Andreasson, K.; Cicek, C.; Enlund, A.; Bergkvist, L. The choice of anaesthetic—Sevoflurane or propofol—And outcome from cancer surgery: A retrospective analysis. Upsala J. Med. Sci. 2014, 119, 251–261. [Google Scholar] [CrossRef]
- Deegan, C.A.; Murray, D.; Doran, P.; Moriarty, D.C.; Sessler, D.I.; Mascha, E.; Kavanagh, B.P.; Buggy, D.J. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg. Anesth. Pain Med. 2010, 35, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gu, X.; Zhu, L.; Wu, G.; Zhou, H.; Song, Y.; Wu, C. Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Medicine 2016, 95, e5479. [Google Scholar] [CrossRef]
- Soltanizadeh, S.; Degett, T.H.; Gögenur, I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: A systematic review. J. Clin. Anesth. 2017, 42, 19–25. [Google Scholar] [CrossRef]
- Carnet Le Provost, K.; Kepp, O.; Kroemer, G.; Bezu, L. Trial watch: Dexmedetomidine in cancer therapy. Oncoimmunology 2024, 13, 2327143. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liang, H.; Yin, H.; Ye, X. Anesthesia-related postoperative oncological surgical outcomes: A comparison of total intravenous anesthesia and volatile anesthesia. A meta-analysis. Videosurgery Other Miniinvasive Tech. 2023, 18, 612–624. [Google Scholar] [CrossRef]
- Yoon, S.; Jung, S.-Y.; Kim, M.-S.M.; Yoon, D.M.; Cho, Y.M.; Jeon, Y. Impact of Propofol-based Total Intravenous Anesthesia Versus Inhalation Anesthesia on Long-term Survival After Cancer Surgery in a Nationwide Cohort. Ann. Surg. 2023, 278, 1024–1031. [Google Scholar] [CrossRef]
- Pang, Q.-Y.; Duan, L.-P.; Jiang, Y.; Liu, H.-L. Comparison of Outcomes After Breast Cancer Surgery Between Inhalational and Propofol-Based Intravenous Anaesthesia: A Systematic Review and Meta-Analysis. J. Pain Res. 2021, 14, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Wu, M.-Y.; Chien, Y.-J.; Su, I.-M.; Wang, S.-C.; Kao, M.-C. Anesthesia and Long-term Oncological Outcomes: A Systematic Review and Meta-analysis. Anesth. Analg. 2021, 132, 623–634. [Google Scholar] [CrossRef]
- Jin, Z.; Li, R.; Liu, J.; Lin, J. Long-term prognosis after cancer surgery with inhalational anesthesia and total intravenous anesthesia: A systematic review and meta-analysis. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 83–94. [Google Scholar]
- Yap, A.; Lopez-Olivo, M.A.; Dubowitz, J.; Hiller, J.; Riedel, B. Anesthetic technique and cancer outcomes: A meta-analysis of total intravenous versus volatile anesthesia. J. Can. Anesth. 2019, 66, 546–561. [Google Scholar] [CrossRef]
- Tang YT, L.; Yao, Y.; Huang, H.; Chen, B. Effects of anesthesia on long-term survival in cancer surgery: A systematic review and meta-analysis. Heliyon 2024, 10, e24791. [Google Scholar] [CrossRef] [PubMed]
- Enlund, M.; Berglund, A.; Enlund, A.; Lundberg, J.; Wärnberg, F.; Wang, D.-X.; Ekman, A.; Ahlstrand, R.; Flisberg, P.; Hedlund, L.; et al. Impact of general anaesthesia on breast cancer survival: A 5-year follow up of a pragmatic, randomised, controlled trial, the CAN-study, comparing propofol and sevoflurane. eClinicalMedicine 2023, 60, 102037. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.J.; Zhang, Y.; Zhang, Y.X.; Zhao, W.; Pan, L.H.; Sun, X.D.; Jia, Z.; Ouyang, W.; Ye, Q.S.; Zhang, F.X.; et al. Long-term survival in older patients given propofol or sevoflurane anaesthesia for major cancer surgery: Follow-up of a multicentre randomised trial. Br. J. Anaesth. 2023, 131, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.; Dubois, B.F.H.; Hollmann, M.W. The Effect of Propofol versus Inhalation Anesthetics on Survival after Oncological Surgery. J. Clin. Med. 2022, 11, 6741. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Manfredi, B.; Bianchi, M.; Panerai, A. Intermittent but not continuous inescapable footshock stress affects immune responses and immunocyte beta-endorphin concentrations in the rat. Brain Behav. Immun. 1994, 8, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Sawada, S.; Yoshioka, I.; Ohashi, Y.; Matsuo, M.; Harimaya, Y.; Tsukada, K.; Saiki, I. Increased surgical stress promotes tumor metastasis. Surgery 2003, 133, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Page, G.G.; Ben-Eliyahu, S.; Yirmiya, R.; Liebeskind, J.C. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain 1993, 54, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Yamaguchi, Y. Autologous tumor killing activity as a prognostic factor in primary resected nonsmall cell carcinoma of the lung. Cancer 1997, 79, 474–481. [Google Scholar] [CrossRef]
- Pintado, M.C.; Unzúe, I.L.; Sanz, R.G.; Alonso, M.D.; Ortega, M.A.; de Mon, M.; Losada, E.N.; Calvo, A.G. Hematological Alterations after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J. Clin. Med. 2023, 12, 4323. [Google Scholar] [CrossRef]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2009, 34, J258–J265. [Google Scholar] [CrossRef]
- Bezu, L.; Bordenave, L.; Suria, S.; Billard, V.; Barlesi, F.; Morice, P. Onco-anaesthesia: From theory to practice. Anesth. Reanim. 2022, 8, 315–330. [Google Scholar]
- Noba, L.; Rodgers, S.; Chandler, C.; Balfour, A.; Hariharan, D.; Yip, V.S. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Improve Clinical Outcomes in Liver Surgery: A Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2020, 24, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-M.; Wang, Y.; Mao, Y.-X.; Wang, W. The Safety and Feasibility of Enhanced Recovery after Surgery in Patients Undergoing Pancreaticoduodenectomy: An Updated Meta-Analysis. BioMed Res. Int. 2020, 2020, 7401276. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.L.; Ye, X.Z.; Zhang, X.D.; Chen, B.C.; Yu, Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: A meta-analysis of randomized controlled trials. Dis. Colon. Rectum. 2013, 56, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.M.; Longhitano, Y.; Zanza, C.; Sener, S.F. Factors affecting the incidence of chronic pain following breast cancer surgery: Preoperative history, anesthetic management, and surgical technique. J. Surg. Oncol. 2020, 122, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Sandrucci, S.; Beets, G.; Braga, M.; Dejong, K.; Demartines, N. Perioperative nutrition and enhanced recovery after surgery in gastrointestinal cancer patients. A position paper by the ESSO task force in collaboration with the ERAS society (ERAS coalition). Eur. J. Surg. Oncol. 2018, 44, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Quiram, B.J.; Crippa, J.; Grass, F.; Lovely, J.K.; Behm, K.T.; Colibaseanu, D.T.; Merchea, A.; Kelley, S.R.; Harmsen, W.S.; Larson, D.W. Impact of enhanced recovery on oncological outcomes following minimally invasive surgery for rectal cancer. Br. J. Surg. 2019, 106, 922–929. [Google Scholar] [CrossRef]
- Mari, G.; Crippa, J.; Costanzi, A.; Mazzola, M.; Rossi, M.; Maggioni, D. ERAS Protocol Reduces IL-6 Secretion in Colorectal Laparoscopic Surgery: Results from a Randomized Clinical Trial. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 444–448. [Google Scholar] [CrossRef]
| Immunological Parameter | Description |
|---|---|
| Neutrophil-to-lymphocyte ratio Platelet-to-lymphocyte ratio Lymphocyte-to-monocyte ratio | Neutrophils: can either promote or inhibit cancer development and growth. Lymphocytes: key components of the adaptive immune system. Platelets: facilitate metastatic spread through production of adhesion proteins, clotting factors, and various interactions. Monocytes: innate immune cells, which can have both pro-tumor and anti-tumor effects. |
| IL-4 | Anti-inflammatory |
| IL-6 | Pro-inflammatory |
| IL-8 | Pro-inflammatory |
| IL-10 | Anti-inflammatory |
| IL-12 | Pro-inflammatory |
| IL-17A | Pro-inflammatory |
| TNF-α | Pro-inflammatory |
| Oxidative stress profile (lipid peroxidation status and antioxidant capacity of plasma) | Lipid peroxidation: oxidative degradation of lipids mediated by free radicals; can cause DNA damage. |
| CRP | Non-specific marker of inflammation. |
| NK and CD8+ T cells | Cytotoxic function. |
| T helper cells | CD4+ helper cells, enhance function of CD8+ cells and macrophages. |
| Caspase 3 | Marker of apoptosis. |
| Cortisol | Glucocorticoid hormone with immunosuppressive effects. |
| HIF-1α | Regulates transcription of genes involved in cell viability and proliferation. |
| VEGF | Growth factor promoting angiogenesis. |
| NF-κB | Involved in regulation of inflammation, cell activation, and proliferation. |
| Outcomes | Immunological | Oncological | |
|---|---|---|---|
| Authors | Parameters | Main Findings | Main Findings |
| Rangel et al. (2021) [104] | Neutrophil-to-lymphocyte ratio | Postoperative neutrophil-to-lymphocyte median rates not significantly different between groups. | Biochemical recurrence-free survival. No significant differences in either outcome between groups. |
| Aboalsoud et al. (2021) [105] | IL-10, TNF-α, caspase 3 | OFA vs. OBA: Increase in IL-10 and caspase 3; decrease in TNF-α. | N/A |
| Titon et al. (2021) [106] | IL-4, IL-12, IL-17A, TNF-α, oxidative stress profile | OFA vs. OBA: Decrease in lipid peroxidation, increase in antioxidant capacity of plasma. No variation in IL-4, IL-17A, and TNF-α. | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezu, L.; Akçal Öksüz, D.; Bell, M.; Buggy, D.; Diaz-Cambronero, O.; Enlund, M.; Forget, P.; Gupta, A.; Hollmann, M.W.; Ionescu, D.; et al. Perioperative Immunosuppressive Factors during Cancer Surgery: An Updated Review. Cancers 2024, 16, 2304. https://doi.org/10.3390/cancers16132304
Bezu L, Akçal Öksüz D, Bell M, Buggy D, Diaz-Cambronero O, Enlund M, Forget P, Gupta A, Hollmann MW, Ionescu D, et al. Perioperative Immunosuppressive Factors during Cancer Surgery: An Updated Review. Cancers. 2024; 16(13):2304. https://doi.org/10.3390/cancers16132304
Chicago/Turabian StyleBezu, Lucillia, Dilara Akçal Öksüz, Max Bell, Donal Buggy, Oscar Diaz-Cambronero, Mats Enlund, Patrice Forget, Anil Gupta, Markus W. Hollmann, Daniela Ionescu, and et al. 2024. "Perioperative Immunosuppressive Factors during Cancer Surgery: An Updated Review" Cancers 16, no. 13: 2304. https://doi.org/10.3390/cancers16132304
APA StyleBezu, L., Akçal Öksüz, D., Bell, M., Buggy, D., Diaz-Cambronero, O., Enlund, M., Forget, P., Gupta, A., Hollmann, M. W., Ionescu, D., Kirac, I., Ma, D., Mokini, Z., Piegeler, T., Pranzitelli, G., Smith, L., & The EuroPeriscope Group. (2024). Perioperative Immunosuppressive Factors during Cancer Surgery: An Updated Review. Cancers, 16(13), 2304. https://doi.org/10.3390/cancers16132304










