Simple Summary
Digital health technologies can help manage the growing cancer burden, but understanding patients’ needs is crucial for these tools to be effective. Our study reviewed existing research on what cancer patients want from digital health technologies. We analysed 128 studies, focusing on web-based platforms, mobile apps, and wearable devices used in cancer care. Patients highlighted the importance of these technologies being easy to use, effective in managing their care, and enhancing communication with healthcare providers. Our findings offer insights for future research to develop digital health tools that meet cancer patients’ preferences, potentially improving their healthcare experience and outcomes.
Abstract
Digital health technologies have the potential to alleviate the increasing cancer burden. Incorporating patients’ perspectives on digital health tools has been identified as a critical determinant for their successful uptake in cancer care. The main objective of this scoping review was to provide an overview of the existing evidence on cancer patients’ perspectives and requirements for patient-facing digital health technologies. Three databases (CINAHL, MEDLINE, Science Direct) were searched and 128 studies were identified as eligible for inclusion. Web-based software/platforms, mobile or smartphone devices/applications, and remote sensing/wearable technologies employed for the delivery of interventions and patient monitoring were the most frequently employed technologies in cancer care. The abilities of digital tools to enable care management, user-friendliness, and facilitate patient–clinician interactions were the technological requirements predominantly considered as important by cancer patients. The findings from this review provide evidence that could inform future research on technology-associated parameters influencing cancer patients’ decisions regarding the uptake and adoption of patient-facing digital health technologies.
1. Introduction
Cancer constitutes a global health issue with 19.3 million new cases occurring in 2020, while cancer incidence is projected to significantly increase in the coming years, reaching 28.4 million new cases annually by 2040 [1]. Meanwhile, advances in early detection and cancer treatment have improved survival rates, leading to steadily rising numbers of long-term cancer survivors [2]. The growing population of people diagnosed and living with cancer together with the complex healthcare needs that this population faces place a substantial strain on health services, often resulting in cancer patients and their caregivers not accessing or receiving timely and adequate care and support [3]. Emerging digital health technologies have the potential to contribute towards alleviating the increasing cancer burden exerted on healthcare services by leveraging technology to improve care quality, accessibility, and cost-effectiveness [4].
Digital health technologies are digital products, encompassing both hardware and software solutions and services, for healthcare and related uses intended to benefit people and the wider health and social care system [5,6]. Such technologies may comprise smartphone applications, electronic medical records, wearable monitoring or reporting devices, decision support systems, and online tools for treating or diagnosing conditions, preventing ill health, or for improving system efficiencies [6,7]. Numerous beneficial applications of digital health technologies to healthcare delivery and practice have been reported, including the remote monitoring of patients’ symptoms and conditions; the provision of electronic decision support, resources, and interventions; and the facilitation of distant patient–clinician communication [7,8]. Based on these, the incorporation of digital health technologies into national health systems worldwide has been identified as a key priority [9].
In cancer care, where patients’ symptom burden tends to be high due to disease progression or treatment-associated side-effects, the use of digital health technologies can facilitate the collection of patient-generated data, including patient-reported outcomes. This can help in overcoming the challenges associated with conventional clinician-led symptom monitoring, which can often lead to the under-reporting of patients’ symptoms [10]. Routine symptom assessment enabled by digital health technologies thus has the potential to improve symptom management, resulting, in turn, in improvements in healthcare resource utilisation and patients’ quality of life compared with standard clinical assessment [10]. A further advantage of digital technology applications is that they can increase the equity of cancer care delivery by extending access to care for patients in remote or rural areas or those living with socioeconomic disadvantages, mitigating, therefore, geographical and socioeconomic disparities in cancer outcomes that are associated with limited access to care [11,12]. Moreover, using digital health technologies to support patient education has the potential to improve compliance with care pathways and aid self-management to address the complex healthcare needs of people diagnosed with cancer [7,13]. Despite these advantages, however, the adoption of digital health technologies remains limited both in healthcare overall and in cancer care in particular [10,14].
Incorporating patients’ perspectives into the development of digital health tools has been identified as a critical determinant for the successful uptake of these technologies in healthcare [15]. Understanding end-users’ needs and preferences for technological innovations has been found to increase their acceptance, feasibility, and long-term use in clinical populations [16,17]. To this end, the active engagement of patients and other key stakeholders throughout all stages of technology development via the use of participatory and iterative approaches is recommended and has been shown to be particularly useful for the development of technologies intended to be used by patients with ongoing healthcare and educational needs, such as those experienced by patients diagnosed with cancer [18,19,20].
In spite of the reported added value of encompassing end-users’ perspectives into the development of technological innovations, there is limited information available to date regarding cancer patients’ needs and perceptions of digital health technologies, while existing evidence has not yet been systematically reviewed and synthesised. The purpose of the present review, therefore, is to provide an overview of the available evidence regarding cancer patients’ requirements for patient-facing digital health technologies, aiming to enhance our understanding of the needs and preferences of this population for the technologies integrated into their care. This comprehension can contribute towards guiding the development of patient-facing digital technologies to ensure that they meet the expectations of cancer patients and do not place additional burdens on their care, thereby improving their uptake and continued use.
2. Materials and Methods
2.1. Review Question and Objectives
The main objective of the scoping review was to systematically map and synthesise the existing evidence on cancer patients’ perspectives and requirements for patient-facing digital health technologies, i.e., digital technologies with which patients interact to participate in healthcare or clinical activities [21]. To form the review question, the Population, Intervention, Comparison, and Outcomes (PICO) framework was adopted (Table 1). The review was registered with Review Registry (ID: reviewregistry1816).

Table 1.
Review question (PICO).
2.2. Search Strategy
The search strategy comprised two steps. A database, MEDLINE (via PubMed), was initially searched to identify primary research studies reporting on cancer patients’ perceptions of digital health technologies. Relevant text words contained in the title, abstract, and authors’ keywords of identified papers and database index terms were compiled to produce a list of search terms. Three databases were then searched, CINAHL, MEDLINE, and Science Direct, using a combination of subject headings and free-text terms for cancer patients, digital health technologies, and user perceptions (see Figure 1 for the search strategy used for MEDLINE).
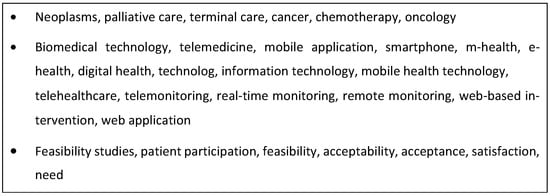
Figure 1.
Search terms applied in database searches.
2.3. Eligibility Criteria
- Articles were included if they met all the following a priori specified criteria:
- Primary research studies.
- Full-text research articles.
- English-language publications.
- Studies conducted with adult (>18) cancer patients.
- Studies reporting cancer patients’ perspectives on patient-facing digital technologies.
Papers were excluded if they were reporting on the following:
- Non-primary studies, including systematic reviews.
- Opinion articles, editorials, or book chapters.
- Case-report studies and studies providing no information about sample size.
- Non-English-language publications.
- Studies with non-adult (<18) cancer patients.
- Studies reporting the use of provider-facing digital health technologies.
- Studies reporting clinicians’/caregivers’ perceptions of the use of patient-facing digital technologies.
2.4. Selection Procedure
The study selection process involved three phases. First, all records yielded by the database searches were reviewed for eligibility by title. Then, a selection process based on abstract information was conducted. Finally, a full-text review of studies that met the inclusion criteria according to the initial two phases was performed. Two reviewers (IL, AMK) independently assessed the obtained records for eligibility. Discrepancies at each stage of study selection were resolved through discussion. Finally, citation chaining (forward and backward) was undertaken to ensure that all relevant publications were identified and included, if eligible.
2.5. Data Extraction
Two reviewers (IL, AMK) independently extracted relevant data on each included study, the technologies used, and user perceptions using a standardised form developed for the purposes of this review. For each study, the following information was extracted and entered into the form: author(s), date of publication, study design, study population, sample size, technology used, and purpose of technology use. Data on users’ perceptions of technologies were also extracted using the same form and were categorised based on a modified version of the technology evaluation categories of the Human, Organization and Technology-fit (HOT-fit) evaluation framework for Health Information Systems [22]. These categories included perceptions relating to the quality of the technological system, its content/available information, its service quality, and other relevant characteristics and features reported by cancer patients. The results of data extraction were compared and any disagreements between reviewers were resolved by consensus.
3. Results
3.1. Search Results
Database searches yielded a total of 7836 records. After duplicates were removed, 7793 studies were screened based on their titles, and 709 studies were screened on the basis of their abstracts. Following the screening of titles and abstracts, 391 potentially eligible articles remained, which were examined in full. Of these studies, 107 met the criteria for inclusion. A further 21 eligible studies were identified through forward and backward citation chaining, resulting in 128 included studies (see Figure 2 or the PRISMA flow diagram of the study selection process [23]).
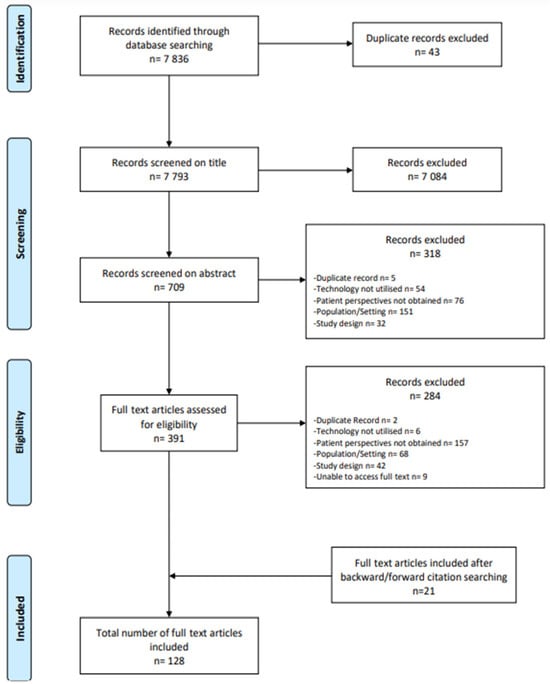
Figure 2.
PRISMA flow diagram of the article screening and selection process [23].
3.2. Description of Included Studies
Overall, the included studies were published within the last 20 years (2002–2022), with the overwhelming majority (n = 110, 85.9%) being published from 2013 onwards (Figure 3). Studies were predominantly conducted in the USA (n = 53), Australia (n = 15), the UK (n = 12), Canada (n = 11), and the Netherlands (n = 9). Different research designs were employed by the included studies, with most (n = 55) being interventional, predominantly full-scale or pilot randomised controlled trials (RCTs), or small-scale observational investigations nested within interventional studies (e.g., an evaluation study nested within an RCT). Data collection in all studies was conducted prospectively. Sample size varied greatly between studies, ranging from 3 to 4737 participants, reflecting the diverse objectives and research designs employed by the included studies. The participant population mostly consisted of breast (n = 38), prostate (n = 14), lung (n = 8), colorectal (n = 8), and head and neck (n = 7) cancer patients receiving or awaiting treatment and survivors. The remaining studies (n = 46) included patients with various cancer types or did not provide type-specific information.
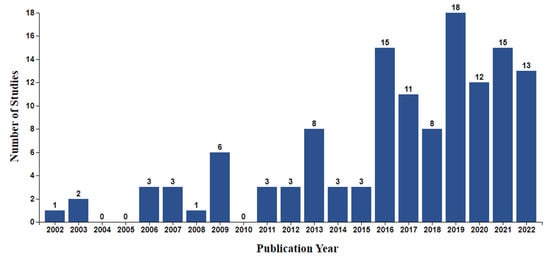
Figure 3.
Number of identified studies by publication year.
3.3. Description of Identified Patient-Facing Digital Technologies
Patient-facing digital technologies identified across the included studies can be broadly categorised as follows: Web-based software/platforms (n = 53), mobile/smartphone devices/applications (n = 33), telephone-based services and tools (n = 13), and remote sensing and wearable technologies (n = 4) [24,25,26,27]. Telephone-based services and tools were mostly identified in studies published before 2014, while newer studies (published between 2015 and 2022) predominantly reported the use of Web- and smartphone-based technologies or remote sensing devices. Sixteen studies reported using more than one patient-facing digital technology, mostly including wearable devices combined with additional technologies such as smartphone applications or Web-based platforms (n = 13). In two studies [28,29], an existing social media platform (i.e., Facebook) was used together with other digital health technologies. Identified wearable technologies in all cases comprised activity-tracking devices which were predominantly wrist-worn (n = 15). One study included an upper-arm wearable sensor [24], whist in another study participants were asked to use an accelerometer on their waist [30]. Other technologies, not classified in the abovementioned categories, were reported in nine studies. These included automated chatbots simulating human conversations through text or voice interactions (n = 3) [31,32,33], a touch-screen computer monitor for self-reporting psychosocial information in an ambulatory cancer clinic (n = 1) [34], an electronic health system facilitating patient-reported outcome data collection (n = 1) [35], a virtual reality platform supporting post-treatment rehabilitation (n = 1) [36], a voice-activated, interactive computer model enabling virtual conversations (n = 1) [37], a non-invasive optical technology for the remote screening of severe neutropenia (n = 1) [38], and a telemonitoring system consisting of a small point-of-care haematology analyser linked to a telecommunication hub facilitating the self-testing of patients’ blood counts (n = 1) [39]. Figure 4 presents the number of included studies by technology category.
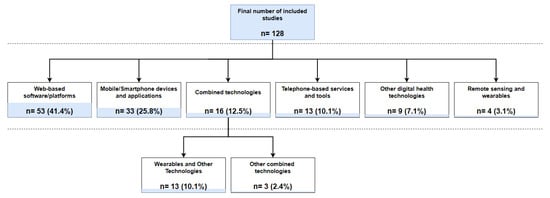
Figure 4.
Number of identified studies by technology category.
The majority of the reported technologies (n = 77) were predominantly used for delivering interventions to cancer patients at various stages of disease progression (Figure 5). Of these, 23 provided interventions aiming to assist the self-management of patients’ symptoms and/or overall conditions. In a further 15 studies, interventions were designed to increase patients’ cancer-related knowledge, mostly by providing information and resources on different treatment and care management options. Technologies were particularly used for educating patients on clinical trial participation in one study [40], whilst in another study cancer-related educational materials were developed both for patients and their partners, thus delivering a couple-focused intervention [41]. Sixteen studies described using digital health technology-assisted interventions for promoting patients’ physical activity and health behaviour habits, while a further twelve studies reported delivering therapeutic or educational interventions for the management of psychosocial symptoms. Assisting patient rehabilitation after surgery or chemotherapy was the focus of technological interventions in five of the included studies in this category, whereas an equal number of studies (n = 5) delivered interventions aimed at improving cancer patients’ medication adherence. One study reported the use of speech-generating devices to support telephone or face-to-face communication after total laryngectomy [42].
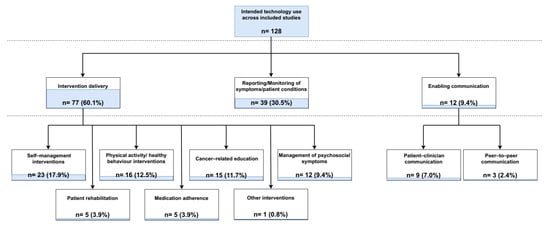
Figure 5.
Number of included studies by intended technology use.
Other intended uses of digital health technologies described in the identified studies included the reporting and/or monitoring of patients’ symptoms and physiological parameters and enabling patient–clinician or peer-to-peer communication. Specifically, the remote reporting and/or monitoring of patients’ conditions, vital signs, and symptoms conducted through self-reported or automatically collected data via wearable technologies was described as the primary intent of digital health technology use in 39 studies. Similarly, the principal purpose of technology employment was the facilitation of remote patient–clinician consultations or peer interactions in nine and three of the included studies, respectively.
3.4. Evaluation of Digital Health Technologies
Cancer patients’ perceptions of digital health technologies were almost exclusively investigated by the included studies in the context of evaluations of existing or newly developed patient-facing technologies/technological interventions following their development (n = 115) (Table 2). Only a small number of studies (n = 13) explored patients’ perspectives in the design and/or development phases of technologies. Assessment objectives differed significantly between studies, with 68 papers reporting two or more objectives. Among these, the most frequently evaluated were user satisfaction (n = 43) and the acceptability (n = 41), feasibility (n = 38), and utility/usability (n = 31) of the technologies. Twenty-three studies described exploring cancer patients’ perceptions and/or experiences of using digital health technologies, while the main objective in five studies was the examination of patients’ technological needs and requirements. Despite a plethora of evaluation objectives being described across the studies, there was a significant overlap in the definitions of assessed concepts, with the terms “acceptability”, “satisfaction”, “utility/usability”, and “user perceptions/experiences” being used interchangeably in a number of studies
In terms of the methods employed for the evaluation of identified technologies, 68 studies reported using mixed (i.e., both qualitative and quantitative) or multiple assessment methods, while in 60 studies a single quantitative or qualitative method was utilised. Patient-reported measures, predominantly questionnaires (n = 68), comprised the most frequently described means of technology evaluation, followed by different metrics, such as technology interaction duration and rates, login data, and completion rates, collected either through participants’ self-reports or extracted from technology tracking data (n = 42). Qualitative methods, and particularly individual or group interviews and textual data provided by patients, were utilised in 32 studies. In most cases (n = 62), evaluation methods (questionnaires, scales, interview guides) were developed or adapted/modified from measures and guides used in previous research for the purposes of individual studies without any prior validation (i.e., ad hoc methods). Thirty-two studies described using validated evaluation measures and existing theoretical frameworks for the development of interview guides. Of these, the most commonly employed were the System Usability Scale (n = 10) [43], the Mobile App Rating Scale (MARS; n = 4) [44], and the Technology Acceptance Model (TAM; n = 3) [45].
The included studies overwhelmingly reported positive findings for the patient-facing technology appraisals performed by cancer patients, with 120 studies describing the evaluated technologies being perceived as acceptable, feasible, and/or useful to patients overall. Only eight studies reported mixed findings, concluding with suggested improvements in distinct technological features spanning from content changes to hardware adjustments to increase the usability and acceptance of technologies [25,46,47,48,49,50,51,52].
3.5. User Perspectives and Requirements
In line with the HOT-fit evaluation framework for Health Information Systems [22], cancer patients’ perceptions of digital health technologies were grouped into four main categories: system, information/content, service, and other requirements. System requirements concern the inherent features of digital health technologies, including the system performance and user interface. Information/content requirements relate to the characteristics of the information delivered to patients through health technologies. Service requirements refer to the extent to which support is provided to patients in the use and handling of technologies. Other requirements cover any additional issues and perspectives raised by cancer patients, not covered by the aforementioned categories. Table 3 and Figure 6 provide an overview of the identified user perceptions and requirements.
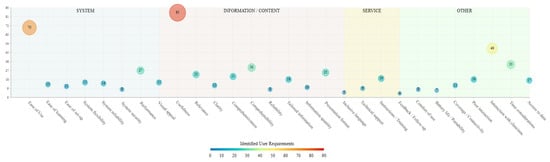
Figure 6.
Identified user patient-facing digital health technology requirements by classification category.
In the system requirements category, ease of use (n = 70) and overall system performance (n = 28) were the technological characteristics most frequently assessed as important by cancer patients. Other system features considered by patients included system reliability in terms of the stability of different features (n = 15) and flexibility (n = 15) in being able to return to or skip certain parts or modules and alter their responses. The ease of setting up technologies for the first time and the attractiveness of the overall design and/or user interface were reported as important characteristics by participants in 11 and 15 studies, respectively. In 13 studies, cancer patients highlighted the importance of being able to easily learn how to operate technologies, whilst the system ensuring that provided patient data are secure and protected was reported as a digital health technology requirement in 8 studies.
In the information/content category, the extent to which the content of the technological intervention was considered useful for the management of patients’ symptoms or overall conditions was the most frequently reported feature among participants of the included studies (n = 86). Using language that is easy to understand and lay terms was raised as an important content feature by participants in 30 studies, while in 26 studies cancer patients noted that alternative means of information presentation, such as videos, pictures, and graphs, are helpful in aiding comprehensibility. The extent to which the presented content is relevant to the patients’ situations and the comprehensiveness of the provided information in terms of covering all important aspects of a given topic were noted by participants in 23 and 21 studies, respectively. In 18 studies, cancer patients reported finding the content of the technological interventions to be tailored to their own needs and situation, which was considered a feature that increased the usability of technologies, whilst in other studies participants commented on the importance of information clarity (n = 12), quantity (n = 10), and reliability (n = 8). Using culturally and gender-sensitive, as well as inclusive, language was noted as an important content characteristic by cancer patients in five studies.
For the service requirements, cancer patients mostly reported the need to receive appropriate instructions and/or training (n = 20) in the use of technologies, either in the form of a training manual or one-to-one sessions with people who could guide them through the process of setting up and interacting with the technologies. Additional service requirements included technical support being available for the duration of interactions with technologies (n = 9) and receiving feedback on whether given tasks were completed in line with the relevant guidance (n = 4).
Further issues reported by cancer patients which could not be grouped in the aforementioned categories included the technology enabling patient–clinician (n = 50) and peer-to-peer (n = 18) interactions. Specifically, patients highlighted the importance of being able to communicate their symptoms or overall condition with the clinical teams involved in their care through health technologies, either via their data being directly shared with their consulting clinicians or by being able to produce alerts in case of emergencies. Furthermore, in 33 studies participants reported that in the development phase of digital health technologies, consideration should be given to the amount of time that needs to be invested by end-users in order for the appropriate data to be collected, especially in the case of cancer patients where increased symptom burden may not allow for lengthy amounts of time to be devoted to technology interactions. In 18 studies, participants commented that they would appreciate having access to aggregate forms and graphical representations of their own data reported through/gathered from health technologies. Further issues were raised related to the coverage/connectivity of health technologies to internet/telecommunication networks (n = 12), and to the limited battery life of medical devices/wearables (n = 8), deterring patients from using technologies due to additional costs and burden. Lastly, comfort of use was reported as a requirement for technological devices, especially wearables, by patients in nine of the included studies.

Table 2.
Study characteristics.
Table 2.
Study characteristics.
| Author(s), Year | Country | Study Design | Study Population | n | Technology | Purpose of Technology Use | Technology Description |
|---|---|---|---|---|---|---|---|
| Abernethy et al., 2009 [53] | USA | Prospective observational study | Metastatic breast cancer patients | 66 | Tablet-based software | Symptom and quality-of-life reporting | Tablets used by patients for symptom and quality-of-life self-reported assessments |
| Admiraal et al., 2017 [54] | Netherlands | RCT | Breast cancer patients | 138 | Web-based platform | Delivery of a tailored psychoeducation programme | Features included assessments of patients’ distress and prevailing problems and psychoeducational materials tailored to their reported needs |
| Aiello et al., 2006 [55] | USA | RCT | Breast cancer patients | 160 | Tablet-based software | Enabling electronic data collection on breast cancer risk factors | Passive digitizer tablet computer with a plastic pointer to complete electronic questionnaires through Microsoft Access |
| Albrecht et al., 2011 [56] | Germany | Mixed methods study | Breast cancer patients | 9 | Video-based decision aid | Providing guidance in patient decision-making and communicating preferences | Adaptation of existing 55 min video on different surgical treatment options (lumpectomy vs. mastectomy) in the German context using synchronised voice-over |
| Allenby et al., 2002 [34] | Australia | RCT | Patients with various cancer types | 451 | Platform accessed through touch-screen computer | Self-reporting of psychosocial information | Touch-screen monitor for patient symptom reporting using software developed utilising the Visual Basic Version 6 programming language; data stored in Microsoft Access 97 databases |
| Allicock et al., 2021 [30] | USA | Pilot RCT | African American breast cancer survivors | 22 | Smartphone-based application, waist-worn wearable (ActiGraph wGT3X-BT) | Improving survivors’ physical activity and diet behaviours | App accessed through Samsung Galaxy Core Prime devices used to complete daily ecological momentary assessments; accelerometer for physical activity monitoring |
| Alshoumr et al., 2021 [57] | Saudi Arabia | Qualitative study | Patients with various cancer types | 22 | Web-based platform and monitor | Providing patient education, enabling communication with clinicians, aiding self-management of care and symptoms | Inpatient portal presented on 55-inch high-resolution monitors placed in front of a patient’s bed in clinical wards |
| Appleyard et al., 2021 [58] | UK | Mixed methods observational study | Advanced prostate cancer patients | 40 | Tablet-based platform | Enabling the electronic collection of patient-reported outcomes | Platform facilitating patient completion of health-related quality-of-life questionnaires on a tablet |
| Badr et al., 2016 [59] | USA | Qualitative study | Oral cancer survivors | 13 | Web-based platform | Improving survivor self-management and survivor/caregiver quality of life | Education for the management of side-effects, facilitation of connections with peers, and provision of self-monitoring features |
| Basch et al., 2007 [60] | USA | Prospective observational study | Cancer patients receiving chemotherapy | 180 | Web-based platform | Enabling the electronic collection of patient-reported outcomes | Platform features include the real-time report generation of patient symptoms and an alert system for clinicians |
| Basch et al., 2017 [61] | USA | Feasibility study nested within an RCT | Cancer patients receiving radiation treatment | 152 | Web-based platform | Enabling patient reporting of symptomatic adverse events | Online patient-reported outcome items for the systematic identification of adverse events in clinical trials |
| Basch et al., 2020 [62] | USA | Multicentre RCT | Patients with various cancer types | 496 | Web-based platform | Enabling remote patient symptom monitoring | Online patient-reported outcome items for the systematic identification of adverse events in clinical trials |
| Beaver et al., 2012 [63] | UK | Exploratory RCT | Colorectal cancer patients | 65 | Telephone service | Delivery of follow-up consultations | Audio-recorded telephone sessions pre-registered on computerised hospital information systems together with patients’ medical records |
| Beaver et al., 2009 [64] | UK | RCT | Breast cancer patients | 374 | Telephone service | Delivery of follow-up consultations | Nurse-led telephone follow-up intervention using a standardised protocol to address various patient concerns, including symptoms, information needs, and psychosocial support, ensuring comprehensive and consistent patient care remotely |
| Bender et al., 2022 [65] | Canada | Pilot feasibility study | Prostate cancer patients | 29 | Web-based application | Delivery of peer support for care management | True North PN: Web-based peer navigation programme for prostate cancer patients using a matching algorithm, private messaging, health resources, and case management |
| Bender et al., 2013 [66] | Canada | Multi-method study | Breast cancer patients | 100 | Web-based synchronous text message communication platform | Delivery of online support groups | Online communities–websites that offer discussion forums or chat rooms to support breast cancer survivors by providing information, symptom management, and emotional support |
| Bennett et al., 2016 [67] | USA | Prospective observational study | Patients with various cancer types | 112 | Web-enabled tablet computer, interactive voice response system (IVRS) | Collection of patient-reported outcomes | IVRS accessed through mobile phones or landlines. Touch-screen tablets provided to users at clinic visits |
| Benze et al., 2019 [68] | Germany | Prospective feasibility study | Advanced cancer patients | 40 | Smartphone-based application | Enabling the electronic collection of patient-reported outcomes | MeQoL app for ePROs in advanced cancer patients (symptoms, pain intensity, quality-of-life data) |
| Bol et al., 2013 [69] | Netherlands | Prospective randomised trial | Older lung cancer patients | 357 | Website | Delivery of cancer-related information | Personalised audio–visual information in addition to text on website satisfaction and recall of cancer-related online information |
| Bolle et al., 2016 [70] | Netherlands | Think-aloud study | Older cancer patients and survivors | 15 | Existing cancer information tools | Providing cancer-related information | 3 cancer information websites, 3 Web-based question prompt lists, 1 decision aid based on the values clarification method |
| Brennan et al., 2022 [71] | Ireland | Prospective feasibility study | Upper gastrointestinal cancer survivors | 12 | Web-based platform | Delivery of a multidisciplinary rehabilitation programme | Clinically tested digital therapy platform for hosting video calls (one-to-one and group), providing exercise prescription, and appointment scheduling |
| Cadmus-Bertram et al., 2019 [72] | USA | Pilot RCT | Breast and colorectal cancer survivors | 50 | Wrist-worn wearable (Fitbit) linked to electronic health record | Monitoring of and education on physical activity | Fitbit activity trackers linked to electronic health records (EHRs), along with email-based coaching and an educational handbook |
| Chaix et al., 2019 [31] | France | Prospective observational study | Breast cancer patients | 4737 | Chatbot | Improving medication adherence | The chatbot provides personalised text responses to user questions using machine learning methods |
| Chee et al., 2017 [73] | USA | Prospective observational study | Asian American breast cancer survivors | 5 | Web-based platform | Delivery of culturally appropriate patient education | Culturally tailored internet cancer support group for Asian American breast cancer survivors. Pilot test for enhancing women’s breast cancer survivorship experience |
| Cheville et al., 2018 [74] | USA | RCT | Late-stage cancer patients | 516 | Web-based platform, pedometer, telephone service | Remote monitoring and management of symptoms and physical activity | Web-based interface and telephone-based IVR used for collection of pain and function patient-reported outcomes; pedometers used for step count |
| Childes et al., 2017 [42] | USA | Online questionnaire survey | Head and neck cancer patients after total laryngectomy | 265 | Text-to-speech-based applications/software programmes accessed using various devices | Providing verbal communication support | Speech-generating devices (SGDs) to support telephone or face-to-face communication post-laryngectomy along with text-to-speech for both face-to-face and phone communication and teletypewriter devices as an alternative communication method for individuals post-laryngectomy |
| Chow et al., 2021 [28] | USA | Pilot RCT | Haematologic malignancy survivors | 41 | Smartphone-based application, wrist-worn wearable (Fitbit), social media platform (Facebook) | Delivery of an intervention to improve diet and physical activity | mHealth-supported intervention utilising a Fitbit wearable wristband for tracking daily steps, the Healthwatch360 app for monitoring dietary intake (sodium, saturated fats, and added sugars), and a private Facebook peer support group for social interaction and educational support among haematologic malignancy survivors |
| Chow et al., 2019 [75] | USA | Prospective observational study | Patients receiving active cancer treatment | 52 | Short Message Service (SMS) over networks of mobile operators, Web-based platform | Enabling distress monitoring | Weekly distress screeners via text message on their personal smartphones, utilising secure links delivered through a Qualtrics SMS Survey tool. The PHQ-4, a validated measure of distress, was completed online, with geolocation data automatically logged upon completion to track screening locations. Automated alerts were triggered for high distress scores |
| Cleeland et al., 2011 [76] | USA | RCT | Patients following cancer-related thoracotomy | 100 | Automated telephone service | Postoperative symptom monitoring | Automated telephone calls monitored postoperative symptoms for thoracotomy patients via interactive voice response system. Symptom alerts were sent to clinicians for severe symptoms via email |
| Collins et al., 2017 [77] | Australia | Pilot RCT | Head and neck cancer patients | 30 | Web-based platform | Delivering acute symptom monitoring, nutritional management, and swallowing and communication rehabilitation | Participants used PCs, smartphones, or tablets with cameras and microphones to connect with RBWH clinicians via a secure telehealth portal, employing videoconferencing units and webRTC technology. The telehealth system included training sessions for both patients and clinicians, ensuring effective use of the technology for remote consultations |
| Crafoord et al., 2020 [78] | Sweden | Mixed methods study | Breast and prostate cancer patients | 149 | Smartphone/tablet-based application | Reducing symptom burden during cancer treatment | App components: symptom self-assessment, evidence-based self-care advice, summaries/graphs of reported symptoms, urgent/persistent system notifications to health professionals |
| Crawford et al., 2019 [79] | USA | Development and feasibility testing study | Patients with various cancer types | 30 | Smartphone-based application | Delivering patient education on oral anticancer medications | The app interfaces with patients’ electronic medical records and is designed based on learning style and adherence theories, providing information through text, pictures, animations, and audio voiceovers (adherence barriers and features for dose scheduling, refill reminders, and feedback collection through reflective questions) |
| Denis et al., 2014 [80] | France | Prospective pilot study | Lung cancer patients | 42 | Web-based platform | Remote symptom monitoring | Patients self-report symptoms weekly via the internet for lung cancer follow-up—Software tracks weight and 10 symptoms, enabling early relapse detection |
| Duffecy et al., 2013 [81] | USA | Prospective pilot study | Post-treatment cancer survivors | 31 | Website | Skill management training for distress education | Components included lessons on basic cognitive behavioural concepts, interactive tools, self-monitoring features, and a discussion board |
| Eakin et al., 2012 [82] | Australia | RCT | Breast cancer patients | 143 | Telephone service | Delivery of an exercise intervention | Telephone-delivered intervention with mixed aerobic and resistance exercise guidance to deliver personalised exercise programs and offer remote support, ensuring accessibility for women living in rural areas |
| Ferguson et al., 2016 [83] | USA | RCT | Breast cancer survivors | 47 | Videoconferencing equipment and platform | Delivery of Cognitive Behavioural Therapy-based training | Videoconference technology (Tandberg centric 1700 MXP units) to deliver Memory and Attention Adaptation Training (MAAT) and supportive therapy (ST) remotely to breast cancer survivors, improving access to cognitive rehabilitation for participants in various locations |
| Finlay et al., 2020 [46] | Australia | RCT | Prostate cancer survivors | 71 | Web-based platform | Delivery of a physical activity intervention | Web-based computer-tailored interventions with different website architectures (free choice vs. tunnelled) to promote physical activity among prostate cancer survivors, with engagement differing based on the navigational structure of the site |
| Foley et al., 2016 [84] | Ireland | RCT | Breast cancer patients | 39 | Tablet-based application | Delivery of information on basic breast cancer biology, different treatments, and surgical methods | App for iPad to assess its impact on anxiety levels in patients undergoing surgery for breast cancer |
| Fu et al., 2016 [85] | USA | Prospective useability study | Breast cancer survivors | 30 | Web- and smartphone-based platform | Delivery of education on self-care strategies for lymphedema symptom management | TOLF: Web and mobile-based health IT system for lymphedema management—avatar technology for self-care strategies and symptom evaluation |
| Galiano-Castillo et al., 2016 [86] | Spain | RCT | Breast cancer survivors | 81 | Web-based platform | Delivery of a tailored exercise programme | Web-based interventions used for cancer survivorship support. Telehealth system with internet-based tailored exercise programme for cancer rehabilitation |
| Galsky et al., 2017 [87] | USA | Single-arm clinical trial | Prostate cancer patients | 15 | Videoconferencing platform | Enabling remote patient research-related visits | Platform used on PI’s desktop and patients’ smartphones |
| Gell et al., 2017 [88] | USA | Single-arm clinical trial | Cancer survivors | 24 | Wrist-worn wearable (Fitbit), SMS over networks of mobile operators, accelerometer (Actigraph GT3X+) | Supporting physical activity | Intervention includes text messages, Fitbit self-monitoring, and health coaching. Measures physical activity with waist-worn accelerometer and portable GPS |
| Gilbertson-White et al., 2019 [89] | USA | Mixed methods study | Patients with various cancer types | 56 | Web-based platform | Enabling symptom self-management | eHealth symptom self-management intervention, OASIS. It features multimedia graphics and uses HTML5 for animations to enhance accessibility for users with limited literacy |
| Girgis et al., 2017 [35] | Australia | Mixed methods study | Patients with various cancer types | 42 | Electronic health system | Collecting and utilising patient-reported outcome measures for personalised care | System components included self-report assessments, links to online self-care resources, health professional review, and access to patient reports |
| Graetz et al., 2018 [90] | USA | Randomised controlled feasibility trial | Ovarian cancer patients | 26 | Web-based platform | Enabling real-time symptom monitoring | Web-based app for postoperative care in gynaecological oncology patients. Reminders for discharge instructions and symptom monitoring through smartphones, iPads, or Web-enabled devices |
| Greenway et al., 2022 [36] | UK | Qualitative study | Head and neck cancer patients | 7 | Virtual reality platform | Enabling access to cancer-related information and resources | WebXR platform ‘recovery’ to simulate a virtual reality experience within a virtual room. This platform allows patients to navigate and interact with targeted resources and specific learning materials related to their cancer journey, featuring natural landscapes and architectural principles to enhance user experience and facilitate self-management of post-treatment recovery needs |
| Greer et al., 2019 [32] | USA | Pilot RCT | Young adults after cancer treatment | 45 | Online chatbot | Delivery of a cognitive and behavioural intervention to increase positive emotions | Vivibot delivers positive psychology skills via prewritten material online. Users interact with an automated system through a decision tree structure |
| Groarke et al., 2021 [91] | Ireland | Nested mixed methods study within an RCT | Cancer survivors with obesity or overweight | 36 | Wrist-worn wearable (Fitbit), SMS over networks of mobile operators | Delivery of a behaviour-change intervention | mHealth intervention using a Fitbit activity monitor and SMS contact for an 8-week physical activity goal-setting programme. The Fitbit activity monitor provided real-time physical activity feedback, while SMS contact delivered personalised goal setting and behavioural prompts, integrating behaviour-change techniques such as self-monitoring, feedback on behaviour, and goal setting |
| Gustavell et al., 2020 [92] | Sweden | Prospective observational study | Patients following pancreatic cancer surgery | 26 | Tablet- and smartphone-based application | Facilitating person-centred care | App features: assessment of self-reported symptoms, risk assessment models for alerts, access to evidence-based self-care, summaries of symptom history |
| Harless et al., 2009 [37] | USA | Prospective observational study | Breast cancer patients | 39 | Voice-activated, interactive computer model | Delivery of educational dialogues | Telehealth intervention using Health Buddy System for head and neck cancer (daily education, guidance, and encouragement) |
| Head et al., 2011 [93] | USA | Mixed methods study | Head and neck cancer patients | 44 | Telemessaging device (Health Buddy® System) | Providing an interface for patient–healthcare provider communication | Device that attached to land phone line; questions displayed on device screen appliance; responses given by pressing buttons below the screen |
| Head et al., 2009 [94] | USA | RCT | Head and neck cancer patients | 75 | Telemessaging device (Health Buddy® System) | Providing an interface for patient–healthcare provider communication | Health Buddy device communicates intervention algorithms for symptom management. Device plugs into telephone line and electrical outlet for operation |
| Heiney et al., 2012 [95] | USA | Evaluation study nested within an RCT | African American breast cancer patients | 39 | Teleconferencing platform | Delivery of therapeutic group sessions | Teleconference support group intervention for African American women with breast cancer utilised structured sessions delivered over 8 weeks, with additional boosters. The intervention featured story sharing and coping strategies, facilitated by two experienced African American group therapists using process-focused leadership techniques |
| Hochstenbach et al., 2016 [96] | Netherlands | Mixed methods study | Cancer outpatients | 11 | Web- and tablet-based application | Enabling self-management of pain | Mobile app for patients with daily monitoring and graphical feedback and nurses with patient data analysis and decision support |
| Jacobsen et al., 2022 [24] | Germany | Prospective observational study | Patients with aggressive haematologic malignancies | 67 | Upper-arm wearable | Vital sign and physical activity monitoring | Wearable including photoplethysmography, temperature probe, and accelerometery sensors; parameters (heart rate, temperature, respiratory rate, physical activity) calculated using proprietary firmware algorithms |
| Kanera et al., 2016 [97] | Netherlands | PCT process evaluation | Early cancer survivors with various cancer types | 23 | Web-based portal | Coping with psychosocial issues and promoting a healthy lifestyle | Portal providing self-management training modules covering return to work, fatigue, anxiety and depression, social relationship and intimacy issues, physical activity, diet, and smoking cessation, and a general information module on residual symptoms |
| Katz et al., 2016 [98] | USA | Prospective pilot study | Patients after pancreatic surgery for any neoplasm | 15 | Videoconferencing via tablet | Clinical follow-up following surgical procedures | Existing videoconferencing software (Vidyo, Hackensack, NJ, USA) used on Apple iPad tablets provided to patients |
| Kearney et al., 2006 [99] | UK | Prospective feasibility study | Cancer patients receiving chemotherapy | 18 | Handheld-computer software | Facilitating patient monitoring and support | Features provided: assessment of self-reported symptoms, pre-defined symptom-scoring algorithm alerting health professionals to the presence of persistent/urgent symptoms, tailored self-care advice, summaries of symptom history |
| Kelleher et al., 2019 [100] | USA | RCT | Patients with breast, lung, prostate, or colorectal cancer | 89 | Videoconferencing platform, website | Delivery of behaviour training in pain coping skills | Four 45 min videoconferencing sessions with a therapist, a website that provides patients with training materials and information, social networking, and daily assessments used to personalise videoconferencing sessions |
| Kenfield et al., 2019 [101] | USA | RCT | Prostate cancer patients | 76 | Website, wrist-worn wearable (Fitbit), text messages | Facilitate adoption of lifestyle changes | Website included information and recommendations on four topic areas (get active, eat well, stop smoking, and find support); wearable used to track activity levels, text messages used to reinforce adoption, and continued repetition of the recommendations |
| Kim et al., 2018 [102] | South Korea | RCT | Breast cancer patients | 72 | Smartphone-based game | Facilitate patient education | 3-week programme using typical multiplayer, social network, and platform-based features |
| Kim et al., 2016 [103] | USA | Qualitative study nested within an RCT | Cancer patients receiving chemotherapy | 12 | Tablet-based application | Promote active patient engagement and improve care coordination | Personalised social network built around a patient for collaboration with others involved in their care to enable patient-centred health |
| Kokts-Porietis et al., 2019 [25] | Canada | Qualitative study | Breast cancer survivors | 28 | Wrist-worm wearable (Polar A360®) | Physical activity monitoring | Activity tracker with a built-in heart rate sensor |
| Kondylakis et al., 2020 [104] | Italy | Prospective pilot study | Breast and prostate cancer patients | 135 | ICT platform | Aid cancer care and symptom management | Integrated platform containing serious games, psychoemotional monitoring, a personal health system, and a decision support tool |
| Kubo et al., 2019 [105] | USA | RCT | Cancer patients receiving chemotherapy | 82 | Website and smartphone-based application | Mindfulness training programme | Commercially available mindfulness programme (HeadspaceTM) |
| Lamaj et al., 2022 [38] | USA, Spain | Mixed methods observational study | Cancer patients receiving chemotherapy | 154 | Medical device based on optical imaging (PointCheck) | Neutropenia monitoring | Non-invasive device operating through imaging the blood flowing through the capillaries in the finger |
| Lambert et al., 2022 [106] | Canada | RCT | Prostate cancer patients | 49 | Web-based platform | Delivery of a psychosocial and physical activity self-management programme | Platform including five modules: needs assessment, goal setting and action planning, coping planning, sources of support and motivational tools, celebrating successes achieved, and an information library |
| Lee et al., 2018 [107] | South Korea | Retrospective review of prospectively collected data | Breast cancer survivors | 88 | Smartphone-based application, wearable | Self-exercise programme provision | Application providing individual aerobic and resistance training programmes provided by physiatrists; pedometer (InBodyBand) for step count |
| Livingston et al., 2006 [108] | Australia | RCT | Male colorectal and prostate cancer patients | 100 | Telephone service | Provide support and care information | Phone calls by nurse counsellors to patients on a range of cancer information and management issues |
| Livingston et al., 2020 [109] | Australia | RCT | Newly diagnosed cancer patients | 43 | Smartphone/tablet-based application | Provide support and care information | Features include information provision on cancer symptom management and care services, self-report questionnaires, medical appointment diary, and scheduling |
| Loh et al., 2022 [110] | USA | Prospective pilot study | Older patients with myeloid neoplasms | 38 | Website, smartphone-based application, wearable device | Home-based individually tailored exercise programme provision and monitoring of physical activity | Web-based clinician dashboard for patient physical activity monitoring, app for self-reporting of physical activity and provision of exercise prescriptions, wearable activity tracker for step count |
| Lopez et al., 2021 [111] | Canada | Qualitative component of a multi-method study | Cancer survivors | 12 | Videoconferencing platform | Delivery of cancer rehabilitation programmes | Publicly funded virtual care platform developed by the Ontario Telemedicine Network |
| Lozano-Lozano et al., 2019 [112] | Spain | Prospective feasibility study | Breast cancer survivors | 80 | Smartphone-based application | Patient monitoring and provision of feedback on healthy eating and physical activity | Features included self-report questionnaires, notifications on daily energy balance, and recommendations on physical activity and diet |
| Lucas et al., 2018 [113] | USA | Qualitative component of a mixed methods study | Brain and lung cancer patients | 3 | Smartphone-based application, wearable device | Remote monitoring of patients’ health status | App for symptom self-reporting; wearable sensor (Mio Alpha Sports Watch) for heart rate and physical activity monitoring |
| Lyons et al., 2015 [114] | USA | Prospective observational study | Breast cancer survivors | 31 | Telephone service | Delivery of an intervention to optimise functional recovery | Telephone-delivered sessions on exercise, managing stress, and functioning better at work and home |
| Ma et al., 2021 [33] | USA | Prospective observational study | Head and neck cancer patients undergoing radiation therapy | 84 | Automated chatbot | Symptom reporting and self-management education provision | Interactive Web-based communication system |
| MacDonald et al., 2020 [115] | Canada | Prospective pilot study | Cancer survivors | 35 | Smartphone-based application, wearable device, telephone service, Web-based platform | Delivery of a rehabilitation and exercise programme | App (Physitrack®) providing progressive exercise prescription, wearable (Fitbit) for activity tracking, telephone calls for health coaching, online learning platform comprising self-management e-modules |
| Mark et al., 2008 [116] | USA | Survey study | Patients with various cancer types | 100 | Tablet-based platform | Enabling patient symptom screening and reporting | Pen-based e/tablet that operates through a wireless network hosting a platform providing symptom assessment questionnaires |
| Matthew et al., 2007 [117] | Canada | RCT | Prostate cancer patients | 152 | Personal digital assistant | Patient health-related quality-of-life monitoring | PDA using stylus for completion of self-reported questionnaires |
| McCann et al., 2009 [118] | UK | RCT | Breast, lung, and colorectal cancer patients | 56 | Mobile-based platform (ASyMS©) | Symptom monitoring and management | Platform developed for completion of self-reported symptom questionnaire and input of physiological data, including a risk model for alerting health professionals to the presence of persistent/urgent symptoms |
| Meropol et al., 2016 [40] | USA | RCT | Patients with various cancer types | 623 | Web-based application | Education on patient participation in clinical trials | Platform delivering tailored educational content based on assessed patients’ knowledge and attitudinal barriers |
| Milbury et al., 2022 [119] | USA | Pilot RCT | Female non-small cell lung cancer patients | 54 | Videoconferencing platform (Zoom) | Mindfulness training or psychoeducation delivery | Platform accessed through patients’ own devices to attend sessions led by specialists certified in mindfulness-based stress reduction |
| Mirkovic et al., 2014 [47] | Norway | Mixed methods study | Patients with various cancer types | 7 | Smartphone- tablet-based application | Enabling the management of health-related issues | App developed using οpen-source framework for building cross-platform mobile apps (PhoneGap) containing four features; messaging with health professionals, symptom assessment, symptom management information, forum to connect with peers |
| Myall et al., 2015 [120] | UK | Qualitative process evaluation of an exploratory RCT | Patients following primary cancer treatment | 12 | Web-based platform | Self-management of cancer-related fatigue | Platform features included self-reported assessments, educational sessions, and guidance for structured activities |
| Nguyen et al., 2017 [26] | Australia | Qualitative study | Breast cancer survivors | 14 | Wearable activity trackers | Increasing physical activity and reducing sedentary behaviour | Six devices used: Fitbit One, Jawbone Up24, Garmin Vivofit2, Garmin Vivosmart, Garmin Vivoactive, and Polar A300; selected for providing a step count function and an associated app |
| Nguyen et al., 2019 [121] | Netherlands | RCT | Newly diagnosed cancer patients | 232 | Mode-tailored websites | Patient education as preparation before consultation on diagnosis and treatment | Four different versions of website developed containing the same information, presented in different modalities (via text, images, and/or patient videos) |
| Nimako et al., 2013 [39] | UK | Prospective pilot study | Oncology patients receiving chemotherapy | 10 | Telemonitoring system | Blood, body temperature, and symptom monitoring | System consisted of a small point of care haematology analyser, coupled to a telecommunication hub (tele-hub), enabling patients to self-test their own blood count. The tele-hub consists of a touch screen and keypad and acts as the patient interface, communicating results to a server |
| O’Brien et al., 2020 [122] | Australia | Online survey | Female breast cancer patients | 202 | Interaction database (IMgateway) | Patient education on complementary and alternative medicine | Database sets out potential interactions between various complementary and alternative medicines and pharmaceutical drugs |
| Ormel et al., 2018 [123] | Netherlands | Randomised feasibility study | Patients with various cancer types | 16 | Smartphone-based application | Self-monitoring of physical activity | Existing GPS fitness-tracking app for iOS and Android |
| Owens et al., 2020 [124] | USA | Qualitative study | African American lung cancer survivors | 12 | Smartphone-based application | Education on strategies to combat symptoms related to lung cancer | App (Breathe Easier) contains a combination of audio-directed breathing practices, meditations, and yoga exercises demonstrated using instructional text and images of African American and Caucasian adults aged 55 years or older performing various poses |
| Ownsworth et al., 2022 [125] | Australia | Mixed methods pilot study | Brain tumour patients | 8 | Videoconferencing platform | Delivery of psychological support | Platform (Metro South telehealth portal) used by health professionals to provide remote consultations to people in their own homes or local health services |
| Pavic et al., 2020 [126] | Switzerland | Prospective observational study | Palliative cancer patients | 30 | Smartphone-based application, wearable | Monitoring of patients’ vital signs, physical activity, and symptoms | App consisted of a patient interface providing digital questionnaires to rate subjective pain and distress, a sensor logging module for recording and transmitting signals from smartphone sensors, and an interface to record wearable sensor signals and transmit these to a secured server. Wearable is a sensor-equipped upper-arm bracelet (Biovotion) measuring physiological and activity parameters |
| Peipert et al., 2021 [127] | USA | RCT | Breast or colorectal cancer patients | 65 | Interactive platform, touch-screen device | Education provision on cancer care and treatment options | Multimedia touch-screen device for data collection; educational software with the option to select modules of interest |
| Pope et al., 2019 [29] | USA | Prospective pilot study | Breast cancer survivors | 10 | Smartphone-based application, social media platform, wearable accelerometer | Monitoring and promoting physical activity and overall health | Commercially available GPS tracking physical activity app (MapMyFitness), Facebook for intervention delivery, Actigraph GT3X+ accelerometer for physical activity assessment |
| Post et al., 2013 [128] | USA | Pilot RCT | Breast cancer patients undergoing chemotherapy | 27 | Personal digital assistant | Delivery of educational videos on symptom communication | Low-tech, non-interactive device used for the delivery of race-concordant videos on how to communicate about pain, depression, and/or fatigue |
| Price and Brunet, 2021 [129] | Canada | Prospective mixed methods study | Young adult cancer survivors | 7 | Videoconferencing platform | Delivery of a behaviour-change intervention for promoting physical activity and fruit and vegetable consumption | Teleconferencing technology of participants’ choosing (e.g., Skype) |
| Purdy et al., 2022 [130] | Canada | Prospective feasibility study | Multiple myeloma patients | 29 | Online application | Delivery of a home exercise programme | Non-commercial app developed by the University of Alberta (HEAL-Me) providing virtually supervised group workouts, independent home workouts, and independent aerobic exercise |
| Puszkiewicz et al., 2016 [131] | UK | Prospective one-arm, pre–post study | Cancer survivors | 11 | Smartphone-based application | Promoting physical activity | Commercial app (GAINFitness) for iOS operating platform providing a physical activity programme based on users’ goals, fitness levels, and equipment they have access to |
| Reilly et al., 2021 [48] | UK | Qualitative component of a pilot RCT | Cutaneous melanoma patients | 13 | Tablet-based application | Enabling total self-skin examination | App hosted on Android tablets including an individualised digital skin map and the ability to send electronic reports of any skin concerns, including photographs, to a remote Dermatology Nurse Practitioner |
| Rezaee et al., 2022 [132] | Iran | Prospective observational study | Breast cancer survivors | 25 | Smartphone-based application | Providing educational content to improve resilience and quality of life | App developed with the component-based programming approach and user interface developed within the Android Studio environment, including features on the calculation of resilience scores, exercise programmes, patient assessments, and experience sharing among peers |
| Richards et al., 2020 [133] | UK | Mixed methods prospective pilot study | Patients after discharge following cancer-related upper gastrointestinal surgery | 40 | Web-based platform | Enabling remote symptom monitoring | System components include a patient website, a Web-based symptom-report questionnaire software, and a Web application interface for the secure transfer of data to electronic health records. Algorithms are programmed into self-report scoring system, allowing severity-specific tailored self-management advice to be provided to patients |
| Rossi et al., 2018 [27] | USA | Prospective observational study | Endometrial cancer survivors | 30 | Wrist-worn wearable (Fitbit) | Physical activity monitoring | Fitbit Alta™ used for step count |
| Ruland et al., 2013 [134] | Norway | Questionnaire-based survey nested within an RCT | Breast and prostate cancer patients | 103 | Web-based application | Illness management support | App developed at the Oslo University Hospital that includes components on symptom assessment, self-management information and activities, information on condition and treatment options, and communication with peers and health professionals |
| Ruland et al., 2003 [135] | Norway | Pilot RCT | Cancer outpatients | 52 | Tablet-based application | Provision of patient support on shared decision-making in symptom management | App components include a symptom assessment tool, shared decision-making, and a care-planning component highlighting to clinicians symptoms patients are experiencing |
| Russell et al., 2019 [136] | Australia | Pilot RCT | Melanoma patients | 69 | Website | Delivery of mindfulness intervention | Features included short videos and downloadable PDF transcript of the videos, MP3 audio files of guided meditations, general information about meditation |
| Skrabal Ross et al., 2022 [137] | Australia | Proof-of-concept trial | Cancer patients receiving oral chemotherapy | 22 | Online SMS gateway, medication adherence monitoring device (MEMS device) | Supporting adherence to oral chemotherapy | SMS consisted of a one-way (no need to reply) message used to provide reminders to take oral chemotherapy and information about the management of side-effects via hyperlinks to portable document format online documents. MEMS consisted of a pill bottle with a cap that contains a microelectronic chip and tracks the date and time the medication bottle cap is opened and, therefore, the assumed dose taken |
| Smith et al., 2022 [138] | Australia | Qualitative study | Adult cancer patients | 13 | Various telephone and video telehealth platforms | Delivery of clinical consultations | Telephone and videoconferencing used to facilitate real-time communication between health professionals, patients, and caregivers during the COVID-19 pandemic |
| Song et al., 2021 [41] | USA | Pilot feasibility study | Prostate cancer patients | 62 | Web-based programme | Delivery of couple-focused patient education | Programme accessible from patients’ preferred devices (e.g., smartphone, tablet, or computer), including modules about how couples can work effectively as a team, assess and manage prostate cancer treatment-related side-effects and symptoms, and improve healthy behaviours, and a social support feature with post-module assignments, a moderated online forum, meetings with a health educator, and a resource centre |
| Spoelstra et al., 2016 [139] | USA | RCT | Patients with various cancer types | 75 | SMS over networks of mobile operators | Promoting adherence to oral anticancer agent medication | Text messages developed according to Social Cognitive Theory using 160 characters or less, delivered through an automated platform storing associated data |
| Stephen et al., 2014 [140] | Canada | Qualitative study | Cancer patients and survivors | 80 | Online chat platform | Delivery of professionally led cancer support groups | Synchronous text communication in password-protected chat rooms |
| Sundberg et al., 2015 [141] | Sweden | Prospective observational study | Prostate cancer patients receiving radiotherapy | 9 | ICT platform for smartphone use | Aiding the early assessment and management of patient-reported symptoms | Platform components include patient symptom assessments, a risk assessment model based on symptom occurrence and frequency sending alerts to nurses by text message if symptoms are of concern, continuous access to self-care advice related to symptoms and links to relevant websites, and symptom history presented in graphs over time |
| Suzuki et al., 2016 [49] | Japan | Prospective observational study | Cancer patients receiving radiation therapy | 152 | Tablet-based software | Delivery of a psychosocial questionnaire | Electronic touch-screen tablet operated via a stylus used for the completion of patient reported outcomes |
| Valle et al., 2017 [142] | USA | Pilot RCT | African American breast cancer survivors | 35 | Wireless scale, wearable activity tracker, Web-based platform | Promoting weight gain prevention through self-regulation behaviours | Activity tracker (Withings Pulse) interfaced with a Bluetooth and Wifi-enabled wireless scale (Withings WS-30, Cambridge, MA) and synced data to a single online account accessed through a mobile app or website which contained graphs of weight and physical activity trends |
| Van Blarigan et al., 2019 [143] | USA | Pilot RCT | Colorectal cancer survivors | 42 | SMS over networks of mobile operators, wrist-worn wearable (Fitbit) | Promoting increases in physical activity | Daily text messages providing physical activity prompts and recommendations; Fitbit Flex wristband for tracking physical activity, including steps, distance, active minutes, and calories burned |
| Van der Linden et al., 2021 [144] | Netherlands | RCT | Brain tumour patients | 62 | Tablet-based application | Delivery of cognitive rehabilitation programme | App containing educational modules on cognitive functions, influences, compensation, attention, planning and control, and memory. In each module, information about cognitive functions is given and compensatory strategies are provided, together with fill-in exercises to practice the strategies |
| Visser et al., 2018 [50] | Netherlands | RCT | Breast cancer patients | 109 | Tablet-based applications | Delivery of support group sessions, clinical contact, and illness-specific information | iPad containing existing apps connected through a shared iCloud account for each group (iBooks for educational materials, FaceTime for remote group sessions, contact app including the email addresses of participants, clinical nurse specialist, and the researcher. Calendar app containing dates of the scheduled video sessions) |
| Vogel et al., 2019 [145] | USA | Pilot RCT | Ovarian cancer patients | 104 | Smartphone-based application | Provide information on the usefulness of genetic counselling | iOS (Apple) app providing educational materials on genetic counselling and motivational messages, positive feedback, videos, graphics, and triggers to encourage app use |
| Vogel et al., 2013 [51] | USA | RCT | Ovarian cancer patients | 35 | Website | Promoting advance care planning | Prototype website developed using Microsoft. NET framework with Ajax to bring together the HTML and CSS at the front end, and Internet Information Services for Microsoft Windows Servers, an SQL database, and SSL encryption at the back end |
| Walle et al., 2020 [146] | Germany | RCT | Patients with solid tumours undergoing cancer therapy | 66 | Videoconferencing application | Delivery of follow-up clinical consultations | The Minxli—Arzt via Video Chat application for smartphones was utilised, including features for scheduling encrypted video calls with verified physicians and a chat function with options to upload pictures |
| Wallwiener et al., 2017 [147] | Germany | Prospective feasibility study | Advanced breast cancer patients | 15 | Web-based platform | Enabling reporting of patient-reported outcomes | Real-time registry containing molecular data adapted to include patient-reported outcomes |
| Wan et al., 2021 [52] | Singapore | Development and evaluation study | Patients undergoing elective colorectal cancer surgeries | 5 | Smartphone-based application | Improving health outcomes for patients and family caregivers | App on BuddyCare platform encompassing the following features: a surgical timeline (29-day perioperative phase where users receive information packages listing important tasks about how to prepare for surgery, postsurgery monitoring, and discharge care), search functionality, introduction to mindfulness-based practices, daily tasks, alerts, reminders, motivational messages |
| Waterland et al., 2021 [148] | Australia | Impact evaluation study | Patients preparing for major cancer surgery | 31 | Videoconferencing platform | Delivery of prehabilitation education session | Zoom used for webinar delivery |
| Weaver et al., 2007 [149] | UK | Prospective observational study | Colon cancer patients | 6 | Mobile telephone-based software | Symptom monitoring and management | Patients self-reported symptoms using the phone keypad; data were automatically transmitted to a dedicated server. Each patient’s cumulative toxicity chart was displayed both on individualised Web pages (for review by the study nurse) and on the patient’s phone (for information). If incoming readings gave rise to concern, alerts were generated according to criteria stored both on the phone and the server, and appropriate management actions were communicated to patients |
| Wickline et al., 2022 [150] | USA | Mixed methods study | Advanced ovarian cancer patients | 134 | Web-based programme | Enabling symptom monitoring and self-management | System where patients can report and track their symptoms, quality-of-life measures, and decision-making preferences during cancer therapy, delivering self-care instructions targeted to reports of moderate–severe symptoms and providing tips on symptom communication with clinicians |
| Wilkie et al., 2003 [151] | USA | Mixed methods study | Cancer inpatients and outpatients | 116 | Desktop-based software | Enabling pain assessment | Microsoft® Windows 95/98 personal desktop computer with a touch-screen (Elo™ monitor) was used to complete the electronic version of the McGill Pain Questionnaire |
| Wu et al., 2021 [152] | UK | Prospective observational study | Patients awaiting cancer treatment | 139 | Telephone or video calls | Delivery of a prehabilitation education programme | Telephones or videoconferencing platforms used to deliver home-based prehabilitation including personalised training exercises, dietary advice, medical optimisation therapies, and psychological support |
| Yap et al., 2013 [153] | Singapore | Prospective observational study | Ambulatory cancer patients | 60 | SMS over networks of mobile operators | Chemotherapy-induced nausea and vomiting management | Series of questions sent via SMS daily for 5 days post-chemotherapy, following a predeveloped clinical algorithm in consultation with clinical pharmacists; each SMS contained several options in which patients were required to respond by selecting the option number that best reflected their symptoms |
| Zini et al., 2019 [154] | Italy | Prospective pilot study | Head and neck cancer patients undergoing concurrent chemo-radiotherapy | 10 | Smartphone-based application | Reporting of clinical parameters, quality of life, and symptoms | App running on Android designed to collect patients’ symptoms, clinical parameters, and provide educational materials, daily self-management suggestions, therapy-cost recording, and peer networking and facilitate interactions with clinicians |

Table 3.
Cancer patients’ perceptions of digital health technologies.
Table 3.
Cancer patients’ perceptions of digital health technologies.
| System | Information/Content | Service | Other | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ease of Use | Ease of Learning | Ease of Set-Up | System Flexibility | System Reliability | System Security | Performance | Visual Appeal | Usefulness | Relevance | Clarity | Comprehensiveness | Comprehensibility | Reliability | Tailored Information | Information Quantity | Presentation Format | Inclusive Language | Technical Support | Instructions/Training | Feedback/Follow-Up | Comfort of Use | Battery Life/Portability | Coverage/Connectivity | Peer Interaction | Interaction with Clinicians | Time Considerations | Access to Data | |
| Abernethy et al., 2009 [53] | X | X | X | X | ||||||||||||||||||||||||
| Admiraal et al., 2017 [54] | X | X | X | |||||||||||||||||||||||||
| Aiello et al., 2006 [55] | X | X | ||||||||||||||||||||||||||
| Albrecht et al., 2011 [56] | X | X | X | X | X | X | ||||||||||||||||||||||
| Allenby et al., 2002 [34] | X | X | X | X | X | X | ||||||||||||||||||||||
| Allicock et al., 2021 [30] | X | X | X | X | X | X | ||||||||||||||||||||||
| Alshoumr et al., 2021 [57] | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Appleyard et al., 2021 [58] | X | X | X | X | ||||||||||||||||||||||||
| Badr et al., 2016 [59] | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||
| Basch et al., 2007 [60] | X | X | X | |||||||||||||||||||||||||
| Basch et al., 2017 [61] | X | X | X | X | ||||||||||||||||||||||||
| Basch et al., 2020 [62] | X | X | X | X | X | X | ||||||||||||||||||||||
| Beaver et al., 2012 [63] | X | X | ||||||||||||||||||||||||||
| Beaver et al., 2009 [64] | X | X | X | |||||||||||||||||||||||||
| Bender et al., 2022 [65] | X | X | X | X | X | |||||||||||||||||||||||
| Bender et al., 2013 [66] | X | X | X | X | X | |||||||||||||||||||||||
| Bennett et al., 2016 [67] | X | X | X | X | ||||||||||||||||||||||||
| Benze et al., 2019 [68] | X | X | X | |||||||||||||||||||||||||
| Bol et al., 2013 [69] | X | X | X | X | ||||||||||||||||||||||||
| Bolle et al., 2016 [70] | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| Brennan et al., 2022 [71] | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Cadmus-Bertram et al., 2019 [72] | X | X | X | |||||||||||||||||||||||||
| Chaix et al., 2019 [31] | X | X | X | X | X | |||||||||||||||||||||||
| Chee et al., 2017 [73] | X | X | X | |||||||||||||||||||||||||
| Cheville et al., 2018 [74] | X | X | X | |||||||||||||||||||||||||
| Childes et al., 2017 [42] | X | X | X | X | ||||||||||||||||||||||||
| Chow et al., 2021 [28] | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Chow et al., 2019 [75] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Cleeland et al., 2011 [76] | X | X | ||||||||||||||||||||||||||
| Collins et al., 2017 [77] | X | X | X | X | ||||||||||||||||||||||||
| Crafoord et al., 2020 [78] | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Crawford et al., 2019 [79] | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| Denis et al., 2014 [80] | X | X | ||||||||||||||||||||||||||
| Duffecy et al., 2013 [81] | X | X | ||||||||||||||||||||||||||
| Eakin et al., 2012 [82] | X | X | ||||||||||||||||||||||||||
| Ferguson et al., 2016 [83] | X | X | ||||||||||||||||||||||||||
| Finlay et al., 2020 [46] | X | X | X | |||||||||||||||||||||||||
| Foley et al., 2016 [84] | X | X | ||||||||||||||||||||||||||
| Fu et al., 2016 [85] | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Galiano-Castillo et al., 2016 [86] | X | X | X | X | X | |||||||||||||||||||||||
| Galsky et al., 2017 [87] | X | X | ||||||||||||||||||||||||||
| Gell et al., 2017 [88] | X | X | X | |||||||||||||||||||||||||
| Gilbertson-White et al., 2019 [89] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Girgis et al., 2017 [35] | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||
| Graetz et al., 2018 [90] | X | X | X | |||||||||||||||||||||||||
| Greenway et al., 2022 [36] | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Greer et al., 2019 [32] | X | X | ||||||||||||||||||||||||||
| Groarke et al., 2021 [91] | X | X | X | |||||||||||||||||||||||||
| Gustavell et al., 2020 [92] | X | X | X | X | X | |||||||||||||||||||||||
| Harless et al., 2009 [37] | X | X | X | X | ||||||||||||||||||||||||
| Head et al., 2011 [93] | X | X | X | X | X | |||||||||||||||||||||||
| Head et al., 2009 [94] | X | X | X | X | ||||||||||||||||||||||||
| Heiney et al., 2012 [95] | X | X | ||||||||||||||||||||||||||
| Hochstenbach et al., 2016 [96] | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Jacobsen et al., 2022 [24] | X | X | ||||||||||||||||||||||||||
| Kanera et al., 2016 [97] | X | X | X | |||||||||||||||||||||||||
| Katz et al., 2016 [98] | X | X | X | |||||||||||||||||||||||||
| Kearney et al., 2006 [99] | X | X | X | X | ||||||||||||||||||||||||
| Kelleher et al., 2019 [100] | X | |||||||||||||||||||||||||||
| Kenfield et al., 2019 [101] | X | X | X | X | X | |||||||||||||||||||||||
| Kim et al., 2018 [102] | X | X | X | |||||||||||||||||||||||||
| Kim et al., 2016 [103] | X | X | X | X | ||||||||||||||||||||||||
| Kokts-Porietis et al., 2019 [25] | X | X | X | |||||||||||||||||||||||||
| Kondylakis et al., 2020 [104] | X | X | ||||||||||||||||||||||||||
| Kubo et al., 2019 [105] | X | X | X | X | X | |||||||||||||||||||||||
| Lamaj et al., 2022 [38] | X | X | X | |||||||||||||||||||||||||
| Lambert et al., 2022 [106] | X | X | X | X | X | |||||||||||||||||||||||
| Lee et al., 2018 [107] | X | X | X | X | ||||||||||||||||||||||||
| Livingston et al., 2006 [108] | X | X | X | X | ||||||||||||||||||||||||
| Livingston et al., 2020 [109] | X | X | X | X | X | |||||||||||||||||||||||
| Loh et al., 2022 [110] | X | X | X | X | ||||||||||||||||||||||||
| Lopez et al., 2021 [111] | X | X | X | |||||||||||||||||||||||||
| Lozano-Lozano et al., 2019 [112] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Lucas et al., 2018 [113] | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| Lyons et al., 2015 [114] | X | X | X | |||||||||||||||||||||||||
| Ma et al., 2021 [33] | X | X | X | |||||||||||||||||||||||||
| MacDonald et al., 2020 [115] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Mark et al., 2008 [116] | X | X | X | |||||||||||||||||||||||||
| Matthew et al., 2007 [117] | X | X | X | |||||||||||||||||||||||||
| McCann et al., 2009 [118] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Meropol et al., 2016 [40] | X | X | X | X | ||||||||||||||||||||||||
| Milbury et al., 2022 [119] | X | X | X | X | X | X | ||||||||||||||||||||||
| Mirkovic et al., 2014 [47] | X | X | X | X | X | X | ||||||||||||||||||||||
| Myall et al., 2015 [120] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Nguyen et al., 2017 [26] | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||
| Nguyen et al., 2019 [121] | X | X | X | |||||||||||||||||||||||||
| Nimako et al., 2013 [39] | X | X | X | X | X | X | ||||||||||||||||||||||
| O’Brien et al., 2020 [122] | X | X | X | X | X | |||||||||||||||||||||||
| Ormel et al., 2018 [123] | X | X | ||||||||||||||||||||||||||
| Owens et al., 2020 [124] | X | X | X | |||||||||||||||||||||||||
| Ownsworth et al., 2022 [125] | X | X | X | X | X | |||||||||||||||||||||||
| Pavic et al., 2020 [126] | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| Peipert et al., 2021 [127] | X | X | ||||||||||||||||||||||||||
| Pope et al., 2019 [29] | X | X | X | |||||||||||||||||||||||||
| Post et al., 2013 [128] | X | X | X | |||||||||||||||||||||||||
| Price and Brunet, 2021 [129] | X | X | X | |||||||||||||||||||||||||
| Purdy et al., 2022 [130] | X | X | X | X | ||||||||||||||||||||||||
| Puszkiewicz et al., 2016 [131] | X | X | X | X | ||||||||||||||||||||||||
| Reilly et al., 2021 [48] | X | X | X | |||||||||||||||||||||||||
| Rezaee et al., 2022 [132] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Richards et al., 2020 [133] | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Rossi et al., 2018 [27] | X | X | X | X | ||||||||||||||||||||||||
| Ruland et al., 2013 [134] | X | X | X | |||||||||||||||||||||||||
| Ruland et al., 2003 [135] | X | X | X | X | X | |||||||||||||||||||||||
| Russell et al., 2019 [136] | X | X | X | X | X | |||||||||||||||||||||||
| Skrabal Ross et al., 2022 [137] | X | X | X | |||||||||||||||||||||||||
| Smith et al., 2022 [138] | X | X | X | X | X | |||||||||||||||||||||||
| Song et al., 2021 [41] | X | X | ||||||||||||||||||||||||||
| Spoelstra et al., 2016 [139] | X | |||||||||||||||||||||||||||
| Stephen et al., 2014 [140] | X | X | X | X | ||||||||||||||||||||||||
| Sundberg et al., 2015 [141] | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Suzuki et al., 2016 [49] | X | X | X | |||||||||||||||||||||||||
| Valle et al., 2017 [142] | X | X | X | |||||||||||||||||||||||||
| Van Blarigan et al., 2019 [143] | X | X | ||||||||||||||||||||||||||
| van der Linden et al., 2021 [144] | X | X | X | X | ||||||||||||||||||||||||
| Visser et al., 2018 [50] | X | X | X | |||||||||||||||||||||||||
| Vogel et al., 2019 [145] | X | X | X | X | X | |||||||||||||||||||||||
| Vogel et al., 2013 [51] | X | X | X | X | X | |||||||||||||||||||||||
| Walle et al., 2020 [146] | X | X | X | X | ||||||||||||||||||||||||
| Wallwiener et al., 2017 [147] | X | X | X | |||||||||||||||||||||||||
| Wan et al., 2021 [52] | X | X | X | X | X | |||||||||||||||||||||||
| Waterland et al., 2021 [148] | X | X | X | |||||||||||||||||||||||||
| Weaver et al., 2007 [149] | X | X | X | |||||||||||||||||||||||||
| Wickline et al., 2022 [150] | X | X | X | X | X | |||||||||||||||||||||||
| Wilkie et al., 2003 [151] | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Wu et al., 2021 [152] | X | X | X | |||||||||||||||||||||||||
| Yap et al., 2013 [153] | X | X | X | X | X | X | ||||||||||||||||||||||
| Zini et al., 2019 [154] | X | X | X | X | X | |||||||||||||||||||||||
4. Discussion
4.1. Main Findings
This scoping review aimed to identify and synthesise existing evidence on cancer patients’ perspectives and requirements for patient-facing digital health technologies. We found 128 studies published between 2002 and 2022 reporting on users’ perceptions and evaluations of patient-centred digital technologies intended to be used as part of cancer patients’ care. A significant surge in publications was observed from 2013 onwards (n = 110, 85.9%), suggesting a growing interest and investment in leveraging digital solutions to address cancer care needs. Identified technologies predominantly comprised Web-based software or platforms (n = 53), mobile or smartphone devices and/or applications (n = 33), remote sensing and wearable technologies (n = 17) used either in combination with other technologies (n = 13) or as standalone digital applications (n = 4), and telephone-based services and tools (n = 13). This diverse range of technologies mirrors the broader technological advancements seen in healthcare, where the emphasis on user-centred design and accessibility has propelled the development and deployment of a wide array of digital tools tailored to specific patient populations [155]. The perceived usefulness of technologies for the management of patients’ symptoms and overall conditions (n = 86), their user-friendliness in terms of ease of use (n = 70), their ability to facilitate patient–clinician interactions (n = 50), and the time investment required to operate the technologies (n = 33) were the most frequently reported technological requirements considered by cancer patients.
The delivery of interventions mostly aiming to educate patients on the self-management of their care (n = 77) and the systematic reporting and/or monitoring of patients’ symptoms and physiological parameters (n = 39) constituted the principal purposes of technology employment among the included studies. Similar findings were reported in previously published reviews investigating the applications of digital technologies in patients living with chronic conditions, such as dementia [156] and serious mental illness [157]. This suggests that people with complex and ongoing healthcare needs may have broadly similar requirements and needs for the digital technologies employed to aid their care regardless of their individual diagnoses.
Limited digital literacy is a common barrier to digital technology adoption among clinical populations [7,15]. Patients need to have the necessary skills to engage with digital tools independently and meaningfully. These skills comprise a patient’s digital literacy [11]. The user-friendliness of technologies (n = 70), receiving appropriate instructions and/or training in setting up and engaging with technologies, and technical support being readily available for the duration of interactions with technologies (n = 9) were identified as key requirements by patients participating in the studies included in this review. These requirements together with end-users’ digital literacy levels ought to be considered by digital technology companies when designing and developing tools for health purposes so that these can accommodate patients with varying digital literacy levels and ensure that all patients can benefit from digital tools [11].
Patients in a large proportion of studies (n = 50) considered the ability of digital technologies to facilitate patient–clinician communication and interaction an important feature of these tools. Previous research has found that the use of digital health interventions can positively influence patient–clinician communication and relationships by enabling patients to feel more comfortable about disclosing information to their clinicians and by reducing the imbalance of power between the patient and clinician [158,159]. For this to be achieved, it is critical that clinicians work collaboratively with patients to establish mutually agreed goals, boundaries, and expectations for technology engagement [160]. Failing to set and adhere to these may lead to the overburdening of clinical staff, patients experiencing disappointment and frustration, and, ultimately, challenged patient–clinician relationships [161,162]. Given than the ethical implications of digital health communication are complex in terms of the boundaries of the patient–clinician relationship and the professional duty of care, there is a need for guidelines on good practice in the use of technology-enabled communication to be established both at the institutional and national levels [158].
In line with previous research, technological features that enable the personalisation of information and care were highly endorsed by cancer patients participating in studies included in the present review [15,163]. In the context of digital technology, personalisation refers to the process of tailoring the user experience of a digital tool to an individual’s specific needs, preferences, behaviours, and characteristics [15]. In this review, the extent to which the content presented through technologies was tailored (n = 18) and relevant (n = 23) to the patients’ individual situations and was perceived as useful for the management of their own care (n = 86) were identified as desirable technological characteristics. The personalisation of information and content received through digital health technologies has been shown to empower patients to have greater ownership over the management of their health and to contribute towards shifting the power balance from health providers making care decisions on behalf of patients to a shared decision-making process [15,164]. It is thus recommended that a personalised approach is undertaken in the development and operation of digital health technologies as it could be pivotal in increasing patient engagement with their care [15].
Almost all studies included in this review (n = 120) reported predominantly positive appraisals of patient-facing digital technologies in terms of acceptability, feasibility, and/or utility. Since studies were not evaluated for their methodological quality, as this was outside the scope of the present review, any reported findings by individual studies should be interpreted with caution. Nevertheless, these findings echo those of other research, suggesting that cancer patients may be overall amenable to the incorporation of digital health technologies into their care [165,166]. Therefore, future research should direct its efforts into exploring strategies for the successful implementation of digital health technologies into routine cancer care practice, including the identification of both individual- and institutional-level barriers and facilitators to the sustainable long-term use of such technologies in this context. The findings from the present review could contribute to this direction by providing evidence to guide investigations into the technology-associated parameters influencing patients’ decisions regarding the adoption of such technologies.
4.2. Strengths and Limitations
A comprehensive approach to locate relevant articles was undertaken in this review, using three databases and a combination of subject headings and free-text terms without applying any date restrictions and performing a rigorous forward and backward citation search for all records meeting the inclusion criteria. Nevertheless, there may have been publications which were missed. The identification of 21 additional eligible publications through citation searching may be suggestive of this. Moreover, the exclusion of grey literature and non-English-language publications may mean that studies providing evidence on cancer patients’ perspectives and requirements for patient-facing digital technologies published outside the traditional academic channels or in languages other than English were also missed. Despite these limitations, a large number of eligible studies were identified and included in this review (n = 128), enabling a rich synthesis of evidence.
In line with the broad scope of this review, studies reporting on cancer patients’ perceptions of digital technologies employed for their care were considered eligible for inclusion regardless of their research design or the methods employed for the assessment of patients’ perceptions. However, more than half of the included studies (n = 68) used either standardised or ad hoc questionnaires as their primary means of evaluating patients’ perceptions of digital health technologies. This means that the technological parameters considered by patients were limited to those included in the measures used by individual studies. It is possible, therefore, that the patient requirements presented in this review do not adequately reflect all technological parameters considered important by cancer patients, and that potentially relevant technological requirements were missed. Furthermore, although no restrictions on cancer type were applied in our search, a large number of the included studies (n = 75) were conducted with breast, prostate, lung, colorectal, and head and neck cancer patients and survivors. Hence, the reported findings may not capture the views of or be applicable to cancer patient and survivor populations outside of those represented in the present review.
5. Conclusions
To the authors’ knowledge, the present scoping review is the first to systematically map and synthesise the existing evidence on cancer patients’ perspectives and requirements for patient-facing digital technologies. An increasing number of studies are using digital technologies to support cancer care. These predominantly comprise Web-based software/platforms, mobile or smartphone devices and/or applications, and remote sensing and wearable technologies, mostly employed for the delivery of self-management interventions and the monitoring of patients’ symptoms and physiological parameters. Several technological features and parameters are considered important by cancer patients. Of these, the most commonly reported include the utility of technologies in enabling the management of patients’ symptoms and overall conditions, their user-friendliness, and their ability to facilitate patient–clinician interactions. The findings from this review provide evidence that could inform future research on technology-associated parameters influencing cancer patients’ decisions regarding the uptake and adoption of patient-facing digital health technologies.
Author Contributions
I.L.—Conceptualisation, Evidence Identification and Extraction, Analyses, Writing—Original Draft and Redrafting. A.-M.K.—Conceptualisation, Evidence Identification and Extraction, Analyses, Writing—Original Draft and Redrafting. S.N.—Supervision, Resources, Writing—Review and Editing. L.A.—Resources, Writing—Review and Editing. N.S.—Resources, Writing—Review and Editing. S.P.—Resources, Writing—Review and Editing. I.K.—Resources, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the RELEVIUM project, which received funding from H2020 under grant agreement No. 101057821 (https://www.releviumproject.eu/ accessed on 18 June 2024).
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Leach, C.R.; Weaver, K.E.; Aziz, N.M.; Alfano, C.M.; Bellizzi, K.M.; Kent, E.E.; Forsythe, L.P.; Rowland, J.H. The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. J. Cancer Surviv. Res. Pract. 2015, 9, 239–251. [Google Scholar] [CrossRef]
- Prager, G.W.; Braga, S.; Bystricky, B.; Qvortrup, C.; Criscitiello, C.; Esin, E.; Sonke, G.S.; Martínez, G.A.; Frenel, J.S.; Karamouzis, M.; et al. Global cancer control: Responding to the growing burden, rising costs and inequalities in access. ESMO Open 2018, 3, e000285. [Google Scholar] [CrossRef]
- Shaffer, K.M.; Turner, K.L.; Siwik, C.; Gonzalez, B.D.; Upasani, R.; Glazer, J.V.; Ferguson, R.J.; Joshua, C.; Low, C.A. Digital health and telehealth in cancer care: A scoping review of reviews. Lancet Digit. Health 2023, 5, e316–e327. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration (FDA). What is Digital Health? 2020. Available online: https://www.fda.gov/medical-devices/digital-health-center-excellence/what-digital-health (accessed on 12 July 2023).
- National Institute for Health and Care Excellence (NICE). Evidence Standards Framework For Digital Health Technologies. 2018. Available online: https://www.nice.org.uk/corporate/ecd7 (accessed on 12 July 2023).
- Kemp, E.; Trigg, J.; Beatty, L.; Christensen, C.; Dhillon, H.M.; Maeder, A.; Williams, P.A.H.; Koczwara, B. Health literacy, digital health literacy and the implementation of digital health technologies in cancer care: The need for a strategic approach. Health Promot. J. Aust. 2021, 32, 104–114. [Google Scholar] [CrossRef]
- Pan, L.; Wu, X.; Lu, Y.; Zhang, H.; Zhou, Y.; Liu, X.; Liu, S.; Yan, Q. Artificial intelligence empowered digital health technologies in cancer survivorship care: A scoping review. Asia Pac. J. Oncol. Nurs. 2022, 9, 100127. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Strategy On Digital Health 2020–2025. 2021. Available online: https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf (accessed on 12 July 2023).
- Parikh, R.B.; Basen-Enquist, K.M.; Bradley, C.; Estrin, D.; Levy, M.; Lichtenfeld, J.L.; Malin, B.; McGraw, D.; Meropol, N.J.; Oyer, R.A.; et al. Digital Health Applications in Oncology: An Opportunity to Seize. J. Natl. Cancer Inst. 2022, 114, 1338–1339. [Google Scholar] [CrossRef]
- Arora, S.; Ryals, C.; Rodriguez, J.A.; Byers, E.; Clewett, E. Leveraging Digital Technology to Reduce Cancer Care Inequities. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 559–566. [Google Scholar] [CrossRef]
- Morris, B.B.; Rossi, B.; Fuemmeler, B. The role of digital health technology in rural cancer care delivery: A systematic review. J. Rural. Health 2022, 38, 493–511. [Google Scholar] [CrossRef]
- Osborn, J.; Ajakaiye, A.; Cooksley, T.; Subbe, C.P. Do mHealth applications improve clinical outcomes of patients with cancer? A critical appraisal of the peer-reviewed literature. Support. Care Cancer 2020, 28, 1469–1479. [Google Scholar] [CrossRef]
- Jimenez, G.; Spinazze, P.; Matchar, D.; Koh Choon Huat, G.; van der Kleij, R.M.J.J.; Chavannes, N.H.; Car, J. Digital health competencies for primary healthcare professionals: A scoping review. Int. J. Med. Inform. 2020, 143, 104260. [Google Scholar] [CrossRef] [PubMed]
- Madanian, S.; Nakarada-Kordic, I.; Reay, S.; Chetty, T.h. Patients’ perspectives on digital health tools. PEC Innov. 2023, 2, 100171. [Google Scholar] [CrossRef] [PubMed]
- Helleman, J.; Johnson, B.; Holdom, C.; Hobson, E.; Murray, D.; Steyn, F.J.; Ngo, S.T.; Henders, A.; Lokeshappa, M.B.; Visser-Meily, J.M.A.; et al. Patient perspectives on digital healthcare technology in care and clinical trials for motor neuron disease: An international survey. J. Neurol. 2022, 269, 6003–6013. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, I.; Stavropoulos, T.G.; Mpaltadoros, L.; Nikolopoulos, S.; Koumanakos, G.; Tsolaki, M.; Kompatsiaris, I.Y. Human Factors and Requirements of People with Cognitive Impairment, Their Caregivers, and Healthcare Professionals for mHealth Apps Including Reminders, Games, and Geolocation Tracking: A Survey-Questionnaire Study. J. Alzheimer’s Dis. Rep. 2021, 5, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Siglen, E.; Vetti, H.H.; Lunde, A.B.F.; Hatlebrekke, T.A.; Strømsvik, N.; Hamang, A.; Hovland, S.T.; Rettberg, J.W.; Steen, V.M.; Bjorvatn, C. Ask Rosa–The making of a digital genetic conversation tool, a chatbot, about hereditary breast and ovarian cancer. Patient Educ. Couns. 2022, 105, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, T.G.; Lazarou, I.; Diaz, A.; Gove, D.; Georges, J.; Manyakov, N.V.; Pich, E.M.; Hinds, C.; Tsolaki, M.; Nikolopoulos, S.; et al. Wearable Devices for Assessing Function in Alzheimer’s Disease: A European Public Involvement Activity About the Features and Preferences of Patients and Caregivers. Front. Aging Neurosci. 2021, 13, 643135. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, K.; Reuzel, R.; Rovers, M.; Tummers, M. An Overview of Stakeholders, Methods, Topics, and Challenges in Participatory Approaches Used in the Development of Medical Devices: A Scoping Review. Int. J. Health Policy Manag. 2023, 12, 6839. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, A.M.; Kadhim, H.; Barnes, S.; Zebrowski, S.E.; Simmonds, A.; Masand, S.N.; Banner, J.; Dupont, M. Accelerating Adoption of Patient-Facing Technologies in Clinical Trials: A Pharmaceutical Industry Perspective on Opportunities and Challenges. Ther. Innov. Regul. Sci. 2019, 53, 8–24. [Google Scholar] [CrossRef]
- Yusof, M.M.; Kuljis, J.; Papazafeiropoulou, A.; Stergioulas, L.K. An evaluation framework for Health Information Systems: Human, organization and technology-fit factors (HOT-fit). Int. J. Med. Inform. 2008, 77, 386–398. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Jacobsen, M.; Rottmann, P.; Dembek, T.A.; Gerke, A.L.; Gholamipoor, R.; Blum, C.; Hartmann, N.U.; Verket, M.; Kaivers, J.; Jäger, P.; et al. Feasibility of Wearable-Based Remote Monitoring in Patients During Intensive Treatment for Aggressive Hematologic Malignancies. JCO Clin. Cancer Inform. 2022, 6, e2100126. [Google Scholar] [CrossRef] [PubMed]
- Kokts-Porietis, R.L.; Stone, C.R.; Friedenreich, C.M.; Froese, A.; McDonough, M.; McNeil, J. Breast cancer survivors’ perspectives on a home-based physical activity intervention utilizing wearable technology. Support. Care Cancer 2019, 27, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Hadgraft, N.T.; Moore, M.M.; Rosenberg, D.E.; Lynch, C.; Reeves, M.M.; Lynch, B.M. A qualitative evaluation of breast cancer survivors’ acceptance of and preferences for consumer wearable technology activity trackers. Support. Care Cancer 2017, 25, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Frechette, L.; Miller, D.; Miller, E.; Friel, C.; Van Arsdale, A.; Lin, J.; Shankar, V.; Kuo, D.Y.S.; Nevadunsky, N.S. Acceptability and feasibility of a Fitbit physical activity monitor for endometrial cancer survivors. Gynecol. Oncol. 2018, 149, 470–475. [Google Scholar] [CrossRef]
- Chow, E.J.; Doody, D.R.; Di, C.; Armenian, S.H.; Baker, K.S.; Bricker, J.B.; Gopal, A.K.; Hagen, A.M.; Ketterl, T.G.; Lee, S.J.; et al. Feasibility of a behavioral intervention using mobile health applications to reduce cardiovascular risk factors in cancer survivors: A pilot randomized controlled trial. J. Cancer Surviv. Res. Pract. 2021, 15, 554–563. [Google Scholar] [CrossRef]
- Pope, Z.; Lee, J.E.; Zeng, N.; Lee, H.Y.; Gao, Z. Feasibility of smartphone application and social media intervention on breast cancer survivors’ health outcomes. Transl. Behav. Med. 2019, 9, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Allicock, M.; Kendzor, D.; Sedory, A.; Gabriel, K.P.; Swartz, M.D.; Thomas, P.; Yudkin, J.S.; Rivers, A. A Pilot and Feasibility Mobile Health Intervention to Support Healthy Behaviors in African American Breast Cancer Survivors. J. Racial Ethn. Health Disparities 2021, 8, 157–165. [Google Scholar] [CrossRef]
- Chaix, B.; Bibault, J.E.; Pienkowski, A.; Delamon, G.; Guillemassé, A.; Nectoux, P.; Brouard, B. When Chatbots Meet Patients: One-Year Prospective Study of Conversations Between Patients With Breast Cancer and a Chatbot. JMIR Cancer 2019, 5, e12856. [Google Scholar] [CrossRef]
- Greer, S.; Ramo, D.; Chang, Y.J.; Fu, M.; Moskowitz, J.; Haritatos, J. Use of the Chatbot “Vivibot” to Deliver Positive Psychology Skills and Promote Well-Being Among Young People After Cancer Treatment: Randomized Controlled Feasibility Trial. JMIR Mhealth Uhealth 2019, 7, e15018. [Google Scholar] [CrossRef]
- Ma, D.; Orner, D.; Ghaly, M.M.; Parashar, B.; Ames, J.W.; Chen, W.C.; Potters, L.; Teckie, S. Automated health chats for symptom management of head and neck cancer patients undergoing radiation therapy. Oral. Oncol. 2021, 122, 105551. [Google Scholar] [CrossRef]
- Allenby, A.; Matthews, J.; Beresford, J.; McLachlan, S.A. The application of computer touch-screen technology in screening for psychosocial distress in an ambulatory oncology setting. Eur. J. Cancer Care 2002, 11, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Girgis, A.; Durcinoska, I.; Levesque, J.V.; Gerges, M.; Sandell, T.; Arnold, A.; Delaney, G.P. eHealth System for Collecting and Utilizing Patient Reported Outcome Measures for Personalized Treatment and Care (PROMPT-Care) Among Cancer Patients: Mixed Methods Approach to Evaluate Feasibility and Acceptability. J. Med. Internet Res. 2017, 19, e330. [Google Scholar] [CrossRef] [PubMed]
- Greenway, K.; Frisone, C.; Placidi, A.; Sanjay, K.; Guest, W.; Winter, S.C.; Shah, K.; Henshall, C. Using immersive technology and architectural design to assist head and neck cancer patients’ recovery from treatment: A focus group and technology acceptance study. Eur. J. Oncol. Nurs. 2022, 62, 102261. [Google Scholar] [CrossRef] [PubMed]
- Harless, W.G.; Zier, M.A.; Harless, M.G.; Duncan, R.C.; Braun, M.A.; Willey, S.; Isaacs, C.; Warren, R.D. Evaluation of a virtual dialogue method for breast cancer patient education. Patient Educ. Couns. 2009, 76, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lamaj, G.; Pablo-Trinidad, A.; Butterworth, I.; Bell, N.; Benasutti, R.; Bourquard, A.; Sanchez-Ferro, A.; Castro-Gonzalez, C.; Jiménez-Ubieto, A.; Baumann, T.; et al. Usability Evaluation of a Noninvasive Neutropenia Screening Device (PointCheck) for Patients Undergoing Cancer Chemotherapy: Mixed Methods Observational Study. J. Med. Internet Res. 2022, 24, e37368. [Google Scholar] [CrossRef] [PubMed]
- Nimako, K.; Lu, S.K.; Ayite, B.; Priest, K.; Winkley, A.; Gunapala, R.; Popat, S.; O’Brien, M.E. A pilot study of a novel home telemonitoring system for oncology patients receiving chemotherapy. J. Telemed. Telecare 2013, 19, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Meropol, N.J.; Wong, Y.N.; Albrecht, T.; Manne, S.; Miller, S.M.; Flamm, A.L.; Benson, A.B., 3rd; Buzaglo, J.; Collins, M.; Egleston, B.; et al. Randomized Trial of a Web-Based Intervention to Address Barriers to Clinical Trials. J. Clin. Oncol. 2016, 34, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Guo, P.; Tan, X.; Chen, R.C.; Nielsen, M.E.; Birken, S.A.; Koontz, B.F.; Northouse, L.L.; Mayer, D.K. Enhancing survivorship care planning for patients with localized prostate cancer using a couple-focused web-based, mHealth program: The results of a pilot feasibility study. J. Cancer Surviv. Res. Pract. 2021, 15, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Childes, J.M.; Palmer, A.D.; Fried-Oken, M.; Graville, D.J. The Use of Technology for Phone and Face-to-Face Communication After Total Laryngectomy. Am. J. Speech-Lang. Pathol. 2017, 26, 99–112. [Google Scholar] [CrossRef]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An Empirical Evaluation of the System Usability Scale. Int. J. Hum. Comput. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile app rating scale: A new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth 2015, 3, e27. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.D. Perceived Usefulness, Perceived Ease of Use, and User Acceptance of Information Technology. MIS Q. 1989, 13, 319–340. [Google Scholar] [CrossRef]
- Finlay, A.; Evans, H.; Vincent, A.; Wittert, G.; Vandelanotte, C.; Short, C.E. Optimising Web-Based Computer-Tailored Physical Activity Interventions for Prostate Cancer Survivors: A Randomised Controlled Trial Examining the Impact of Website Architecture on User Engagement. Int. J. Environ. Res. Public. Health 2020, 17, 7920. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, J.; Kaufman, D.R.; Ruland, C.M. Supporting cancer patients in illness management: Usability evaluation of a mobile app. JMIR Mhealth Uhealth 2014, 2, e33. [Google Scholar] [CrossRef]
- Reilly, F.; Contstable, L.; Brant, W.; Rahman, K.; Durrani, A.; Burrows, N.; Proby, C.; Allan, J.; Johnston, M.; Johnston, D.; et al. Achieving integrated self-directed Cancer aftercare (ASICA) for melanoma: How a digital intervention to support total skin self-examination was used by people treated for cutaneous melanoma. BMC Cancer 2021, 21, 1217. [Google Scholar] [CrossRef]
- Suzuki, E.; Mackenzie, L.; Sanson-Fisher, R.; Carey, M.; D’Este, C.; Asada, H.; Toi, M. Acceptability of a Touch Screen Tablet Psychosocial Survey Administered to Radiation Therapy Patients in Japan. Int. J. Behav. Med. 2016, 23, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.; Prins, J.B.; Jansen, L.; Radema, S.A.; Schlooz, M.S.; van Dalen, T.; van Laarhoven, H.W.M. Group medical consultations (GMCs) and tablet-based online support group sessions in the follow-up of breast cancer: A multicenter randomized controlled trial. Breast 2018, 40, 181–188. [Google Scholar] [CrossRef]
- Vogel, R.I.; Petzel, S.V.; Cragg, J.; McClellan, M.; Chan, D.; Dickson, E.; Jacko, J.A.; Sainfort, F.; Geller, M.A. Development and pilot of an advance care planning website for women with ovarian cancer: A randomized controlled trial. Gynecol. Oncol. 2013, 131, 430–436. [Google Scholar] [CrossRef]
- Wan, S.W.; Chong, C.S.; Toh, E.L.; Lim, S.H.; Loi, C.T.; Lew, Y.F.H.; Chua, M.C.H.; Jee, X.P.; Liu, G.; Zhu, L.; et al. A Theory-Based, Multidisciplinary Approach to Cocreate a Patient-Centric Digital Solution to Enhance Perioperative Health Outcomes Among Colorectal Cancer Patients and Their Family Caregivers: Development and Evaluation Study. J. Med. Internet Res. 2021, 23, e31917. [Google Scholar] [CrossRef]
- Abernethy, A.P.; Herndon, J.E.; Wheeler, J.L.; Day, J.M.; Hood, L.; Patwardhan, M.; Shaw, H.; Lyerly, H.K. Feasibility and Acceptability to Patients of a Longitudinal System for Evaluating Cancer-Related Symptoms and Quality of Life: Pilot Study of an e/Tablet Data-Collection System in Academic Oncology. J. Pain. Symptom Manag. 2009, 37, 1027–1038. [Google Scholar] [CrossRef]
- Admiraal, J.M.; van der Velden, A.W.G.; Geerling, J.I.; Burgerhof, J.G.M.; Bouma, G.; Walenkamp, A.M.E.; de Vries, E.G.E.; Schröder, C.P.; Reyners, A.K.L. Web-Based Tailored Psychoeducation for Breast Cancer Patients at the Onset of the Survivorship Phase: A Multicenter Randomized Controlled Trial. J. Pain. Symptom Manag. 2017, 54, 466–475. [Google Scholar] [CrossRef]
- Aiello, E.J.; Taplin, S.; Reid, R.; Hobbs, M.; Seger, D.; Kamel, H.; Tufano, J.; Ballard-Barbash, R. In a randomized controlled trial, patients preferred electronic data collection of breast cancer risk-factor information in a mammography setting. J. Clin. Epidemiol. 2006, 59, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.; Simon, D.; Buchholz, A.; Reuter, K.; Frosch, D.; Seebauer, L.; Härter, M. How does a German audience appraise an American decision aid on early stage breast cancer? Patient Educ. Couns. 2011, 83, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Alshoumr, B.; Yu, P.; Hailey, D.; Bindayel, F.; Alanazi, S.; Alshammary, S. Understanding cancer patients’ use and perceptions of inpatient portal: A case study at a tertiary hospital in Saudi Arabia. Int. J. Med. Inform. 2021, 148, 104398. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, S.E.; Larkin, M.J.W.; Stewart, E.M.; Minton, O.; Gilbert, D.C. Digital Medicine in Men with Advanced Prostate Cancer–A Feasibility Study of Electronic Patient-reported Outcomes in Patients on Systemic Treatment. Clin. Oncol. 2021, 33, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Badr, H.; Lipnick, D.; Diefenbach, M.A.; Posner, M.; Kotz, T.; Miles, B.; Genden, E. Development and usability testing of a web-based self-management intervention for oral cancer survivors and their family caregivers. Eur. J. Cancer Care 2016, 25, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Artz, D.; Iasonos, A.; Speakman, J.; Shannon, K.; Lin, K.; Pun, C.; Yong, H.; Fearn, P.; Barz, A.; et al. Evaluation of an Online Platform for Cancer Patient Self-reporting of Chemotherapy Toxicities. J. Am. Med. Inform. Assoc. 2007, 14, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Pugh, S.L.; Dueck, A.C.; Mitchell, S.A.; Berk, L.; Fogh, S.; Rogak, L.J.; Gatewood, M.; Reeve, B.B.; Mendoza, T.R.; et al. Feasibility of Patient Reporting of Symptomatic Adverse Events via the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) in a Chemoradiotherapy Cooperative Group Multicenter Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 409–418. [Google Scholar] [CrossRef]
- Basch, E.; Stover, A.M.; Schrag, D.; Chung, A.; Jansen, J.; Henson, S.; Carr, P.; Ginos, B.; Deal, A.; Spears, P.A.; et al. Clinical Utility and User Perceptions of a Digital System for Electronic Patient-Reported Symptom Monitoring During Routine Cancer Care: Findings From the PRO-TECT Trial. JCO Clin. Cancer Inform. 2020, 4, 947–957. [Google Scholar] [CrossRef]
- Beaver, K.; Campbell, M.; Williamson, S.; Procter, D.; Sheridan, J.; Heath, J.; Susnerwala, S. An exploratory randomized controlled trial comparing telephone and hospital follow-up after treatment for colorectal cancer. Color. Dis. 2012, 14, 1201–1209. [Google Scholar] [CrossRef]
- Beaver, K.; Tysver-Robinson, D.; Campbell, M.; Twomey, M.; Williamson, S.; Hindley, A.; Susnerwala, S.; Dunn, G.; Luker, K. Comparing hospital and telephone follow-up after treatment for breast cancer: Randomised equivalence trial. BMJ (Clin. Res. Ed.) 2009, 338, a3147. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.L.; Flora, P.K.; Soheilipour, S.; Dirlea, M.; Maharaj, N.; Parvin, L.; Matthew, A.; Catton, C.; Jamnicky, L.; Pollock, P.; et al. Web-Based Peer Navigation for Men with Prostate Cancer and Their Family Caregivers: A Pilot Feasibility Study. Curr. Oncol. 2022, 29, 4285–4299. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.L.; Katz, J.; Ferris, L.E.; Jadad, A.R. What is the role of online support from the perspective of facilitators of face-to-face support groups? A multi-method study of the use of breast cancer online communities. Patient Educ. Couns. 2013, 93, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.V.; Dueck, A.C.; Mitchell, S.A.; Mendoza, T.R.; Reeve, B.B.; Atkinson, T.M.; Castro, K.M.; Denicoff, A.; Rogak, L.J.; Harness, J.K.; et al. Mode equivalence and acceptability of tablet computer-, interactive voice response system-, and paper-based administration of the U.S. National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Health Qual. Life Outcomes 2016, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Benze, G.; Nauck, F.; Alt-Epping, B.; Gianni, G.; Bauknecht, T.; Ettl, J.; Munte, A.; Kretzschmar, L.; Gaertner, J. PROutine: A feasibility study assessing surveillance of electronic patient reported outcomes and adherence via smartphone app in advanced cancer. Ann. Palliat. Med. 2019, 8, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Bol, N.; Smets, E.M.; Rutgers, M.M.; Burgers, J.A.; de Haes, H.C.; Loos, E.F.; van Weert, J.C. Do videos improve website satisfaction and recall of online cancer-related information in older lung cancer patients? Patient Educ. Couns. 2013, 92, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Bolle, S.; Romijn, G.; Smets, E.M.; Loos, E.F.; Kunneman, M.; van Weert, J.C. Older Cancer Patients’ User Experiences With Web-Based Health Information Tools: A Think-Aloud Study. J. Med. Internet Res. 2016, 18, e208. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Sadeghi, F.; O’Neill, L.; Guinan, E.; Smyth, L.; Sheill, G.; Smyth, E.; Doyle, S.L.; Timon, C.M.; Connolly, D.; et al. Telehealth Delivery of a Multi-Disciplinary Rehabilitation Programme for Upper Gastro-Intestinal Cancer: ReStOre@Home Feasibility Study. Cancers 2022, 14, 2707. [Google Scholar] [CrossRef] [PubMed]
- Cadmus-Bertram, L.; Tevaarwerk, A.J.; Sesto, M.E.; Gangnon, R.; Van Remortel, B.; Date, P. Building a physical activity intervention into clinical care for breast and colorectal cancer survivors in Wisconsin: A randomized controlled pilot trial. J. Cancer Surviv. Res. Pract. 2019, 13, 593–602. [Google Scholar] [CrossRef]
- Chee, W.; Lee, Y.; Im, E.O.; Chee, E.; Tsai, H.M.; Nishigaki, M.; Yeo, S.A.; Schapira, M.M.; Mao, J.J. A culturally tailored Internet cancer support group for Asian American breast cancer survivors: A randomized controlled pilot intervention study. J. Telemed. Telecare 2017, 23, 618–626. [Google Scholar] [CrossRef]
- Cheville, A.L.; Moynihan, T.; Basford, J.R.; Nyman, J.A.; Tuma, M.L.; Macken, D.A.; Therneau, T.; Satelel, D.; Kroenke, K. The rationale, design, and methods of a randomized, controlled trial to evaluate the effectiveness of collaborative telecare in preserving function among patients with late stage cancer and hematologic conditions. Contemp. Clin. Trials 2018, 64, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.I.; Drago, F.; Kennedy, E.M.; Chambers, N.; Sheffield, C.; Cohn, W.F. Examining the feasibility, acceptability, and potential utility of mobile distress screening in adult cancer patients. Psycho-Oncology 2019, 28, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S.; Wang, X.S.; Shi, Q.; Mendoza, T.R.; Wright, S.L.; Berry, M.D.; Malveaux, D.; Shah, P.K.; Gning, I.; Hofstetter, W.L.; et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: A randomized controlled clinical trial. J. Clin. Oncol. 2011, 29, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Burns, C.L.; Ward, E.C.; Comans, T.; Blake, C.; Kenny, L.; Greenup, P.; Best, D. Home-based telehealth service for swallowing and nutrition management following head and neck cancer treatment. J. Telemed. Telecare 2017, 23, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Crafoord, M.T.; Fjell, M.; Sundberg, K.; Nilsson, M.; Langius-Eklöf, A. Engagement in an Interactive App for Symptom Self-Management during Treatment in Patients With Breast or Prostate Cancer: Mixed Methods Study. J. Med. Internet Res. 2020, 22, e17058. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.Y.; Boyd, A.D.; Nayak, A.K.; Venepalli, N.K.; Cuellar, S.; Wirth, S.M.; Hsu, G.I.H. Patient-centered design in developing a mobile application for oral anticancer medications. J. Am. Pharm. Assoc. 2019, 59 (Suppl. S2), S86–S95.e1. [Google Scholar] [CrossRef]
- Denis, F.; Viger, L.; Charron, A.; Voog, E.; Dupuis, O.; Pointreau, Y.; Letellier, C. Detection of lung cancer relapse using self-reported symptoms transmitted via an internet web-application: Pilot study of the sentinel follow-up. Support. Care Cancer 2014, 22, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Duffecy, J.; Sanford, S.; Wagner, L.; Begale, M.; Nawacki, E.; Mohr, D.C. Project onward: An innovative e-health intervention for cancer survivors. Psycho-Oncology 2013, 22, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Eakin, E.G.; Lawler, S.P.; Winkler, E.A.; Hayes, S.C. A randomized trial of a telephone-delivered exercise intervention for non-urban dwelling women newly diagnosed with breast cancer: Exercise for health. Ann. Behav. Med. 2012, 43, 229–238. [Google Scholar] [CrossRef]
- Ferguson, R.J.; Sigmon, S.T.; Pritchard, A.J.; LaBrie, S.L.; Goetze, R.E.; Fink, C.M.; Garrett, A.M. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer 2016, 122, 1782–1791. [Google Scholar] [CrossRef]
- Foley, N.M.; O’Connell, E.P.; Lehane, E.A.; Livingstone, V.; Maher, B.; Kaimkhani, S.; Cil, T.; Relihan, N.; Bennett, M.W.; Redmond, H.P.; et al. PATI: Patient accessed tailored information: A pilot study to evaluate the effect on preoperative breast cancer patients of information delivered via a mobile application. Breast 2016, 30, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.R.; Axelrod, D.; Guth, A.A.; Wang, Y.; Scagliola, J.; Hiotis, K.; Rampertaap, K.; El-Shammaa, N. Usability and feasibility of health IT interventions to enhance Self-Care for Lymphedema Symptom Management in breast cancer survivors. Internet Interv. 2016, 5, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Galiano-Castillo, N.; Cantarero-Villanueva, I.; Fernández-Lao, C.; Ariza-García, A.; Díaz-Rodríguez, L.; Del-Moral-Ávila, R.; Arroyo-Morales, M. Telehealth system: A randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer 2016, 122, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Shahin, M.; Jia, R.; Shaffer, D.R.; Gimpel-Tetra, K.; Tsao, C.K.; Baker, C.; Leiter, A.; Holland, J.; Sablinski, T.; et al. Telemedicine-Enabled Clinical Trial of Metformin in Patients With Prostate Cancer. JCO Clin. Cancer Inform. 2017, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gell, N.M.; Grover, K.W.; Humble, M.; Sexton, M.; Dittus, K. Efficacy, feasibility, and acceptability of a novel technology-based intervention to support physical activity in cancer survivors. Support. Care Cancer 2017, 25, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson-White, S.; Yeung, C.W.; Saeidzadeh, S.; Tykol, H.; Vikas, P.; Cannon, A. Engaging Stakeholders in the Development of an eHealth Intervention for Cancer Symptom Management for Rural Residents. J. Rural. Health 2019, 35, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Graetz, I.; Anderson, J.N.; McKillop, C.N.; Stepanski, E.J.; Paladino, A.J.; Tillmanns, T.D. Use of a web-based app to improve postoperative outcomes for patients receiving gynecological oncology care: A randomized controlled feasibility trial. Gynecol. Oncol. 2018, 150, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Groarke, J.M.; Richmond, J.; Mc Sharry, J.; Groarke, A.; Harney, O.M.; Kelly, M.G.; Walsh, J.C. Acceptability of a Mobile Health Behavior Change Intervention for Cancer Survivors With Obesity or Overweight: Nested Mixed Methods Study Within a Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e18288. [Google Scholar] [CrossRef] [PubMed]
- Gustavell, T.; Sundberg, K.; Langius-Eklöf, A. Using an Interactive App for Symptom Reporting and Management Following Pancreatic Cancer Surgery to Facilitate Person-Centered Care: Descriptive Study. JMIR Mhealth Uhealth 2020, 8, e17855. [Google Scholar] [CrossRef]
- Head, B.A.; Keeney, C.; Studts, J.L.; Khayat, M.; Bumpous, J.; Pfeifer, M. Feasibility and Acceptance of a Telehealth Intervention to Promote Symptom Management during Treatment for Head and Neck Cancer. J. Support. Oncol. 2011, 9, e1–e11. [Google Scholar] [CrossRef][Green Version]
- Head, B.A.; Studts, J.L.; Bumpous, J.M.; Gregg, J.L.; Wilson, L.; Keeney, C.; Scharfenberger, J.A.; Pfeifer, M.P. Development of a telehealth intervention for head and neck cancer patients. Telemed. J. E-Health 2009, 15, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Heiney, S.P.; Adams, S.A.; Wells, L.M.; Johnson, H.; King, J.M. Participant evaluation of teleconference support for African American women with breast cancer. Cancer Nurs. 2012, 35, E24–E30. [Google Scholar] [CrossRef] [PubMed]
- Hochstenbach, L.M.J.; Zwakhalen, S.M.G.; Courtens, A.M.; van Kleef, M.; de Witte, L.P. Feasibility of a mobile and web-based intervention to support self-management in outpatients with cancer pain. Eur. J. Oncol. Nurs. 2016, 23, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kanera, I.M.; Willems, R.A.; Bolman, C.A.; Mesters, I.; Zambon, V.; Gijsen, B.C.; Lechner, L. Use and Appreciation of a Tailored Self-Management eHealth Intervention for Early Cancer Survivors: Process Evaluation of a Randomized Controlled Trial. J. Med. Internet Res. 2016, 18, e229. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.; Slack, R.; Bruno, M.; McMillan, J.; Fleming, J.B.; Lee, J.E.; Bednarski, B.; Papadopoulos, J.; Matin, S.F. Outpatient virtual clinical encounters after complex surgery for cancer: A prospective pilot study of “TeleDischarge”. J. Surg. Res. 2016, 202, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Kearney, N.; Kidd, L.; Miller, M.; Sage, M.; Khorrami, J.; McGee, M.; Cassidy, J.; Niven, K.; Gray, P. Utilising handheld computers to monitor and support patients receiving chemotherapy: Results of a UK-based feasibility study. Support. Care Cancer 2006, 14, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.A.; Winger, J.G.; Dorfman, C.S.; Ingle, K.K.; Moskovich, A.A.; Abernethy, A.P.; Keefe, F.J.; Samsa, G.P.; Kimmick, G.G.; Somers, T.J. A behavioral cancer pain intervention: A randomized noninferiority trial comparing in-person with videoconference delivery. Psycho-Oncology 2019, 28, 1671–1678. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Van Blarigan, E.L.; Ameli, N.; Lavaki, E.; Cedars, B.; Paciorek, A.T.; Monroy, C.; Tantum, L.K.; Newton, R.U.; Signorell, C.; et al. Feasibility, Acceptability, and Behavioral Outcomes from a Technology-enhanced Behavioral Change Intervention (Prostate 8): A Pilot Randomized Controlled Trial in Men with Prostate Cancer. Eur. Urol. 2019, 75, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.M.; Shin, H.; Jang, J.S.; Kim, Y.I.; Han, D.H. A Mobile Game for Patients With Breast Cancer for Chemotherapy Self-Management and Quality-of-Life Improvement: Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e273. [Google Scholar] [CrossRef]
- Kim, K.K.; Bell, J.F.; Bold, R.; Davis, A.; Ngo, V.; Reed, S.C.; Joseph, J.G. A Personal Health Network for Chemotherapy Care Coordination: Evaluation of Usability Among Patients. Stud. Health Technol. Inform. 2016, 225, 232–236. [Google Scholar]
- Kondylakis, H.; Bucur, A.; Crico, C.; Dong, F.; Graf, N.; Hoffman, S.; Koumakis, L.; Manenti, A.; Marias, K.; Mazzocco, K.; et al. Patient empowerment for cancer patients through a novel ICT infrastructure. J. Biomed. Inform. 2020, 101, 103342. [Google Scholar] [CrossRef]
- Kubo, A.; Kurtovich, E.; McGinnis, M.; Aghaee, S.; Altschuler, A.; Quesenberry, C., Jr.; Kolevska, T.; Avins, A.L. A Randomized Controlled Trial of mHealth Mindfulness Intervention for Cancer Patients and Informal Cancer Caregivers: A Feasibility Study Within an Integrated Health Care Delivery System. Integr. Cancer Ther. 2019, 18, 1534735419850634. [Google Scholar] [CrossRef]
- Lambert, S.D.; Duncan, L.R.; Culos-Reed, S.N.; Hallward, L.; Higano, C.S.; Loban, E.; Katz, A.; De Raad, M.; Ellis, J.; Korman, M.B.; et al. Feasibility, Acceptability, and Clinical Significance of a Dyadic, Web-Based, Psychosocial and Physical Activity Self-Management Program (TEMPO) Tailored to the Needs of Men with Prostate Cancer and Their Caregivers: A Multi-Center Randomized Pilot Trial. Curr. Oncol. 2022, 29, 785–804. [Google Scholar] [CrossRef]
- Lee, H.; Uhm, K.E.; Cheong, I.Y.; Yoo, J.S.; Chung, S.H.; Park, Y.H.; Lee, J.Y.; Hwang, J.H. Patient Satisfaction with Mobile Health (mHealth) Application for Exercise Intervention in Breast Cancer Survivors. J. Med. Syst. 2018, 42, 254. [Google Scholar] [CrossRef]
- Livingston, P.; White, V.; Hayman, J.; Hill, D. How acceptable is a referral and telephone-based outcall programme for men diagnosed with cancer? A feasibility study. Eur. J. Cancer Care 2006, 15, 467–475. [Google Scholar] [CrossRef]
- Livingston, P.M.; Heckel, L.; Orellana, L.; Ashley, D.; Ugalde, A.; Botti, M.; Pitson, G.; Woollett, A.; Chambers, S.K.; Parente, P.; et al. Outcomes of a randomized controlled trial assessing a smartphone Application to reduce unmet needs among people diagnosed with CancEr (ACE). Cancer Med. 2020, 9, 507–516. [Google Scholar] [CrossRef]
- Loh, K.P.; Sanapala, C.; Watson, E.E.; Jensen-Battaglia, M.; Janelsins, M.C.; Klepin, H.D.; Schnall, R.; Culakova, E.; Vertino, P.; Susiarjo, M.; et al. A single-arm pilot study of a mobile health exercise intervention (GO-EXCAP) in older patients with myeloid neoplasms. Blood Adv. 2022, 6, 3850–3860. [Google Scholar] [CrossRef]
- Lopez, C.J.; Edwards, B.; Langelier, D.M.; Chang, E.K.; Chafranskaia, A.; Jones, J.M. Delivering Virtual Cancer Rehabilitation Programming During the First 90 Days of the COVID-19 Pandemic: A Multimethod Study. Arch. Phys. Med. Rehabil. 2021, 102, 1283–1293. [Google Scholar] [CrossRef]
- Lozano-Lozano, M.; Cantarero-Villanueva, I.; Martin-Martin, L.; Galiano-Castillo, N.; Sanchez, M.J.; Fernández-Lao, C.; Postigo-Martin, P.; Arroyo-Morales, M. A Mobile System to Improve Quality of Life Via Energy Balance in Breast Cancer Survivors (BENECA mHealth): Prospective Test-Retest Quasiexperimental Feasibility Study. JMIR Mhealth Uhealth 2019, 7, e14136. [Google Scholar] [CrossRef]
- Lucas, A.R.; Bass, M.B.; Rothrock, N.E.; O’Connor, M.L.; Sorkin, M.R.; Nawyn, J.; Albinali, F.; Wagner, L.I. Development of an eHealth System to Capture and Analyze Patient Sensor and Self-Report Data: Mixed-Methods Assessment of Potential Applications to Improve Cancer Care Delivery. JMIR Med. Inform. 2018, 6, e46. [Google Scholar] [CrossRef]
- Lyons, K.D.; Hull, J.G.; Kaufman, P.A.; Li, Z.; Seville, J.L.; Ahles, T.A.; Kornblith, A.B.; Hegel, M.T. Development and initial evaluation of a telephone-delivered, behavioral activation, and problem-solving treatment program to address functional goals of breast cancer survivors. J. Psychosoc. Oncol. 2015, 33, 199–218. [Google Scholar] [CrossRef]
- MacDonald, A.M.; Chafranskaia, A.; Lopez, C.J.; Maganti, M.; Bernstein, L.J.; Chang, E.; Langelier, D.M.; Obadia, M.; Edwards, B.; Oh, P.; et al. CaRE @ Home: Pilot Study of an Online Multidimensional Cancer Rehabilitation and Exercise Program for Cancer Survivors. J. Clin. Med. 2020, 9, 3092. [Google Scholar] [CrossRef]
- Mark, T.L.; Fortner, B.; Johnson, G. Evaluation of a tablet PC technology to screen and educate oncology patients. Support. Care Cancer 2008, 16, 371–378. [Google Scholar] [CrossRef]
- Matthew, A.G.; Currie, K.L.; Irvine, J.; Ritvo, P.; Santa Mina, D.; Jamnicky, L.; Nam, R.; Trachtenberg, J. Serial personal digital assistant data capture of health-related quality of life: A randomized controlled trial in a prostate cancer clinic. Health Qual. Life Outcomes 2007, 5, 38. [Google Scholar] [CrossRef]
- McCann, L.; Maguire, R.; Miller, M.; Kearney, N. Patients’ perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur. J. Cancer Care 2009, 18, 156–164. [Google Scholar] [CrossRef]
- Milbury, K.; Kroll, J.; Chen, A.; Antonoff, M.B.; Snyder, S.; Higgins, H.; Yang, C.C.; Li, Y.; Bruera, E. Pilot Randomized Controlled Trial in Women With Non-Small Cell Lung Cancer to Assess the Feasibility of Delivering Group-Based Psychosocial Care via Videoconference. Integr. Cancer Ther. 2021, 20, 15347354211052520. [Google Scholar] [CrossRef]
- Myall, M.; May, C.R.; Grimmett, C.; May, C.M.; Calman, L.; Richardson, A.; Foster, C.L. RESTORE: An exploratory trial of a web-based intervention to enhance self-management of cancer-related fatigue: Findings from a qualitative process evaluation. BMC Med. Inform. Decis. Mak. 2015, 15, 94. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Smets, E.M.; Bol, N.; Loos, E.F.; van Laarhoven, H.W.; Geijsen, D.; van Berge Henegouwen, M.I.; Tytgat, K.M.; van Weert, J.C. Tailored Web-Based Information for Younger and Older Patients with Cancer: Randomized Controlled Trial of a Preparatory Educational Intervention on Patient Outcomes. J. Med. Internet Res. 2019, 21, e14407. [Google Scholar] [CrossRef]
- O’Brien, K.; Moore, A.; Percival-Smith, S.; Venkatraman, S.; Grubacevic, V.; Scoble, J.; Gilham, L.; Greenway, T.; Coghill, K.; Wale, J. An investigation into the usability of a drug-complementary medicines interactions database in a consumer group of women with breast cancer. Eur. J. Integr. Med. 2020, 33, 101004. [Google Scholar] [CrossRef]
- Ormel, H.L.; van der Schoot, G.G.F.; Westerink, N.L.; Sluiter, W.J.; Gietema, J.A.; Walenkamp, A.M.E. Self-monitoring physical activity with a smartphone application in cancer patients: A randomized feasibility study (SMART-trial). Support. Care Cancer 2018, 26, 3915–3923. [Google Scholar] [CrossRef]
- Owens, O.L.; Smith, K.N.; Beer, J.M.; Gallerani, D.G.; McDonnell, K.K. A Qualitative Cultural Sensitivity Assessment of the Breathe Easier Mobile Application for Lung Cancer Survivors and Their Families. Oncol. Nurs. Forum 2020, 47, 331–341. [Google Scholar] [CrossRef]
- Ownsworth, T.; Cubis, L.; Prasad, T.; Foote, M.; Kendall, M.; Oram, J.; Chambers, S.; Pinkham, M.B. Feasibility and acceptability of a telehealth platform for delivering the Making Sense of Brain Tumour programme: A mixed-methods pilot study. Neuropsychol. Rehabil. 2022, 32, 378–406. [Google Scholar] [CrossRef]
- Pavic, M.; Klaas, V.; Theile, G.; Kraft, J.; Tröster, G.; Guckenberger, M. Feasibility and Usability Aspects of Continuous Remote Monitoring of Health Status in Palliative Cancer Patients Using Wearables. Oncology 2020, 98, 386–395. [Google Scholar] [CrossRef]
- Peipert, J.D.; Lad, T.; Khosla, P.G.; Garcia, S.F.; Hahn, E.A. A Low Literacy, Multimedia Health Information Technology Intervention to Enhance Patient-Centered Cancer Care in Safety Net Settings Increased Cancer Knowledge in a Randomized Controlled Trial. Cancer Control J. Moffitt Cancer Cent. 2021, 28, 10732748211036783. [Google Scholar] [CrossRef]
- Post, D.M.; Shapiro, C.L.; Cegala, D.J.; David, P.; Katz, M.L.; Krok, J.L.; Phillips, G.S.; McAlearney, A.S.; Lehman, J.S.; Hicks, W.; et al. Improving symptom communication through personal digital assistants: The CHAT (Communicating Health Assisted by Technology) project. J. Natl. Cancer Inst. Monogr. 2013, 2013, 153–161. [Google Scholar] [CrossRef]
- Price, J.; Brunet, J. Feasibility and acceptability of a telehealth behavior change intervention for promoting physical activity and fruit and vegetable consumption among rural-living young adult cancer survivors. J. Psychosoc. Oncol. 2021, 39, 715–733. [Google Scholar] [CrossRef]
- Purdy, G.M.; Venner, C.P.; Tandon, P.; McNeely, M.L. Feasibility of a tailored and virtually supported home exercise program for people with multiple myeloma using a novel eHealth application. Digit. Health 2022, 8, 20552076221129066. [Google Scholar] [CrossRef]
- Puszkiewicz, P.; Roberts, A.L.; Smith, L.; Wardle, J.; Fisher, A. Assessment of Cancer Survivors’ Experiences of Using a Publicly Available Physical Activity Mobile Application. JMIR Cancer 2016, 2, e7. [Google Scholar] [CrossRef]
- Rezaee, R.; Asadi, S.; Yazdani, A.; Rezvani, A.; Kazeroon, A.M. Development, usability and quality evaluation of the resilient mobile application for women with breast cancer. Health Sci. Rep. 2022, 5, e708. [Google Scholar] [CrossRef]
- Richards, H.S.; Blazeby, J.M.; Portal, A.; Harding, R.; Reed, T.; Lander, T.; Chalmers, K.A.; Carter, R.; Singhal, R.; Absolom, K.; et al. A real-time electronic symptom monitoring system for patients after discharge following surgery: A pilot study in cancer-related surgery. BMC Cancer 2020, 20, 543. [Google Scholar] [CrossRef]
- Ruland, C.M.; Maffei, R.M.; Børøsund, E.; Krahn, A.; Andersen, T.; Grimsbø, G.H. Evaluation of different features of an eHealth application for personalized illness management support: Cancer patients’ use and appraisal of usefulness. Int. J. Med. Inform. 2013, 82, 593–603. [Google Scholar] [CrossRef]
- Ruland, C.M.; White, T.; Stevens, M.; Fanciullo, G.; Khilani, S.M. Effects of a computerized system to support shared decision making in symptom management of cancer patients: Preliminary results. J. Am. Med. Inform. Assoc. JAMIA 2003, 10, 573–579. [Google Scholar] [CrossRef]
- Russell, L.; Ugalde, A.; Orellana, L.; Milne, D.; Krishnasamy, M.; Chambers, R.; Austin, D.W.; Livingston, P.M. A pilot randomised controlled trial of an online mindfulness-based program for people diagnosed with melanoma. Support. Care Cancer 2019, 27, 2735–2746. [Google Scholar] [CrossRef]
- Skrabal Ross, X.; Gunn, K.M.; Suppiah, V.; Patterson, P.; Boyle, T.; Carrington, C.; Tan, S.L.; Ryan, M.; Joshi, R.; Olver, I. A smartphone program to support adherence to oral chemotherapy in people with cancer: Proof-of-concept trial. Asia-Pac. J. Clin. Oncol. 2022, 18, e378–e387. [Google Scholar] [CrossRef]
- Smith, S.J.; Smith, A.B.; Kennett, W.; Vinod, S.K. Exploring cancer patients’, caregivers’, and clinicians’ utilisation and experiences of telehealth services during COVID-19: A qualitative study. Patient Educ. Couns. 2022, 105, 3134–3142. [Google Scholar] [CrossRef]
- Spoelstra, S.L.; Given, C.W.; Sikorskii, A.; Coursaris, C.K.; Majumder, A.; DeKoekkoek, T.; Schueller, M.; Given, B.A. Proof of Concept of a Mobile Health Short Message Service Text Message Intervention That Promotes Adherence to Oral Anticancer Agent Medications: A Randomized Controlled Trial. Telemed. J. E-Health 2016, 22, 497–506. [Google Scholar] [CrossRef]
- Stephen, J.; Collie, K.; McLeod, D.; Rojubally, A.; Fergus, K.; Speca, M.; Turner, J.; Taylor-Brown, J.; Sellick, S.; Burrus, K.; et al. Talking with text: Communication in therapist-led, live chat cancer support groups. Soc. Sci. Med. 2014, 104, 178–186. [Google Scholar] [CrossRef]
- Sundberg, K.; Eklöf, A.L.; Blomberg, K.; Isaksson, A.K.; Wengström, Y. Feasibility of an interactive ICT-platform for early assessment and management of patient-reported symptoms during radiotherapy for prostate cancer. Eur. J. Oncol. Nurs. 2015, 19, 523–528. [Google Scholar] [CrossRef]
- Valle, C.G.; Deal, A.M.; Tate, D.F. Preventing weight gain in African American breast cancer survivors using smart scales and activity trackers: A randomized controlled pilot study. J. Cancer Surviv. Res. Pract. 2017, 11, 133–148. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Chan, H.; Van Loon, K.; Kenfield, S.A.; Chan, J.M.; Mitchell, E.; Zhang, L.; Paciorek, A.; Joseph, G.; Laffan, A.; et al. Self-monitoring and reminder text messages to increase physical activity in colorectal cancer survivors (Smart Pace): A pilot randomized controlled trial. BMC Cancer 2019, 19, 218. [Google Scholar] [CrossRef]
- Van der Linden, S.D.; Rutten, G.M.; Dirven, L.; Taphoorn, M.J.B.; Satoer, D.D.; Dirven, C.M.F.; Sitskoorn, M.M.; Gehring, K. eHealth cognitive rehabilitation for brain tumor patients: Results of a randomized controlled trial. J. Neuro-Oncol. 2021, 154, 315–326. [Google Scholar] [CrossRef]
- Vogel, R.I.; Niendorf, K.; Petzel, S.; Lee, H.; Teoh, D.; Blaes, A.H.; Argenta, P.; Rivard, C.; Winterhoff, B.; Lee, H.Y.; et al. A patient-centered mobile health application to motivate use of genetic counseling among women with ovarian cancer: A pilot randomized controlled trial. Gynecol. Oncol. 2019, 153, 100–107. [Google Scholar] [CrossRef]
- Walle, T.; Erdal, E.; Mühlsteffen, L.; Singh, H.M.; Gnutzmann, E.; Grün, B.; Hofmann, H.; Ivanova, A.; Köhler, B.C.; Korell, F.; et al. Completion rate and impact on physician-patient relationship of video consultations in medical oncology: A randomised controlled open-label trial. ESMO Open 2020, 5, e000912. [Google Scholar] [CrossRef]
- Wallwiener, M.; Heindl, F.; Brucker, S.Y.; Taran, F.A.; Hartkopf, A.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; Ettl, J.; et al. Implementation and Feasibility of Electronic Patient-Reported Outcome (ePRO) Data Entry in the PRAEGNANT Real-Time Advanced and Metastatic Breast Cancer Registry. Geburtshilfe Frauenheilkd 2017, 77, 870–878. [Google Scholar] [CrossRef]
- Waterland, J.L.; Chahal, R.; Ismail, H.; Sinton, C.; Riedel, B.; Francis, J.J.; Denehy, L. Implementing a telehealth prehabilitation education session for patients preparing for major cancer surgery. BMC Health Serv. Res. 2021, 21, 443. [Google Scholar] [CrossRef]
- Weaver, A.; Young, A.M.; Rowntree, J.; Townsend, N.; Pearson, S.; Smith, J.; Gibson, O.; Cobern, W.; Larsen, M.; Tarassenko, L. Application of mobile phone technology for managing chemotherapy-associated side-effects. Ann. Oncol. 2007, 18, 1887–1892. [Google Scholar] [CrossRef]
- Wickline, M.; Wolpin, S.; Cho, S.; Tomashek, H.; Louca, T.; Frisk, T.; Templin, J.; Loechl, A.; Goff, B.; Berry, D. Usability and acceptability of the electronic self-assessment and care (eSAC) program in advanced ovarian cancer: A mixed methods study. Gynecol. Oncol. 2022, 167, 239–246. [Google Scholar] [CrossRef]
- Wilkie, D.J.; Judge, M.K.; Berry, D.L.; Dell, J.; Zong, S.; Gilespie, R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J. Pain. Symptom Manag. 2003, 25, 213–224. [Google Scholar] [CrossRef]
- Wu, F.; Rotimi, O.; Laza-Cagigas, R.; Rampal, T. The Feasibility and Effects of a Telehealth-Delivered Home-Based Prehabilitation Program for Cancer Patients during the Pandemic. Curr. Oncol. 2021, 28, 2248–2259. [Google Scholar] [CrossRef]
- Yap, K.Y.; Low, H.X.; Koh, K.S.; Un, M.; Shih, V.; Chan, A. Feasibility and acceptance of a pharmacist-run tele-oncology service for chemotherapy-induced nausea and vomiting in ambulatory cancer patients. Telemed. J. E-Health 2013, 19, 387–395. [Google Scholar] [CrossRef]
- Zini, E.M.; Lanzola, G.; Quaglini, S.; Bossi, P.; Licitra, L.; Resteghini, C. A pilot study of a smartphone-based monitoring intervention on head and neck cancer patients undergoing concurrent chemo-radiotherapy. Int. J. Med. Inform. 2019, 129, 404–412. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Torkamani, A.; Butte, A.J.; Glicksberg, B.S.; Schuller, B.; Rodriguez, B.; Ting, D.S.W.; Bates, D.; Schaden, E.; Peng, H.; et al. The promise of digital healthcare technologies. Front. Public. Health 2023, 11, 1196596. [Google Scholar] [CrossRef]
- Di Lorito, C.; Bosco, A.; Rai, H.; Craven, M.; McNally, D.; Todd, C.; Booth, V.; Cowley, A.; Howe, L.; Harwood, R.H. A systematic literature review and meta-analysis on digital health interventions for people living with dementia and Mild Cognitive Impairment. Int. J. Geriatr. Psychiatry 2022, 37, gps5730. [Google Scholar] [CrossRef]
- Batra, S.; Baker, R.A.; Wang, T.; Forma, F.; DiBiasi, F.; Peters-Strickland, T. Digital health technology for use in patients with serious mental illness: A systematic review of the literature. Med. Devices 2017, 10, 237–251. [Google Scholar] [CrossRef]
- Ignatowicz, A.; Slowther, A.M.; Elder, P.; Bryce, C.; Hamilton, K.; Huxley, C.; Forjaz, V.; Sturt, J.; Griffiths, F. Ethical implications of digital communication for the patient-clinician relationship: Analysis of interviews with clinicians and young adults with long term conditions (the LYNC study). BMC Med. Ethics 2018, 19, 11. [Google Scholar] [CrossRef]
- Qudah, B.; Luetsch, K. The influence of mobile health applications on patient-healthcare provider relationships: A systematic, narrative review. Patient Educ. Couns. 2019, 102, 1080–1089. [Google Scholar] [CrossRef]
- Lordon, R.J.; Mikles, S.P.; Kneale, L.; Evans, H.L.; Munson, S.A.; Backonja, U.; Lober, W.B. How patient-generated health data and patient-reported outcomes affect patient–clinician relationships: A systematic review. Health Inform. J. 2020, 26, 2689–2706. [Google Scholar] [CrossRef]
- Gammon, D.; Strand, M.; Eng, L.S.; Børøsund, E.; Varsi, C.; Ruland, C. Shifting Practices Toward Recovery-Oriented Care Through an E-Recovery Portal in Community Mental Health Care: A Mixed-Methods Exploratory Study. J. Med. Internet Res. 2017, 19, e145. [Google Scholar] [CrossRef]
- Wannheden, C.; Åberg-Wennerholm, M.; Dahlberg, M.; Revenäs, Å.; Tolf, S.; Eftimovska, E.; Brommels, M. Digital Health Technologies Enabling Partnerships in Chronic Care Management: Scoping Review. J. Med. Internet Res. 2022, 24, e38980. [Google Scholar] [CrossRef]
- Bally, E.L.S.; Cheng, D.; van Grieken, A.; Ferri Sanz, M.; Zanutto, O.; Carroll, A.; Darley, A.; Roozenbeek, B.; Dippel, D.W.J.; Raat, H. Patients’ Perspectives Regarding Digital Health Technology to Support Self-management and Improve Integrated Stroke Care: Qualitative Interview Study. J. Med. Internet Res. 2023, 25, e42556. [Google Scholar] [CrossRef]
- Schofield, P.; Shaw, T.; Pascoe, M. Toward Comprehensive Patient-Centric Care by Integrating Digital Health Technology With Direct Clinical Contact in Australia. J. Med. Internet Res. 2019, 21, e12382. [Google Scholar] [CrossRef]
- Kabukye, J.K.; Kakungulu, E.; Keizer, N.d.; Cornet, R. Digital health in oncology in Africa: A scoping review and cross-sectional survey. Int. J. Med. Inform. 2022, 158, 104659. [Google Scholar] [CrossRef]
- Curry, J.; Patterson, M.; Greenley, S.; Pearson, M.; Forbes, C.C. Feasibility, acceptability, and efficacy of online supportive care for individuals living with and beyond lung cancer: A systematic review. Support. Care Cancer 2021, 29, 6995–7011. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).