Exploring the Survival Determinants in Recurrent Ovarian Cancer: The Role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Inclusion Criteria:
- Age ≥ 18 years
- Histologically confirmed diagnosis of the first recurrence of epithelial OC
- Status post-surgery for primary OC with adjuvant platinum-based CTH
- Recurrence confirmed via radiology with/without elevated CA-125 and HE4 levels
- Al least one measurable target lesion per RECIST v 1.1
- ECOG performance score of 0–2, indicating ability for self-care and ambulatory for over 50% of waking hours
- Adequate organ and bone marrow function for CRS and HIPEC treatment
- Deemed suitable for CRS and HIPEC by a multidisciplinary team
- Written informed consent for treatment
- Exclusion Criteria:
- Diagnosis of non-epithelial OC like germ cell or stromal tumors
- Stage IV disease marked by extra-abdominal metastasis
- Previous treatment with CRS and HIPEC for OC
- Serious cardiac, lung, kidney, or liver conditions posing surgery risks
- Other active primary cancers
- ECOG status of 3 or higher, indicating severe incapacitation
- Underlying medical or psychiatric conditions that will make the administration of therapy hazardous
- Dementia, psychiatric, or substance abuse disorders that would interfere with treatment
- Known untreated, symptomatic, or actively progressing central nervous system metastases
- Known acute hepatitis B, known chronic hepatitis B infection with active untreated disease, or known hepatitis C infection
- Life expectancy under 6 months
2.1. Platinum Sensitivity
2.2. The Peritoneal Carcinomatosis Index and Cytoreduction Completeness
2.3. HIPEC Procedure
2.4. AGO Score
2.5. Statistical Analysis
3. Results
3.1. Study Group Characteristic
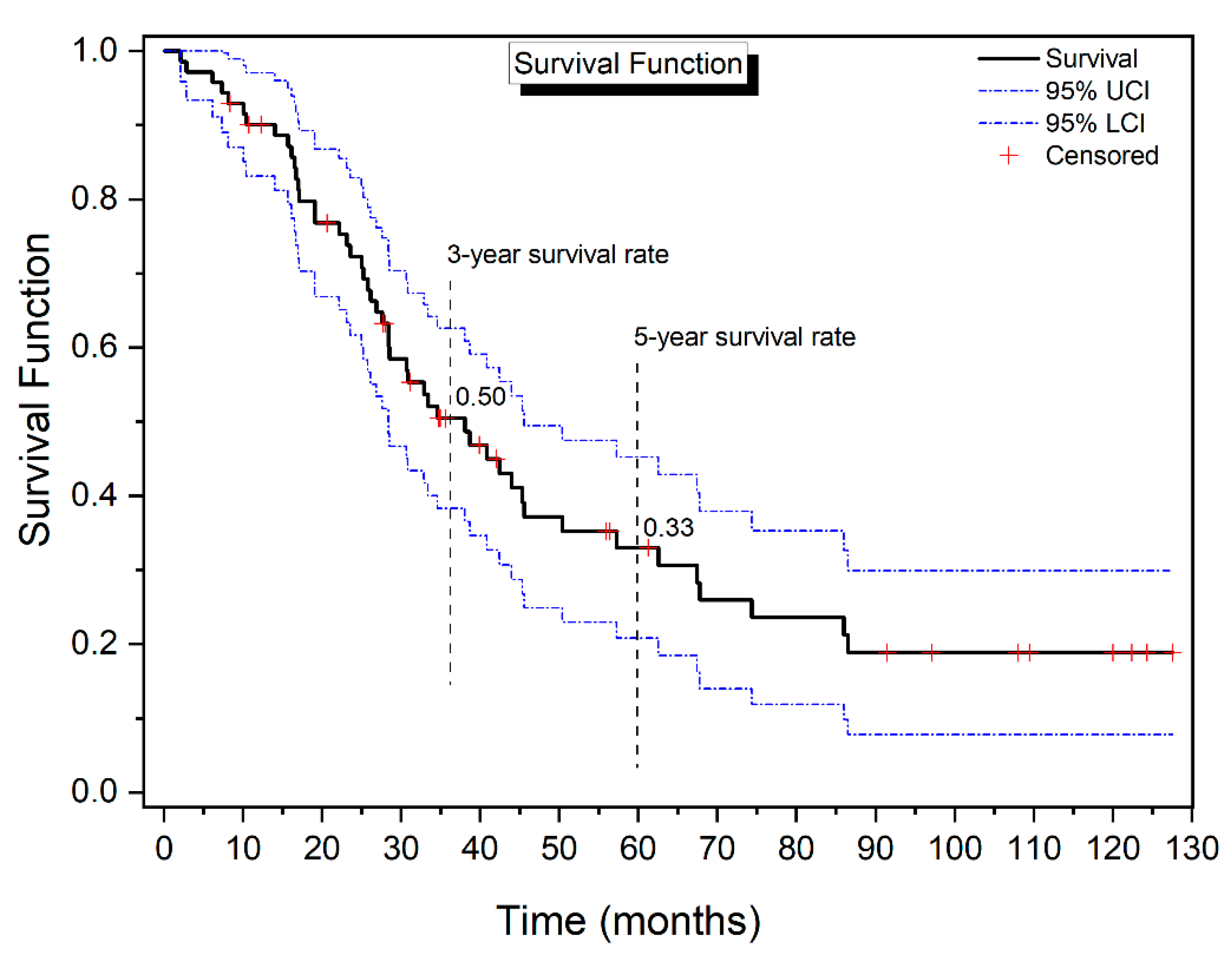
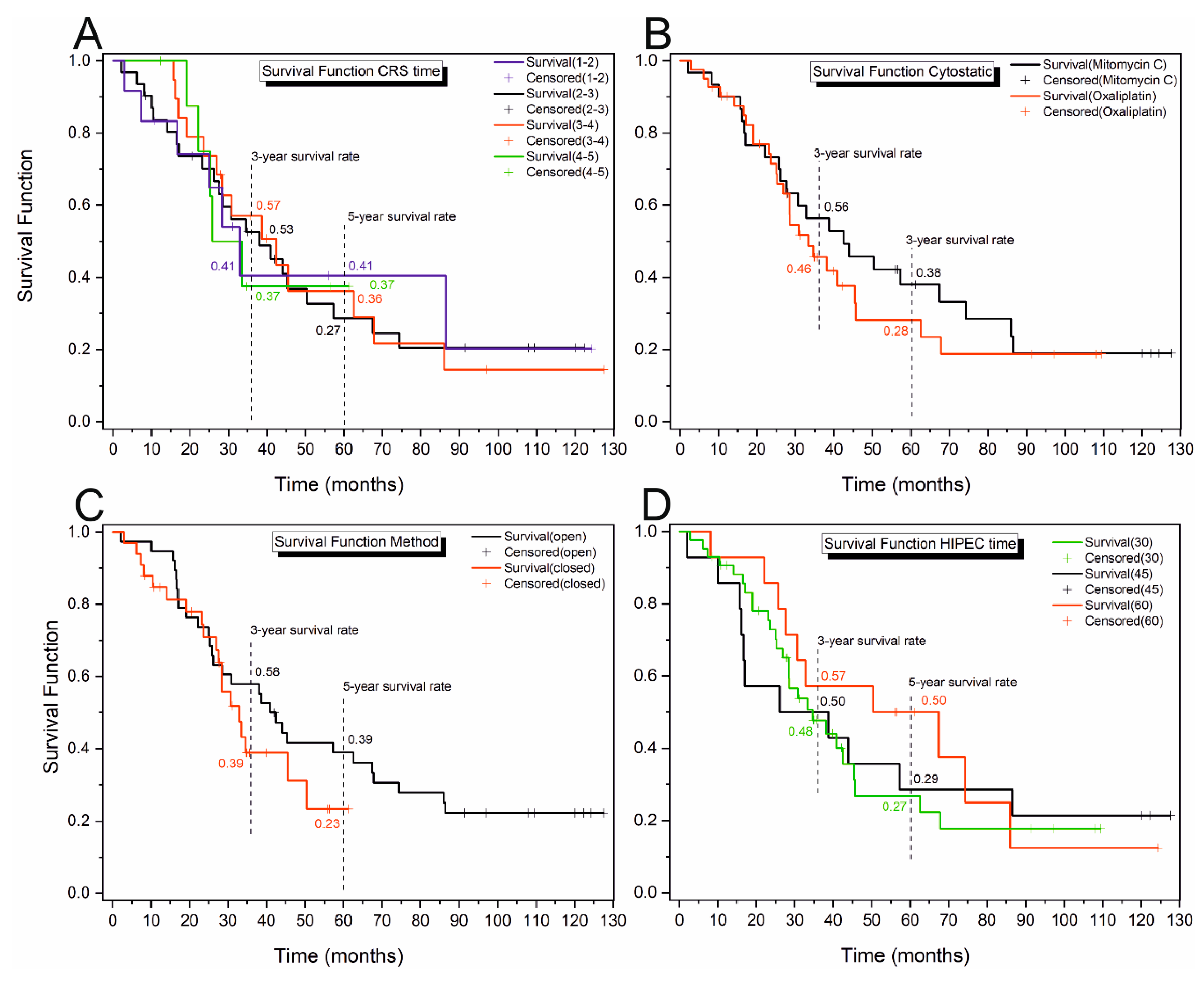
3.2. Kaplan–Meier Analysis
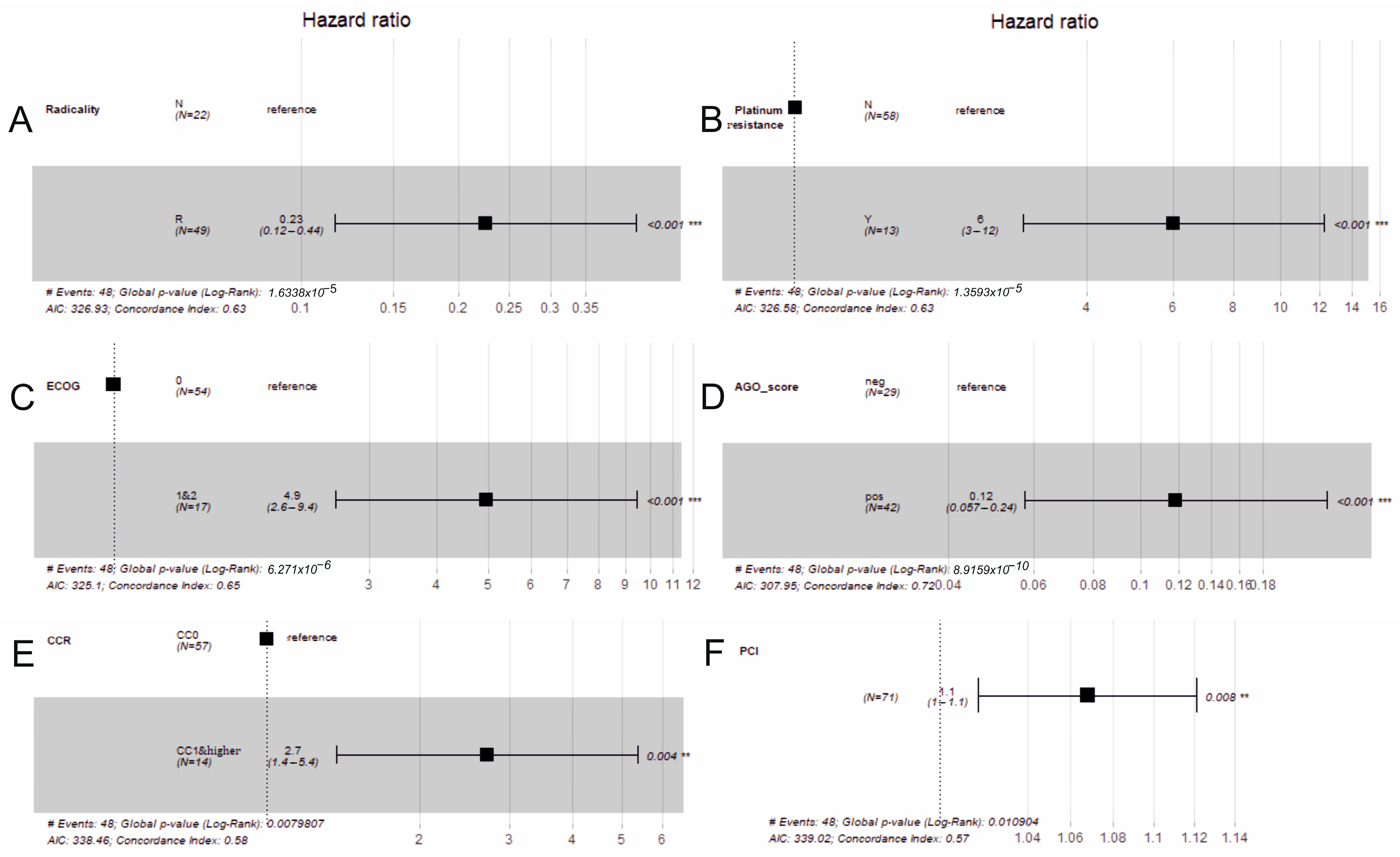
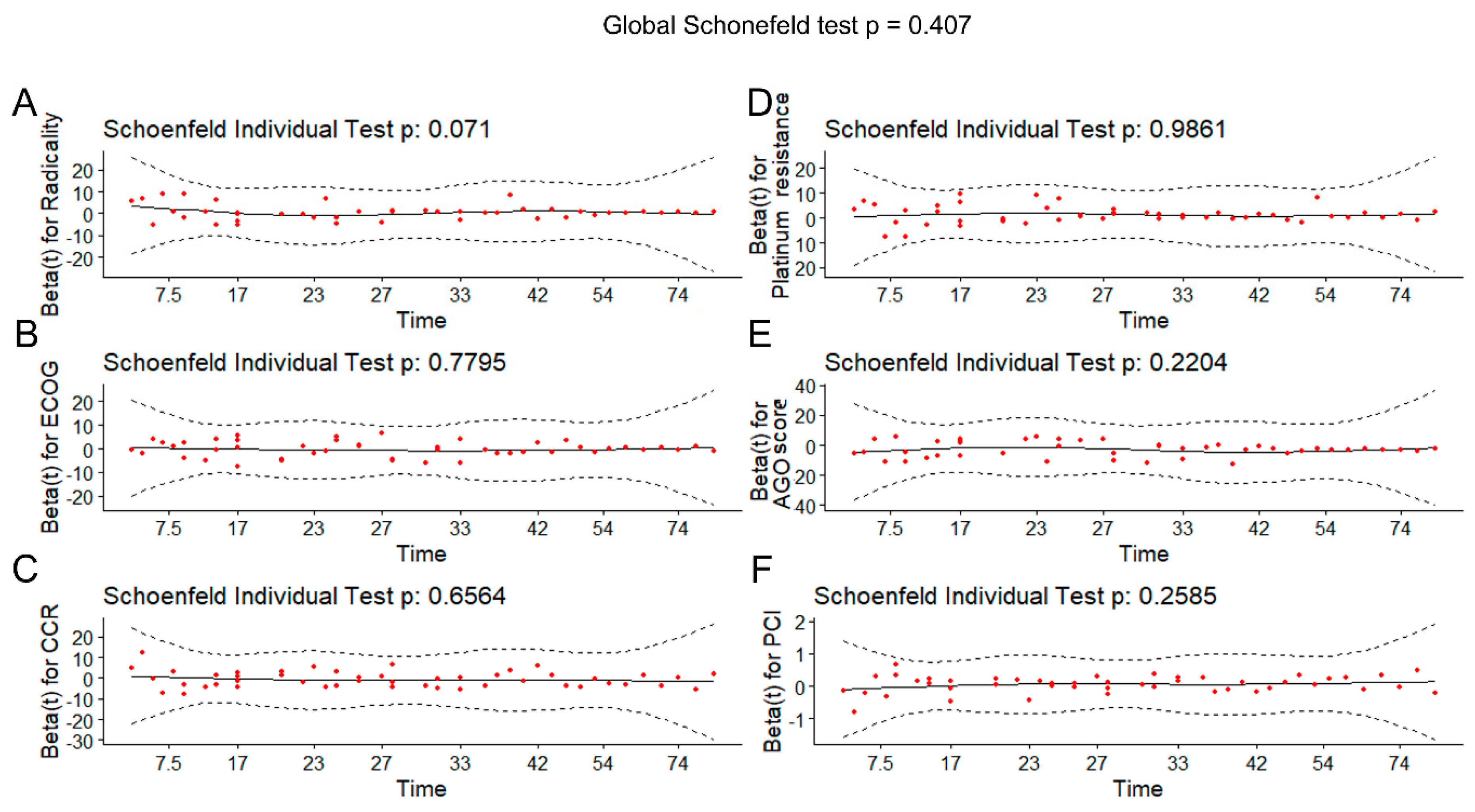
3.3. Cox Regression
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heintz, A.; Odicino, F.; Maisonneuve, P.; Quinn, M.; Benedet, J.; Creasman, W.; Ngan, H.; Pecorelli, S.; Beller, U. Carcinoma of the ovary. Int. J. Gynecol. Obstet. 2006, 95, S161–S192. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, L.A.; Huang, B.; Miller, R.W.; Tucker, T.; Goodrich, S.T.; Podzielinski, I.; DeSimone, C.P.; Ueland, F.R.; van Nagell, J.R.; Seamon, L.G. Ten-year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012, 120, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2023, 41, 4077–4083. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Hahmann, M.; Lueck, H.-J.; Poelcher, M.; Wimberger, P.; Ortmann, O.; Canzler, U.; Richter, B.; Wagner, U.; Hasenburg, A. Surgery for recurrent ovarian cancer: Role of peritoneal carcinomatosis: Exploratory analysis of the DESKTOP I Trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann. Surg. Oncol. 2009, 16, 1324–1330. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Reuss, A.; Hasenburg, A.; Scambia, G.; Cibula, D.; Mahner, S.; Vergote, I.; Reinthaller, A.; Burges, A.; et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: The Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int. J. Gynecol. Cancer 2011, 21, 289–295. [Google Scholar] [CrossRef]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586. [Google Scholar] [CrossRef]
- Ghirardi, V.; Trozzi, R.; Giudice, E.; Scambia, G.; Fagotti, A. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in the Management of Advanced Ovarian Cancer. A Literature Review. Eur. J. Gynaecol. Oncol. 2022, 43, 335–340. [Google Scholar]
- Koole, S.; van Stein, R.; Sikorska, K.; Barton, D.; Perrin, L.; Brennan, D.; Zivanovic, O.; Mosgaard, B.J.; Fagotti, A.; Colombo, P.-E.; et al. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int. J. Gynecol. Cancer 2020, 30, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO–ESMO–ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, J.; Prodromidou, A. Narrative review of hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with advanced ovarian cancer: A critical reappraisal of the current evidence. J. Gastrointest. Oncol. 2021, 12 (Suppl. S1), S182. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.J.; van Driel, W.J.; Zivanovic, O. The necessity to adhere to evidence-based indications for hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Definition of Platinum Sensitive Cancer-NCI Dictionary of Cancer Terms-NCI. 2011. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/platinum-sensitive-cancer (accessed on 20 April 2024).
- Rawert, F.L.; Luengas-Würzinger, V.; von Spee, S.C.-G.; Baransi, S.; Schuler, E.; Carrizo, K.; Dizdar, A.; Mallmann, P.; Lampe, B. The importance of the Peritoneal Cancer Index (PCI) to predict surgical outcome after neoadjuvant chemotherapy in advanced ovarian cancer. Arch. Gynecol. Obstet. 2022, 306, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.D.; Alderman, R.; Chang, D.; Edwards, G.D.; Esquivel, J.; Sebbag, G.; Steves, M.A.; Sugarbaker, P.H. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann. Surg. Oncol. 1999, 6, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sheng, L.; Chen, D. Perspective Chapter: Using Effect Sizes to Study the Survival Difference between Two Groups; IntechOpen: London, UK, 2023. [Google Scholar]
- Chen, J.; Qiu, L.; Wang, B.; Zeng, J.; Chen, Z. Statistical methods for comparing survival rates at a fixed time point. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi 2015, 36, 186–188. [Google Scholar] [PubMed]
- Recurrent Ovarian Cancer; Hematology Oncology Associates of Fredericksburg: Fredericksburg, VA, USA, 2024.
- Spiliotis, J.; Halkia, E.; Lianos, E.; Kalantzi, N.; Grivas, A.; Efstathiou, E.; Giassas, S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: A prospective randomized phase III study. Ann. Surg. Oncol. 2015, 22, 1570–1575. [Google Scholar] [CrossRef]
- Zivanovic, O.; Chi, D.S.; Zhou, Q.; Iasonos, A.; Konner, J.A.; Makker, V.; Grisham, R.N.; Brown, A.K.; Nerenstone, S.; Diaz, J.P.; et al. Secondary cytoreduction and carboplatin hyperthermic intraperitoneal chemotherapy for platinum-sensitive recurrent ovarian cancer: An MSK team ovary phase II study. J. Clin. Oncol. 2021, 39, 2594. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Y.; Shi, Y.; Yao, S.; Dai, M.; Cai, H. Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) for platinum-sensitive recurrence epithelial ovarian cancer with HRR mutation: A phase III randomized clinical trial. Technol. Cancer Res. Treat. 2022, 21, 15330338221104565. [Google Scholar] [CrossRef]
- Bhatt, A.; Glehen, O.; Zivanovic, O.; Brennan, D.; Nadeau, C.; Van Driel, W.; Bakrin, N. The 2022 PSOGI international consensus on HIPEC regimens for peritoneal malignancies: Epithelial ovarian cancer. Ann. Surg. Oncol. 2023, 30, 8115–8137. [Google Scholar] [CrossRef]
- Jänicke, F.; Hölscher, A.; Kuhn, W.; Von Hugo, R.; Pache, L.; Siewert, J.R.; Graef, H. Radical surgical procedure improves survival time in patients with recurrent ovarian cancer. Cancer 1992, 70, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Asp, M.; Malander, S.; Bengtsson, J.; Sartor, H.; Kannisto, P. Prognostic value of peritoneal cancer index after complete Cytoreductive surgery in advanced ovarian cancer. Anticancer Res. 2022, 42, 2541–2551. [Google Scholar] [CrossRef]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Graham, J.; Gabra, H.; Sharma, R. Evolving concepts in the management of drug resistant ovarian cancer: Dose dense chemotherapy and the reversal of clinical platinum resistance. Cancer Treat. Rev. 2013, 39, 153–160. [Google Scholar] [CrossRef]
- Bodnar, L.; Knapp, P.; Sznurkowski, J.; Mądry, R.; Gąsowska-Bodnar, A.; Blecharz, P.; Sikorska, M.; Timorek, A.; Ptak-Chmielewska, A.; Jach, R.; et al. EP804 Prognostic role of second-line platinum-based therapy in recurrent platinum resistant ovarian cancer patients–data from real-world clinical practice in poland. Int. J. Gynecol. Cancer 2019, 29 (Suppl. S4), A443–A444. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Romito, A.; Iacobelli, V.; Sassu, C.M.; Corrado, G.; Ricci, C.; Scambia, G.; Fagotti, A. Chemotherapy resistance in epithelial ovarian cancer: Mechanisms and emerging treatments. Semin. Cancer Biol. 2021, 77, 144–166. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Beutel, B.; Alesina, P.F.; Lorenz, D.; Boergers, A.; Heitz, F.; Hils, R.; Kurzeder, C.; Traut, A.; du Bois, A. Prognostic and predictive value of the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) score in surgery for recurrent ovarian cancer. Gynecol. Oncol. 2014, 132, 537–541. [Google Scholar] [CrossRef]
- Muallem, M.Z.; Gasimli, K.; Richter, R.; Almuheimid, J.; Nasser, S.; Braicu, E.I.; Sehouli, J. AGO score as a predictor of surgical outcome at secondary cytoreduction in patients with ovarian cancer. Anticancer Res. 2015, 35, 3423–3429. [Google Scholar]
- Armbrust, R.; Richter, R.; Woopen, H.; Hilpert, F.; Harter, P.; Sehouli, J. Impact of health-related quality of life (HRQoL) on short-term mortality in patients with recurrent ovarian, fallopian or peritoneal carcinoma (the NOGGO-AGO QoL Prognosis-Score-Study): Results of a meta-analysis in 2209 patients. ESMO Open 2021, 6, 100081. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, R.; Massuger, L.F.; Van Gorp, T.; IntHout, J.; Zusterzeel, P.L.; Kruitwagen, R.F. External validation of two prediction models of complete secondary cytoreductive surgery in patients with recurrent epithelial ovarian cancer. Gynecol. Oncol. 2015, 137, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Tagliabue, E.; Signorelli, M.; Ditto, A.; Martinelli, F.; Chiappa, V.; Mosca, L.; Sabatucci, I.; Maggiore, U.L.R.; Lorusso, D.; et al. A score system for complete cytoreduction in selected recurrent ovarian cancer patients undergoing secondary cytoreductive surgery: Predictors-and nomogram-based analyses. J. Gynecol. Oncol. 2018, 29, e40. [Google Scholar] [CrossRef] [PubMed]
- Tentes, A.-A.; Tripsiannis, G.; Markakidis, S.; Karanikiotis, C.; Tzegas, G.; Georgiadis, G.; Avgidou, K. Peritoneal cancer index: A prognostic indicator of survival in advanced ovarian cancer. Eur. J. Surg. Oncol. (EJSO) 2003, 29, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Chéreau, E.; Ballester, M.; Selle, F.; Cortez, A.; Daraï, E.; Rouzier, R. Comparison of peritoneal carcinomatosis scoring methods in predicting resectability and prognosis in advanced ovarian cancer. Am. J. Obstet. Gynecol. 2010, 202, 178.e1–178.e10. [Google Scholar] [CrossRef] [PubMed]
- Elzarkaa, A.A.; Shaalan, W.; Elemam, D.; Mansour, H.; Melis, M.; Malik, E.; Soliman, A.A. Peritoneal cancer index as a predictor of survival in advanced stage serous epithelial ovarian cancer: A prospective study. J. Gynecol. Oncol. 2018, 29, e47. [Google Scholar] [CrossRef] [PubMed]
- Gasimli, K.; Braicu, E.I.; Richter, R.; Chekerov, R.; Sehouli, J. Prognostic and predictive value of the peritoneal cancer index in primary advanced epithelial ovarian cancer patients after complete cytoreductive surgery: Study of tumor bank ovarian cancer. Ann. Surg. Oncol. 2015, 22, 2729–2737. [Google Scholar] [CrossRef]
- Fagan, P.J.; Gomes, N.; Heath, O.M.; Chandrasekaran, D.; Yao, S.-E.; Satchwell, L.; George, A.; Banerjee, S.; Sohaib, A.; Barton, D.P.; et al. The peritoneal cancer index as a predictor of complete cytoreduction at primary and interval cytoreductive surgery in advanced ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 1757–1763. [Google Scholar] [CrossRef]
- Lomnytska, M.; Karlsson, E.; Jonsdottir, B.; Lejon, A.-M.; Stålberg, K.; Poromaa, I.S.; Silins, I.; Graf, W. Peritoneal cancer index predicts severe complications after ovarian cancer surgery. Eur. J. Surg. Oncol. 2021, 47, 2915–2924. [Google Scholar] [CrossRef]
- Jónsdóttir, B.; Lomnytska, M.; Poromaa, I.S.; Silins, I.; Stålberg, K. EP879 Peritoneal cancer index (PCI) is a strong predictor of operability in ovarian cancer. BMJ Spec. J. 2019, 29, A475–A477. [Google Scholar]


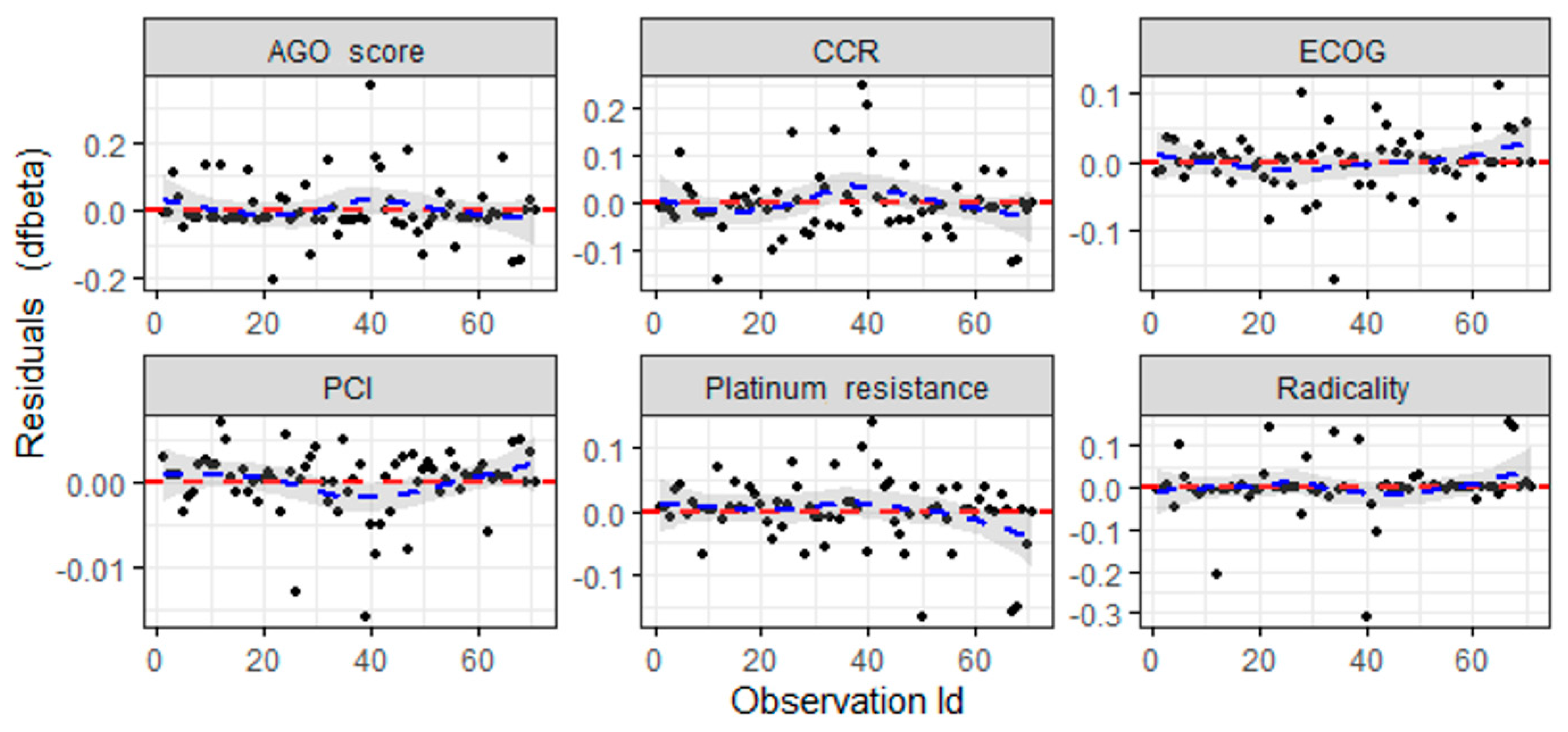
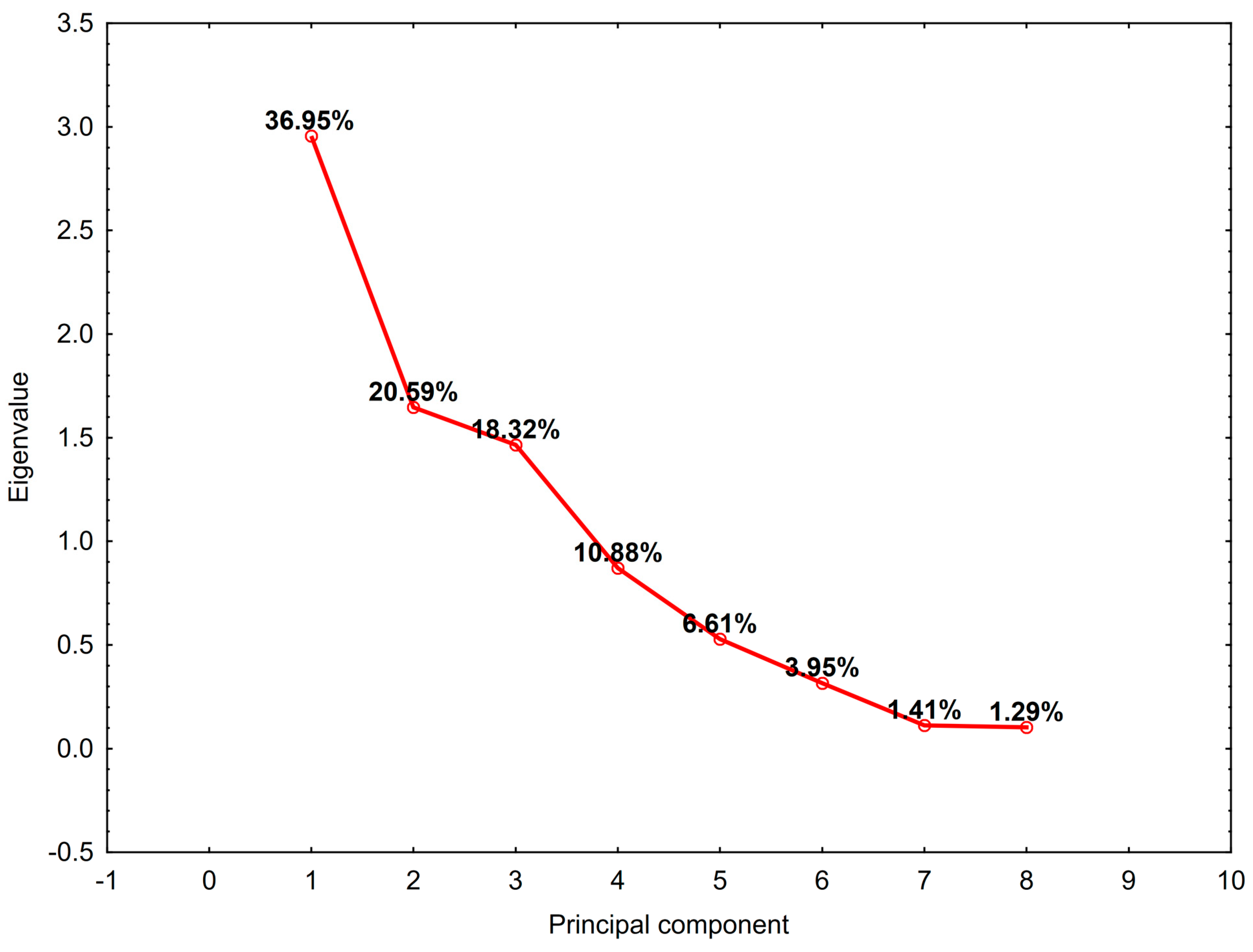
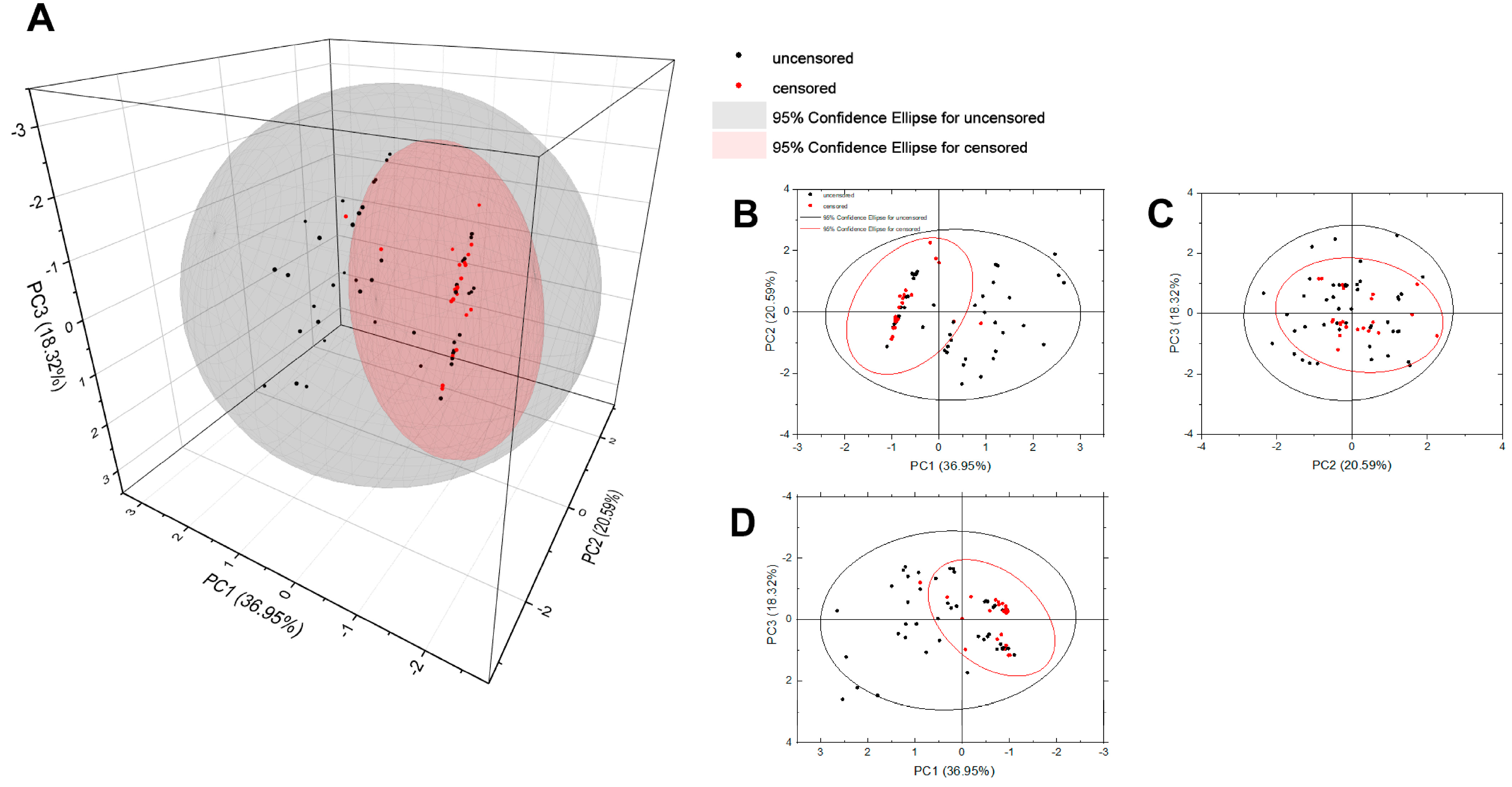
| Characteristics | Median | Q1 | Q3 |
|---|---|---|---|
| Survival time (months) | 31.00 | 19.10 | 56.00 |
| CRS time (minutes) | 150.00 | 120.00 | 210.00 |
| HIPEC time (minutes) | 30.00 | 30.00 | 45.00 |
| PCI | 5.00 | 4.00 | 11.00 |
| Characteristic | Number of Patients n | Percentage | |
|---|---|---|---|
| Radicality | N | 22 | 31% |
| R | 49 | 69% | |
| Platinum resistance | Y | 13 | 18% |
| N | 58 | 82% | |
| ECOG | 0 | 54 | 76% |
| 1 and 2 | 17 | 24% | |
| AGO score | Positive | 42 | 59% |
| Negative | 29 | 41% | |
| CCR | 0 | 57 | 80% |
| 1 and higher | 14 | 20% | |
| Cytostatic | Mitomycin C | 30 | 42% |
| Oxaliplatin | 41 | 58% | |
| HIPEC method | Open | 38 | 54% |
| Closed | 33 | 46% | |
| Percent Failures | Estimate | 95% L.C.I. | 95% U.C.I. |
|---|---|---|---|
| 25 | 23.10 | 16.67 | 27.60 |
| 50 | 38.10 | 28.37 | 45.57 |
| 75 | 74.37 | 45.57 | 86.53 |
| Variable | Mean Estimated Survival Time ± SE (CI) (Months) | Quartile Estimates (CI) | Statistics and p-Value | |||
|---|---|---|---|---|---|---|
| 25% | 50% | 75% | ||||
| Radicality | N | 24.50 ± 2.23 (20.13; 28.87) | 17 (14; 25) | 25 (17; 28) | 28 (27; 42) | χ2 = 22.61 p < 0.001 ES = 0.54 (large) |
| R | 65.33 ± 6.98 (51.64; 79.02) | 26 (22; 41) | 57 (39; 86) | - | ||
| Platinum resistance | Y | 17.87 ± 3.71 (10.59; 25.57) | 10 (3; 17) | 17 (10; 23) | 24 (16; 25) | χ2 = 30.61 p < 0.001 ES = 0.49 (large) |
| N | 60.29 ± 6.11 (48.30; 72.27) | 27.6 (22; 33) | 44 (33; 67) | 87 (63; 86) | ||
| ECOG | 0 | 64.00 ± 6.56 (51.14; 76.86) | 28 (22; 38) | 50 (35; 74) | - | χ2 = 20.86 p < 0.001 ES = −0.55 (large) |
| 1 and 2 | 20.86 ± 3.25 (14.49; 27.23) | 10 (3; 17) | 17 (10; 27) | 27 (17; 42) | ||
| AGO score | Positive | 74.32 ± 7.33 (59.95; 88.86) | 38 (26; 57) | 67 (45; 87) | - | χ2 = 44.40 p < 0.001 ES = 0.86 (large) |
| Negative | 22.09 ± 2.18 (17.81; 26.37) | 16 (7; 19) | 24 (17; 27) | 28 (25; 33) | ||
| CCR | 0 | 58.71 ± 6.23 (46.50; 70.91) | 25 (17; 31) | 44 (30; 67) | 87 (63; 87) | χ2 = 8.609 p = 0.004 ES = 0.29 (medium) |
| 1 and higher | 25.47 ± 4.09 (17.44; 33.50) | 16 (3; 27) | 25 (16; 33) | 33 (25; 42) | ||
| CRS time (h) | 1–2 | 54.92 ± 14.82 (25.87; 83.96) | 17 (7; 33) | 33 (17; 87) | 87 (28; 87) | χ2 = 0.017 p = 0.999 ES = not relevant |
| 2–3 | 50.69 ± 7.73 (35.55; 65.84) | 17 (10; 30) | 38 (26; 57) | 67 (44; 74) | ||
| 3–4 | 52.55 ± 9.15 (34.61; 70.50) | 24 (17; 39) | 42 (27; 68) | 68 (42; 86) | ||
| 4–5 | 38.67 ± 6.32 (26.27; 51.06) | 22 (19; 33) | 26 (22; 33) | - | ||
| Cytostatic | Mitomycin C | 56.24 ± 7.91 (40.74; 71.74) | 22 (16; 33) | 42 (26; 74) | 86 (50; 87) | χ2 = 0.349 p = 0.554 ES = −0.09 (small) |
| Oxaliplatin | 45.88 ± 6.25 (33.63; 58.13) | 23 (17; 28) | 33 (27; 46) | 63 (41; 67) | ||
| Method | Open | 57.30 ± 6.98 (43.62; 70.98) | 22 (17; 31) | 41 (26; 67) | 86 (57; 87) | χ2 = 1.204 p = 0.272 ES = −0.11 (small) |
| Closed | 35.35 ± 3,59 (28.31; 42.40) | 23 (10; 28) | 33 (28; 50) | 50 (33; 50) | ||
| HIPEC time (min) | 30 | 45.58 ± 6.03 (33.77; 57.40) | 24 (17; 28) | 35 (28; 45) | 62 (41; 68) | χ2 = 0.603 p = 0.740 ES30–60 = 0.09 (small) ESrest = not relevant |
| 45 | 50.91 ± 12.08 (27.25; 74.58) | 16 (10; 39) | 39 (16; 86) | 86 (26; 86) | ||
| 60 | 58.12 ± 9.95 (38.62; 77.63) | 28 (22; 67) | 67 (28; 86) | 86 (50; 86) | ||
| PCI | 0–4 a | 63.14 ± 10.23 (43.10; 83.19) | 25 (17; 45) | 45 (28; 65) | - | χ2 = 9.403 p = 0.024 ES0–4–15&under = −0.10 (small) ESrest = not relevant |
| 5–9 a | 50.21 ± 9.89 (30.83; 69.58) | 17 (10; 28) | 29 (17; 44) | 74 (31; 74) | ||
| 10–14 a | 53.54 ± 6.92 (39.98; 67.10) | 31 (23; 50) | 50 (35; 67) | 68 (50; 86) | ||
| 15 and above b | 21.67 ± 3.19 (15.42; 27.93) | 16 (2; 25) | 19 (16; 26) | 27 (19; 33) | ||
| Group | 3-Year Survival Rate (CI) | 5-Year Survival Rate (CI) | |
|---|---|---|---|
| Method | Open | 0.579 (0.557; 0.600) | 0.390 (0.358; 0.422) |
| Closed | 0.395 (0.349; 0.441) | 0.237 (0.154; 0.330) | |
| Statistics and p-value | χ2 = 1.866 p = 0.172 | χ2 = 1.061 p = 0.303 | |
| Cytostatic | Mitomycin C | 0.563 (0.533; 0.591) | 0.380 (0.337; 0.423) |
| Oxaliplatin | 0.458 (0.428; 0.487) | 0.283 (0.233; 0.336) | |
| Statistics and p-value | χ2 = 0.716 p = 0.397 | χ2 = 0.566 p = 0.452 | |
| HIPEC time (min) | 30–45 | 0.483 (0.463; 0.503) | 0.274 (0.240; 0.308) |
| 60 | 0.587 (0.528; 0.641) | 0.513 (0.444; 0.578) | |
| Statistics and p-value | χ2 = 0.539 p = 0.463 | χ2 = 3.034 p = 0.041 | |
| Variable | Reference Level | β | HR | (95% CI for HR) | Wald Test | p-Value |
|---|---|---|---|---|---|---|
| Radicality | Non-radical | −1.50 | 0.23 | (0.12; 0.44) | 19.00 | <0.001 |
| Platinum resistance | No | 1.79 | 6.01 | (2.95; 12.2) | 18.93 | <0.001 |
| ECOG | 0 | 1.60 | 4.90 | (2.6; 9.4) | 23.00 | <0.001 |
| AGO_score | Negative | −2.10 | 0.12 | (0.057; 0.24) | 34.00 | <0.001 |
| CCR | 1 and higher | 0.99 | 2.71 | (1.37; 5.37) | 7.04 | 0.004 |
| CRS_time | - | 0.01 | 1.00 | (1.00; 1.01) | 0.15 | 0.879 |
| Cytostatic | Mitomycin C | 0.18 | 1.20 | (0.67; 2.1) | 0.37 | 0.550 |
| Method | Closed | −0.34 | 0.71 | (0.38; 1.3) | 1.20 | 0.280 |
| HIPEC_time | - | −0.01 | 0.99 | (0.97; 1) | 0.76 | 0.380 |
| PCI | - | 0.07 | 1.10 | (1; 1.1) | 7.10 | 0.008 |
| Variable | Reference | β ± SE | HR (CI) | -HR | z | p-Value |
|---|---|---|---|---|---|---|
| Radicality | Non-radical | 0.552 ± 0.095 | 1.738 (0.746; 5.942) | 0.576 | 1.076 | 0.282 |
| Platinum resistance | No | 1.274 ± 0.045 | 3.574 (1.476; 8.635) | 0.279 | 2.823 | 0.005 |
| ECOG | 0 | −0.237 ± 0.036 | 0.788 (0.311; 2.000) | 1.269 | −0.500 | 0.617 |
| AGO score | Negative | −2.858 ± 0.027 | 0.057 (0.013; 0.249) | 17.418 | −3.815 | 0.001 |
| CCR | 1 and higher | −0.592 ± 0.043 | 0.553 (0.191; 1.604) | 1.809 | −1.090 | 0.276 |
| PCI | - | 0.045 ± 0.015 | 1.047 (0.987; 1.121) | 0.964 | 1.314 | 0.189 |
| Variable | χ2 | df | p-Value |
|---|---|---|---|
| Radicality | 3.259692 | 1 | 0.071 |
| Platinum resistance | 0.000302 | 1 | 0.986 |
| ECOG | 0.078403 | 1 | 0.779 |
| AGO score | 1.501777 | 1 | 0.220 |
| CCR | 0.197870 | 1 | 0.656 |
| PCI | 1.277033 | 1 | 0.258 |
| GLOBAL | 6.146453 | 6 | 0.407 |
| General KMO | Radicality | ECOG | AGO Score | CRS Time | CCR | Cytostatic | HIPEC Time | PCI | Method | Platinum Resistance |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.492 | 0.455 | 0.490 | 0.540 | 0.581 | 0.653 | 0.423 | 0.445 | 0.567 | 0.073 | 0.038 |
| Variable | PC1 | PC2 | PC3 |
|---|---|---|---|
| Radicality | −0.633 | 0.154 | 0.350 |
| ECOG | 0.565 | −0.019 | −0.463 |
| AGO score | −0.816 | 0.106 | 0.479 |
| CRS time | 0.316 | 0.561 | 0.448 |
| CCR | 0.368 | 0.709 | −0.082 |
| Cytostatic | 0.529 | −0.548 | 0.605 |
| HIPEC time | −0.458 | 0.508 | −0.591 |
| PCI | 0.504 | 0.693 | 0.292 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gęca, K.; Litwiński, J.; Ostrowski, T.; Świetlicka, I.; Polkowski, W.P.; Skórzewska, M. Exploring the Survival Determinants in Recurrent Ovarian Cancer: The Role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Cancers 2024, 16, 2150. https://doi.org/10.3390/cancers16112150
Gęca K, Litwiński J, Ostrowski T, Świetlicka I, Polkowski WP, Skórzewska M. Exploring the Survival Determinants in Recurrent Ovarian Cancer: The Role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Cancers. 2024; 16(11):2150. https://doi.org/10.3390/cancers16112150
Chicago/Turabian StyleGęca, Katarzyna, Jakub Litwiński, Tomasz Ostrowski, Izabela Świetlicka, Wojciech P. Polkowski, and Magdalena Skórzewska. 2024. "Exploring the Survival Determinants in Recurrent Ovarian Cancer: The Role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy" Cancers 16, no. 11: 2150. https://doi.org/10.3390/cancers16112150
APA StyleGęca, K., Litwiński, J., Ostrowski, T., Świetlicka, I., Polkowski, W. P., & Skórzewska, M. (2024). Exploring the Survival Determinants in Recurrent Ovarian Cancer: The Role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Cancers, 16(11), 2150. https://doi.org/10.3390/cancers16112150







