Simple Summary
Oestrogen and progesterone are two sex hormones that are important in the development of cancer of the inner lining of the uterus and endometrial cancer (EC). Oestrogen binds to the oestrogen receptor (ER) and progesterone to the progesterone receptor (PR). The presence of these receptors is important because the response to hormonal therapy is higher when the receptors are present. However, as EC grows and spreads throughout the body, ER and PR may be lost. In this study, we found that tumours that have spread to other organs throughout the blood and tumours that have spread in the abdomen have a relatively high presence of ER/PR, while tumours located in the lymph nodes have a lower presence of PR. ER/PR-IHC were not lower in tumours that had previously been treated with radiotherapy. This might influence the application of hormonal therapy in the future.
Abstract
Background: Response to hormonal therapy in advanced and recurrent endometrial cancer (EC) can be predicted by oestrogen and progesterone receptor immunohistochemical (ER/PR-IHC) expression, with response rates of 60% in PR-IHC > 50% cases. ER/PR-IHC can vary by tumour location and is frequently lost with tumour progression. Therefore, we explored the relationship between ER/PR-IHC expression and tumour location in EC. Methods: Pre-treatment tumour biopsies from 6 different sites of 80 cases treated with hormonal therapy were analysed for ER/PR-IHC expression and classified into categories 0–10%, 10–50%, and >50%. The ER pathway activity score (ERPAS) was determined based on mRNA levels of ER-related target genes, reflecting the actual activity of the ER receptor. Results: There was a trend towards lower PR-IHC (33% had PR > 50%) and ERPAS (27% had ERPAS > 15) in lymphogenic metastases compared to other locations (p = 0.074). Hematogenous and intra-abdominal metastases appeared to have high ER/PR-IHC and ERPAS (85% and 89% ER-IHC > 50%; 64% and 78% PR-IHC > 50%; 60% and 71% ERPAS > 15, not significant). Tumour grade and previous radiotherapy did not affect ER/PR-IHC or ERPAS. Conclusions: A trend towards lower PR-IHC and ERPAS was observed in lymphogenic sites. Verification in larger cohorts is needed to confirm these findings, which may have implications for the use of hormonal therapy in the future.
1. Introduction
Endometrial cancer (EC) is the most frequent malignancy of the female genital tract in industrialised countries [1]. The incidence of EC is increasing due to risk factors such as prolonged life expectancy and obesity [2]. Most women are diagnosed with EC at an early stage, resulting in a favourable prognosis with a 5-year survival rate of 80–90% [3,4]. However, a minority of patients diagnosed with advanced or metastatic EC have a significantly worse outcome, with a 5-year survival rate of only 19% [4]. Up to 20% of patients will develop local recurrence or metastatic disease [3,4,5,6,7]. Curative treatment options for metastatic EC are limited to women who only have locoregional metastases or isolated metastases [1,8,9]. For women with more advanced disease, only palliative options remain and are limited to the following: chemotherapy and hormonal therapy (HT) or novel therapies, including immunotherapy and other targeted treatment options [9,10,11].
Both response rate (RR) and toxicity are important in determining the most suitable systemic therapy for a patient. Chemotherapy with carboplatin/paclitaxel yields an RR of 60% and a progression-free survival of about 13 months. However, it is associated with serious toxicity in at least 35% of patients [1,12,13,14,15,16]. The addition of immunotherapy to chemotherapy in women with mismatch repair-deficient tumours increased for the 12-month progression-free survival from 38% to 74% [17]. However, toxicity was also increased compared with the chemotherapy alone group [17]. Hormonal therapy shows an RR of 20–40% in unselected patients, but in responders, it can provide a durable effect lasting several years with minimal side effects [18,19,20,21,22,23].
Unopposed oestrogen exposure plays a pivotal role in EC development. In the normal endometrium, as well as in endometrial cancer, progesterone counteracts oestrogen-induced proliferation and tumour growth. However, it is currently not completely clear if oestrogen is responsible for tumour growth in all ECs, making the application of progesterone potentially ineffective. Immunohistochemical expression of the oestrogen receptor and progesterone receptor (ER/PR-IHC) is a well-established predictive biomarker for response to hormonal therapy, with the highest RR in patients expressing both ER and PR [19,23,24,25,26]. However, ER-IHC does not necessarily reflect ER activity [27,28]. Besides this, receptor status is still not routinely assessed prior to treatment, and there is no consensus on the optimal cut-off value for hormone receptor positivity (ER/PR) in EC [20,21,29]. In addition, the molecular classification has further challenged the position of hormonal therapy [30,31,32,33].
The Prediction of Response to Hormonal therapy in advanced and recurrent Endometrial Cancer (PROMOTE) study showed an association between the ER, PR and ER pathway activity (ERPAS) and the outcome [24,28]. The response was only observed in patients with a pre-treatment tumour expression of ER/PR > 50%. In the group with a tumour expression of PR > 50% and ERPAS > 15 an RR of 57.6% was achieved. However, there was considerable variability among the samples in terms of tumour location and expression profile.
Primary well-differentiated tumours are typically sensitive to hormonal therapy; however, they may lose their hormone sensitivity over time andin response to applied adjuvant treatment such as radiotherapy and/or chemotherapy [34,35,36,37,38,39]. Changes in hormone receptor expression from primary tumours to metastases have been reported in up to 50% of cases [35,36,37]. As metastases often exhibit heterogeneity compared to the primary tumour, the reassessment of hormone receptor status within metastases is recommended [23,34,35,36]. The analysis of ER/PR-IHC expression and ERPAS at different tumour locations can improve the understanding of cancer spread and help refine patient selection for hormonal therapy [33]. Therefore, we evaluated ER/PR-IHC expression and ERPAS between different tumour locations in advanced and recurrent EC collected in the PROMOTE-study [24].
2. Materials and Methods
2.1. Design and Setting
The current study used samples collected from patients in the PROMOTE-study [24]. Patients were included if they were treated with any type of hormonal therapy for advanced or recurrent EC and an available pretreatment tumour biopsy was required. Concurrent therapy was not permitted, and a minimum of 6 months of documented follow-up after the initiation of hormonal treatment was required. Patients receiving hormonal therapy for other indications, patients receiving adjuvant hormonal therapy, and patients with endometrial (stromal) sarcoma were excluded. All participating centres obtained approval from the institutional review board or national ethics committee approval and obtained patient consent according to local regulations. The Radboudumc Institutional Review Board approval number was 2017-3803.
2.2. Patients
Baseline characteristics, including the patient characteristics age, BMI, and the tumour characteristics histology and grade (grade 1–2: low grade; grade 3: high grade), FIGO stage, and biopsied tumour location, were retrieved from patient records. Primary surgical treatment was classified as hysterectomy with bilateral salpingo-oophorectomy, with or without lymph node dissection. Some patients received (adjuvant) radiotherapy or chemotherapy. Radiotherapy could include either vaginal brachytherapy (VBT) and/or external beam radiotherapy (EBRT). All types of hormonal therapy were included (progestin, tamoxifen, aromatase inhibitor). Details of the hormonal therapy used, including type and dosage, were documented.
2.3. Tumour Locations
All biopsied tumour sites were classified according to the hypothesised route of cancer spread based on the study by Kurra et al. [40] as follows:
- Uterine biopsies from patients with advanced EC (cervix, uterus);
- Lymphogenic tumour location (inguinal lymph node, para-aortic lymph node, pelvic lymph node, supraclavicular lymph node);
- Hematogenous tumour location (liver, lung);
- Intra-abdominal tumour location (colon, peritoneum, omentum);
- Port-site tumour location (surgical scar, umbilical cord);
- Vaginal vault tumour location from recurrent EC patients.
2.4. Immunohistochemical Staining and Scoring
The immunohistochemical (IHC) analysis of ER and PR expression was performed on 4 µm tumour-containing sections of formalin-fixed paraffin-embedded (FFPE) tumour blocks [27]. Additional details, including the antibodies used, are provided in Supplementary Text S1 [24]. Two investigators (WvW and JB) independently assessed the percentage of tumour cells expressing nuclear ERα and PR (Figure 1). In the case of a disagreement, the final score was determined in a consensus meeting. Both reviewers were blinded to the clinical and pathological data.
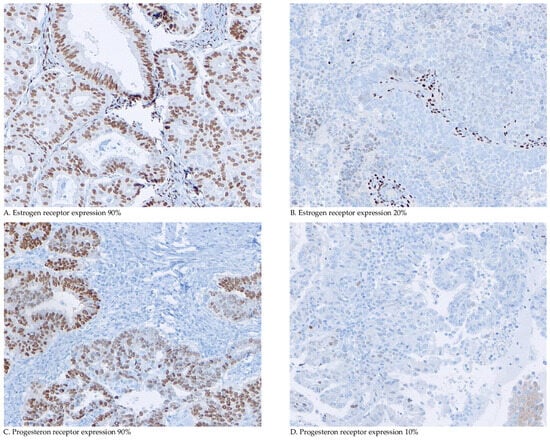
Figure 1.
Microscopic immunohistochemical oestrogen and progesterone receptor staining and scoring. Representative examples of immunohistochemical analyses of oestrogen and progesterone receptor (ER/PR-IHC) and percentage of tumour cells expressing nuclear ERα and PR.
2.4.1. RNA Isolation
The tissue of interest was micro- or macrodissected from two consecutive 10 µm FFPE sections. Subsequently, RNA was then extracted using the miRNeasy FFPE kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and as fully described in Supplementary Text S1.
2.4.2. ER Pathway Activity Test
A knowledge-based Bayesian computational model, previously shown to have predictive value in breast cancer, was used to assess ER pathway activity, as outlined in Supplementary Text S1 and Supplementary Figure S1. [24]. Briefly, the model utilises the mRNA expression levels of ER target genes measured in tissue samples, estimated by InnoSIGN using OncoSIGNalTM (Philips Molecular Pathway Diagnostics, Eindhoven, The Netherlands) to infer the probability of a transcriptionally active ER transcription. A score of 0 indicates the lowest odds of pathway activity, while 100 represents the maximum odds inferred by the model.
Institutional review boards at all participating centres approved the study prior to initiation. In accordance with the code of conduct for the responsible use of human tissue in medical research, patient consent was not required for this study [41].
2.5. Outcome Measures
The primary outcome was defined as ER-IHC and PR-IHC expression and ERPAS according to the route of cancer spread [40]. In the PROMOTE-study, cut-offs for ER-IHC, PR-IHC and ERPAS were defined based on response. This resulted in three different categories for ER-IHC and PR-IHC: ≤10%, >10–50% and >50%. For ERPAS, this resulted in two different categories: ≤15 and >15. [24]
2.6. Statistical Analysis
Clinical and pathological parameters of the different tumour locations were compared using Pearson χ2 for categorical variables, and the Kruskal–Wallis test was used for continuous variables due to non-normal distribution of age, BMI, ER-IHC, PR-IHC and ERPAS in all subgroups, assessed by the Shapiro–Wilk test. Post hoc analysis with Bonferroni correction was performed for significant differences identified by the Chi-square test.
Differences in receptor status and pathway activity between uterine biopsies and other locations, as well as between lymphogenic and other locations, were assessed using Fisher’s exact test with cut-offs of 10% and 50% for ER-IHC and PR-IHC and a cut-off of 15 for ERPAS due to low expected cell counts.
Fisher’s exact test was used to compare immunohistochemistry and ERPAS between the uterine biopsies of advanced EC and metastasis/recurrence at different locations.
Differences in receptor status and pathway activity between the samples with and without prior applied radiotherapy in the vaginal vault samples were analysed using the independent samples t-test or the Mann–Whitney U test, depending on subgroup normality, assessed by the Shapiro–Wilk test.
All tests were two-tailed, and p-values of <0.05 were considered significant. SPSS (version 25.0 for Microsoft, SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
3. Results
3.1. Patients
A total of 105 eligible patients were included in the PROMOTE-study (Figure 2). Due to the exclusion of cases with insufficient tumour tissue (n = 15), biopsies during HT (n = 1), stage I/II EC (n = 1), incomplete IHC analysis (n = 3), and HT were given to sites other than the biopsied site (n = 5), and a total of 80 samples were included for analysis. In two patients with recurrent EC, two different biopsies from two different sites were included.
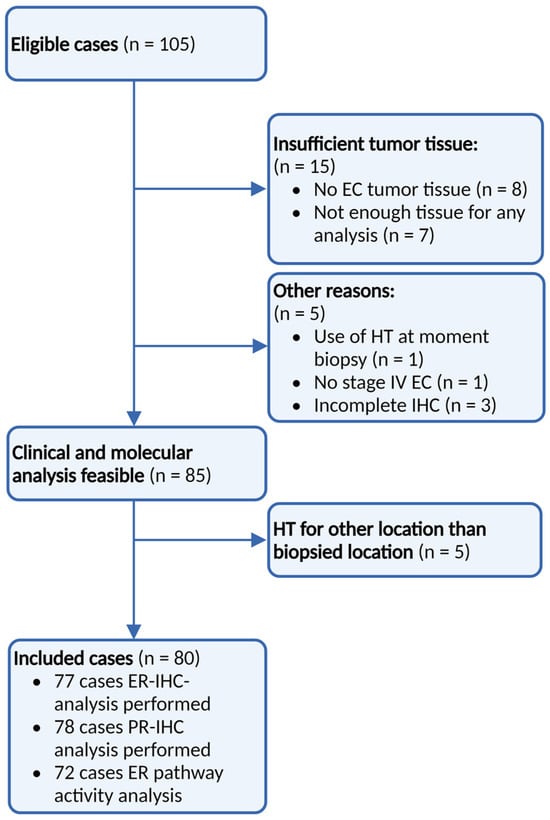
Figure 2.
Patient inclusion. Identification of all women treated with any type of hormonal therapy for advanced and recurrent EC from 2012 up to 2016 using a retrospective search of hospital databases. ER: Oestrogen receptor, PR: progesterone receptor, IHC: immunohistochemistry, and HT: hormonal therapy.
Clinicopathological data of the included patients (n = 80) are shown in Table 1. In total, 54 (67.5%) patients were treated for recurrent EC and 26 (32.5%) for advanced EC. The mean age of all patients was 71.9 years (SD = 9.6), and the mean body mass index (BMI) was 30.4 (SD = 7.8). Most patients (n = 61, 82.4%) were diagnosed with grade 1–2 tumours, and 13 were diagnosed (16.7%) with grade 3 tumours.

Table 1.
Clinicopathological characteristics of included patients. SD: standard deviation, BMI: body mass index, EEC: endometrioid-type endometrial cancer, NEEC: non-endometrioid endometrial cancer, ER: oestrogen receptor (0–100%), PR: progesterone receptor (0–100%), ERPAS: oestrogen receptor pathway activity score (0–100%). Progestin therapy includes the following: medroxyprogesterone acetate and megestrol. Aromatase inhibitors include the following: letrozole, anastrazole and exemestane. p-value: <0.05 is considered significant. * Biopsies from exclusively advanced EC patients. a available for n = 57, b analysis for radiotherapy yes/no, c analysis for chemotherapy yes/no, d analysis for progestin vs. tamoxifen/aromatase inhibitor therapy.
3.2. Samples
The tumour biopsies are classified according to the route of cancer spread, illustrated in Figure 3. Within all tumour locations, 12 biopsies (15%) were uterine biopsies from patients with advanced EC. The remaining 78 biopsies (85%) were derived from tumour locations with advanced stage (n = 14) or recurrent EC (n = 54) with 14 hematogenous (17.5%), 12 lymphogenic (15.0%), and 9 intra-abdominal (11.3%). In addition, 5 biopsies were port-site (6.3%), and 28 were vaginal vault biopsies (35.0%) (exclusively recurrent EC). A total of 33 biopsies were taken from patients who had previously received radiotherapy, either vaginal brachytherapy (VBT) and/or external beam radiation therapy (EBRT). Sixteen biopsies were taken outside of the radiation fields. As a result, 17 biopsies taken from locations within the previously irradiated field (vaginal vault biopsies: n = 16, lymphogenic location: n = 1) were included in this analysis. A total of 63 biopsies from non-irradiated sites were included.
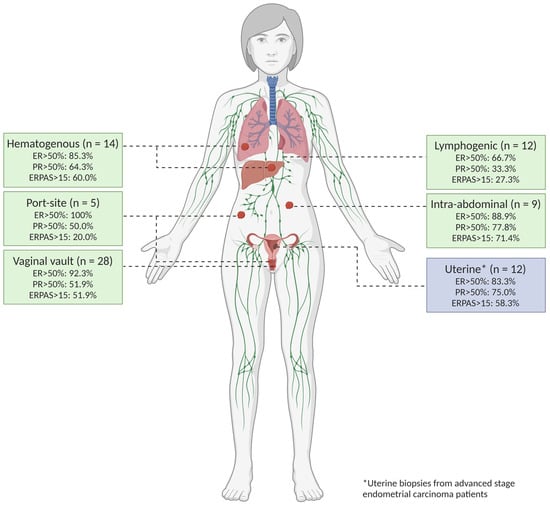
Figure 3.
Tumour locations. All biopsied tumour locations were classified according to the hypothesised route of spread based on an article by Kurra et al. [40].
3.3. Expression of ER/PR-IHC and ER Pathway in Relation to Pattern of Spread
Within the cohort, ER-IHC expression was assessed in 77 biopsies, with PR-IHC in 78 biopsies and ERPAS analysis in 72 biopsies. Within all biopsies, the mean ER-IHC expression was 77.3% (n = 77), PR-IHC 49.5% (n = 78) and ERPAS 16.2% (n = 72) (Table 1). The results of the ER/PR-IHC and ERPAS analysis are illustrated in Figure 4.
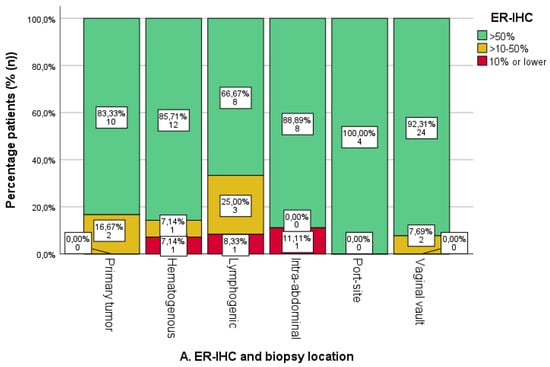
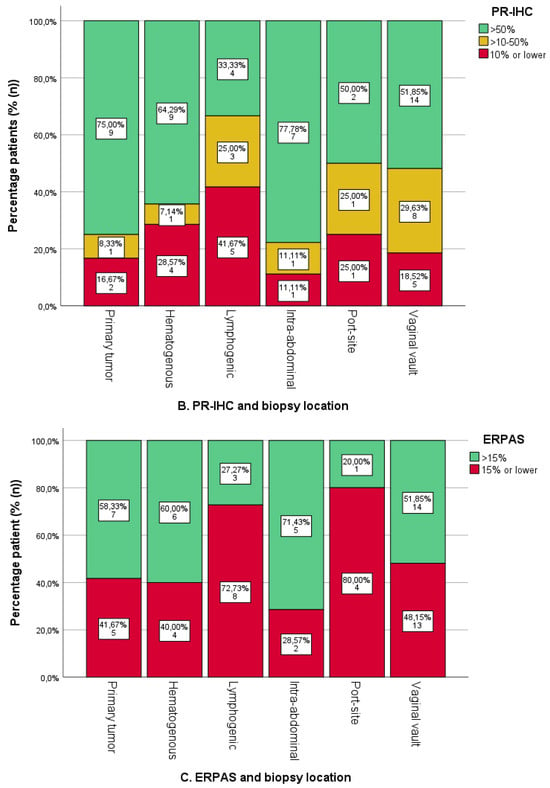
Figure 4.
Oestrogen (ER) and progesterone receptor (PR) immunohistochemistry (IHC) categories and ER pathway activity score categories per sampled tumour location. The number and percentage of patients in each category are shown on the labels. (A). ER-IHC expression was categorised as ≤10%, 10–50%, and >50% in relation to tumour location. (B). PR-IHC expression categorised as ≤10%, 10–50%, and >50% in relation to tumour location. (C). ER pathway activity score with cut-off >15 (as the previously identified optimal cut-off value in the PROMOTE study [24]). * Uterine biopsies from patients with advanced-stage endometrial cancer.
The ER-IHC expression was generally high in both uterine and metastatic locations (Figure 4A). An absence of ER-IHC expression was found in three cases of hematogenous, lymphogenic and intra-abdominal biopsies, respectively. Lymphogenic locations showed the highest proportion of ER-IHC expression <50%, yet differences were not significant.
PR-IHC expression was high in uterine biopsies, with 75.0% (n = 9/12) of cases showing >50% expression (Figure 4B). Among the other locations, lymphogenic locations had the highest proportion of <50% PR-IHC expression, but this was not significantly different from all other biopsies combined (p = 0.074). Intra-abdominal and hematogenous locations had the highest percentage of PR-IHC > 50% (71.4%, n = 5/7 and 69.2%, n = 9/13, respectively).
A total of 58.3% of uterine biopsies were ERPAS > 15 (Figure 4C). Lymphogenic sites were ERPAS-positive in 27.3% (n = 3/11) with port-site locations in 20.0% (n = 1/5). This was lower compared to other locations, although not significant (p = 0.294). ERPAS > 15 was the highest in intra-abdominal (71.4%, n = 5/7) and hematogenous locations (60%, n = 6/10).
3.4. Expression of ER/PR-IHC and ER Pathway Activity in Relation to Tumour Grade
ER-IHC and PR-IHC expression was stratified by grade in all non-uterine locations (n = 63/74, 85.1%), as shown in Figure 5A,B. There were no differences in ER/PR-IHC expression between low- and high-grade EC. However, the numbers for high grades were relatively low (ER-IHC n = 7/60, PR-IHC n = 7/61). The ERPAS was comparable for low- and high-grade tumours, ERPAS > 15 in 50.0% (n = 24/48) and 28.6% (n = 2/7), respectively (X2 p = 0.289) (Figure 5C).
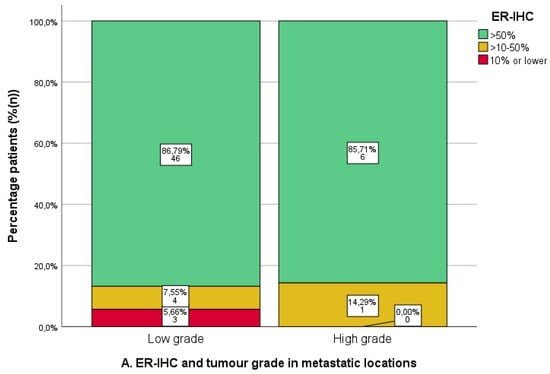
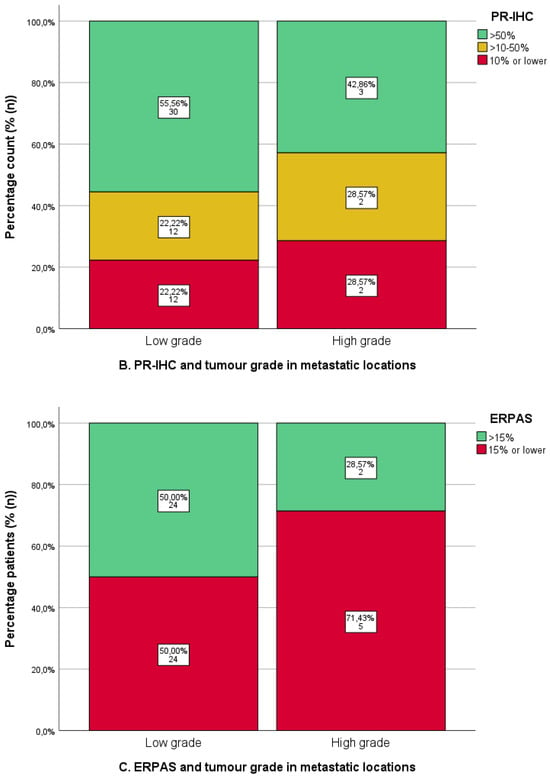
Figure 5.
Immunohistochemical analysis of oestrogen (ER) and progesterone receptor (PR) immunohistochemistry (IHC) and ER -pathway activity score in metastatic locations in relation to tumour grade. Low grade: tumour grade 1 or 2. High grade: tumour grade 3. (A). ER-IHC expression categorised as ≤10%, 10–50%, and >50%. (B). PR-IHC expression categorised as ≤10%, 10–50%, and >50%. (C). ER pathway activity score with cut-off >15 (as the previously identified optimal cut-off value in the PROMOTE study [24]).
Although not significant, tumour biopsies from the lymphogenic locations tended to have higher-grade tumours, with 36.4% (n = 4/11) having grade 3 tumours, of which only 50% (n = 2/4) had PR-IHC > 50% and 33.3% (n = 1/3) had ERPAS > 15 (Supplementary Table S1).
Analysis of endometrioid endometrial cancer (EEC) versus non-endometrioid endometrial cancer (NEEC) was not possible due to the small number of NEEC biopsies (n = 4/80, 5.0%).
3.5. Previous Radiotherapy and IHC
As shown in Figure 5, there was no difference in ER-IHC between samples with and without prior RT (Figure 6A). PR-IHC > 50% occurred in 43.8% of samples from previously irradiated samples (n = 7/16) compared to 61.3% of non-irradiated samples (n = 38/62) (X2 p = 0.329) (Figure 6B). Similarly, ERPAS > 15 was present in 43.8% of previously irradiated samples (n = 7/16) compared to 51.8% of non-irradiated locations (n = 29/56) (X2 p = 0.571) (Figure 6C). These differences were not significant.

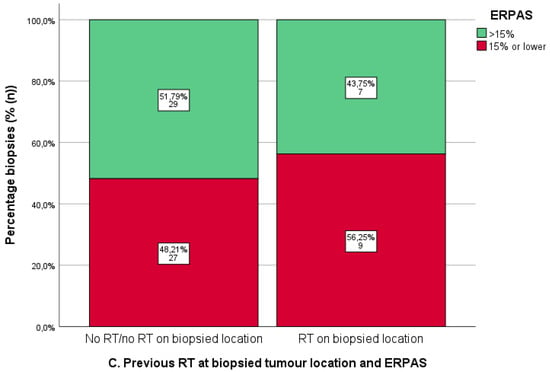
Figure 6.
Immunohistochemistry for oestrogen receptor (ER-IHC) (A) and progesterone receptor (PR) (B) and oestrogen receptor pathway activity score (ERPAS) (C) in tumour locations with and without prior radiotherapy (RT). Predetermined cut-offs of ER-IHC and PR-IHC (>50%, >10–50%, ≤10%) and ERPAS > 15 are shown by the different categories (as the previously identified optimal cut-off value in the PROMOTE study [24]).
Figure 6: Radiotherapy at tumour locations with and without prior RT and ER/PR-IHC and ERPAS.
4. Discussion
The primary objective of this study was to evaluate ER/PR-IHC expression and ER pathway activity (ERPAS) in different tumour locations in a cohort of women treated with hormonal therapy [24]. Overall, ER/PR-IHC expression was high at different tumour locations. Interestingly, lymphogenic locations showed the lowest PR-IHC expression and ERPAS activity of all locations, although these differences were not significant. There was no difference in ER/PR-IHC expression or ERPAS between low- and high-grade EC. Finally, prior radiotherapy did not affect ER/PR-IHC or ERPAS results.
Previous studies have shown that ER-IHC and PR-IHC are lower in metastases than in primary tumours [36,42]. In one study, PR-IHC < 10% occurred significantly more commonly in EEC metastases than in primary tumours, occurring in 65% and 14% of tumours, respectively [35]. In non-endometrioid endometrial cancer (NEEC), PR-IHC loss was seen in 65% of primary tumours and 94% of metastatic tumours [35]. Finally, the loss of ER/PR-IHC in pre-operative endometrial biopsies has been reported to be associated with lymphogenic tumour spread [5]. The distant metastases of EECs showed significantly lower ER-IHC expression than intra-abdominal metastases [36,42]. Although metastases may be heterogeneous compared to the primary tumour, 75% of patients with multiple metastatic lesions show homogeneous PR-IHC expression in all metastases, whereas 25% of cases show the heterogeneous loss of PR-IHC in at least one lesion [35].
4.1. Metastatic Locations and Immunohistochemical Expression
In our study, a relatively high overall percentage of ER/PR-IHC was present in metastatic sites. Lymphogenic locations appeared to have lower PR-IHC expression and ERPAS, whereas port-site locations showed average levels of PR-IHC > 50% and ERPAS > 15% was very low. On the other hand, hematogenous and intra-abdominal locations appeared to have higher PR-IHC and ERPAS. Since no differences in the proportions of endometrioid and non-endometrioid, or in low-grade and high-grade tumours between these groups, were found, we hypothesise that differences in PR-IHC and ERPAS may reflect different pathways of tumour progression and metastasis.
Loss of PR-IHC during cancer progression is known to correlate with markers of aggressive disease and predicts poor survival [35,43]. Progesterone counteracts oestrogen-induced effects, such as the promotion of proliferation and inhibition of apoptosis and the modulation of the tumour suppressor function. Due to its strong tumour suppressor function, the loss of PR-IHC has been shown to play a role in epithelial-to-mesenchymal-transition (EMT) [44]. EMT is an important driver of cancer progression as it increases the invasive and migratory capacity of cancer cells [43,44,45]. In non-progressive EC, intact progesterone signalling appears to be an important factor in stimulating immunosuppression and inhibiting EMT [45]. Furthermore, the lower expression of PR-IHC in metastases compared to the corresponding primary tumour suggests a role for PR-IHC loss in EMT [35,42].
The activity of the ER pathway, which has been extensively studied in breast cancer, is relevant as it is associated with ER/PR expression by analysing the downstream RNAs of ER-related target genes from which the actual oestrogen-induced tumour growth can be inferred [27,28]. However, only a subset of ER-positive tumours actually has high ER pathway activity, and levels of ER-IHC and ER pathway activity are poorly correlated [27,28]. Therefore, the ER-IHC is not a sufficiently specific biomarker of functional ER pathway activity [27,28]. By contrast, the ER pathway activity has shown a good correlation with PR-IHC, suggesting an association between PR negativity and the inactivation of oestrogen-responsive genes in tumour progression [24]. Furthermore, ERPAS was found to be lower in patients with stage 1 EEC who developed a recurrence compared to stage 1 EEC cases without a recurrence, suggesting an association between ERPAS inactivation and tumour progression [24,27,46]. Similar to breast cancer, low ERPAS is associated with adverse outcomes [32].
We observed the lowest PR-IHC and ERPAS expression within lymphogenic and port-site locations, in line with the literature, suggesting an association with EMT. Although not significant, tumour biopsies from the lymphogenic locations tended to have higher-grade tumours, which may partially explain the reduced hormone receptor expression profiles. The loss of PR-IHC and ERPAS may reflect the involvement of EMT in lymphogenic locations or be related to dedifferentiation. It is known that dedifferentiation can occur in recurrent EC, leading to higher tumour grade and the loss of PR-IHV expression [34].
However, the loss of PR-IHC and ERPAS is not universally present in all metastases, as suggested by higher levels of PR-IHC and ERPAS in hematogenous and intra-abdominal locations. This may suggest that EMT is not the only mechanism driving metastases or that EMT occurs in these lesions without PR-IHC and ERPAS loss.
4.2. Radiotherapy and ER/PR-IHC and ERPAS
Both EBRT and VBT are frequently applied in EC as adjuvant therapy for local control [7,47] or with curative intent in isolated vaginal vault recurrences [48,49]. Due to the retrospective nature of this study and unclear radiation fields, this analysis mainly analysed vaginal vault biopsies. When comparing ER/PR-IHC and ERPAS between irradiated and non-irradiated biopsies, no significant differences were observed. There is limited literature on endometrial activity or changes in IHC after radiotherapy. Soslow et al. suggested that adjuvant RT does not alter hormone receptor expression in EC, which is consistent with the results of this study [34]. Furthermore, some residual functional endometrium was retained in cervical cancer treated with curative radiotherapy [50]. This implies that the application of HT can be considered either before or after RT, depending on ER/PR-IHC, which is also in line with our findings.
4.3. Strengths and Weaknesses
This study is the first analysis of ER/PR-IHC expression and ERPAS at different tumour locations in patients with advanced and recurrent disease. Inherent to the retrospective character of this study, there are some limitations that need to be addressed. As the metastatic and recurrent tumour biopsies were not matched to the primary tumour, comparisons within individual patients were not feasible.
Due to the inclusion criteria of the PROMOTE-study, in which only patients receiving hormonal therapy were eligible, this cohort represents a selection of women with advanced stage or recurrent EC. As a result, this cohort is characterised by relatively high ER/PR-IHC expression and a relatively higher number of EECs. Only 13% of the biopsies were high-grade tumours, compared to 40–50% reported in the literature [35]. The limited number of cases included in this study hindered the power of the subgroup analysis to draw significant conclusions. In addition, biopsies were taken from the most accessible tumour location and, therefore, a small number of samples from more difficult locations such as the lung, liver and lymph nodes could be included. However, this reflects daily clinical practice. Finally, variability in the diagnostic process, treatment and follow-up amongst patients further complicates interpretation. The relationship between ER/PR-IHC end ERPAS, tumour location and response to HT would have been a good addition to this study but was hampered by the RECIST criteria (Response evaluation criteria in solid tumors), which only described the target lesion. Data on the response of the specific biopsied location were, therefore, incomplete and not included in this study.
4.4. Clinical and Future Perspectives
The efficacy of hormonal therapy depends mainly on the percentage of PR-IHC expression in the tumour [19,24,25,26]. Therefore, it is important to acquire a biopsy from a metastatic site prior to initiating hormonal therapy to assess receptor status, as changes in receptor status may have occurred during the metastatic process. Based on the results of this study, it appears that lymphogenic metastases may have lower PR-IHC expression and ERPAS, potentially resulting in the lower efficacy of HT.
However, receptor status is not routinely assessed prior to the initiation of therapy. Typically, tumour biopsies are obtained from the most accessible location, often the uterus, in advanced EC. In lymphogenic locations, if HT is the preferred option, a lymphogenic biopsy is indicated, although this can be challenging due to anatomical difficulties (blood vessels, lymph nodes). In addition, the reliance on a single target lesion for the response assessment according to the RECIST guidelines prompts consideration of whether the biopsy should be obtained from this specific target lesion. Consideration should be given to obtaining biopsies from multiple locations, as a single biopsy may not reflect ER/PR-IHC and ERPAS from all metastases. This approach ensures the histological confirmation of advanced disease and clarifies potential tumour heterogeneity at metastatic sites, particularly in cases of lymphatic spread, where PR-IHC loss is more common. Less invasive methods, such as 18F-fluoroestradiol-PET (FES-PET), are also being investigated and may provide a solution in difficult biopsy locations, such as lymphogenic locations.
The prospective analysis of ER/PR-ICH and ERPAS by tumour location and their correlation with treatment response is essential to improve the selective use of hormonal therapy in advanced and recurrent EC. In addition, ERPAS has been shown to be useful in predicting response to hormonal therapy and in identifying hormone-driven tumour growth in metastatic lesions, justifying its inclusion alongside IHC assessment [24].
These recommendations will be incorporated into the PROMOTE-Prospective study, which aims to improve predictive biomarkers and patient selection in advanced and recurrent EC. By tailoring the use of hormonal therapy based on refined clinical and molecular criteria rather than solely on the experience of the treating physician, the use of hormonal therapy in EC can be optimised, resulting in greater cost-effectiveness.
5. Conclusions
In this study, an overall high expression of ER/PR and ERPAS was observed across different tumour locations. Comparing all tumour locations, there was a trend towards lower expression in lymphogenic locations compared to other locations. This potentially reflects the different mechanisms of cancer spread and may indicate reduced sensitivity to hormonal therapy. ER/PR-IHC expression was not significantly affected by tumour grade or previous radiotherapy. Larger studies are needed to confirm the differences in hormone receptor presence and activity between tumour locations and possible implications for endometrial cancer treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16112084/s1, Figure S1: Schematic representation of ER pathway model network; Text S1: Complete protocol for IHC expression procedure and RNA isolation; Table S1: Tumour grade, immunohistochemistry analysis, ER pathway activity score and tumour locations.
Author Contributions
Conceptualisation: J.M.A.P., M.M.W.L. and W.J.v.W.; Methodology M.M.W.L. and W.J.v.W.; Formal analysis, M.M.W.L., W.J.v.W., D.M.K., A.v.d.S., A.R. and J.M.A.P.; Investigation: J.M.A.P., M.M.W.L., W.J.v.W., J.B., D.M.K. and A.v.d.S.; Resources: R.I.L., K.L., H.J.v.B., H.T., D.B., H.M.J.W., L.R.C.W.v.L., R.Y., C.K., P.O.W., K.G., A.A.v.G., E.B., V.W., S.S. and A.G.Z.E.; Data curation, M.M.W.L. and W.J.v.W.; Original draft preparation: M.M.W.L.; Writing: M.M.W.L., W.J.v.W. and J.M.A.P.; Review and editing: M.M.W.L., W.J.v.W., R.I.L., J.B., K.L., H.J.v.B., H.T., D.B., H.M.J.W., L.R.C.W.v.L., R.Y., C.K., P.O.W., K.G., A.A.v.G., E.B., V.W., S.S., A.G.Z.E., D.M.K., A.v.d.S., A.R. and J.M.A.P.; Visualisation, M.M.W.L.; Supervision: W.J.v.W. and J.M.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
InnoSIGN performed the ER pathway analysis free of charge. InnoSIGN was not granted access to patient details and did not have a role in the interpretation or representation of the results.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board and/or national Ethics Committee of all participating centres (Radboud university medical centre, Rijnstate hospital, Canisius Wilhelmina Hospital, Maastricht University Medical Centre+, Oslo University Hospital, Erasmus Medical Centre Rotterdam, Netherlands Cancer Institute, Catharina Hospital, Amsterdam University Medical Centres, University Medical Center Groningen, Haukeland University Hospital, University Medical Center Utrecht, Royal Cornwall Hospital NHS Trust, ASST Spedali Civili di Brescia, Masaryk Unisersity and University Hospital Brno) (The Radboudumc Institutional Review Board protocol code 2017-3803).
Informed Consent Statement
Patient consent was waived due to the retrospective design of the study. The Radboudumc Institutional Review Board protocol code is as follows: 2017-3803.
Data Availability Statement
The raw data of this study can be made available upon reasonable request. Please contact the authors for requests.
Acknowledgments
The authors would like to thank the donation of Annelise Bråthen which was used to process the tissue blocks originating from Oslo University Hospital. European Network for Individualized Treatment in Endometrial Cancer (ENITEC) supported the collaboration for the current study and facilitated the exchange of data and samples according to the General Data Protection Regulation (GDPR) within Europe.
Conflicts of Interest
Diederick Keizer and Anja van de Stolpe are currently or have been employed by InnoSIGN. The other authors have no potential conflicts of interest.
References
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. Cancer of the corpus uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 37–50. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Uterine Cancer. Surveillance, Epidemiology, and End Results Program Cancer Statistics Review 2014–2020. Available online: https://seer.cancer.gov/statfacts/html/corp.html#.Xx63P5vaNyU.link (accessed on 27 May 2024).
- Trovik, J.; Wik, E.; Werner, H.M.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur. J. Cancer 2013, 49, 3431–3441. [Google Scholar] [CrossRef]
- Van der Putten, L.J.; Visser, N.C.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. L1CAM expression in endometrial carcinomas: An ENITEC collaboration study. Br. J. Cancer 2016, 115, 716–724. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; Nout, R.A.; Lybeert, M.L.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, E.; van de Poll-Franse, L.V.; van Putten, W.L.; et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e631–e638. [Google Scholar] [CrossRef] [PubMed]
- Rütten, H.; Verhoef, C.; van Weelden, W.J.; Smits, A.; Dhanis, J.; Ottevanger, N.; Pijnenborg, J.M.A. Recurrent Endometrial Cancer: Local and Systemic Treatment Options. Cancers 2021, 13, 6275. [Google Scholar] [CrossRef]
- Tronconi, F.; Nero, C.; Giudice, E.; Salutari, V.; Musacchio, L.; Ricci, C.; Carbone, M.V.; Ghizzoni, V.; Perri, M.T.; Camarda, F.; et al. Advanced and recurrent endometrial cancer: State of the art and future perspectives. Crit. Rev. Oncol. Hematol. 2022, 180, 103851. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Mahdi, H.; Chelariu-Raicu, A.; Slomovitz, B.M. Immunotherapy in endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 351–357. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Radiother. Oncol. 2015, 117, 559–581. [Google Scholar] [CrossRef]
- Hoskins, P.J.; Swenerton, K.D.; Pike, J.A.; Wong, F.; Lim, P.; Acquino-Parsons, C.; Lee, N. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: A phase II study. J. Clin. Oncol. 2001, 19, 4048–4053. [Google Scholar] [CrossRef]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef]
- Pectasides, D.; Xiros, N.; Papaxoinis, G.; Pectasides, E.; Sykiotis, C.; Koumarianou, A.; Psyrri, A.; Gaglia, A.; Kassanos, D.; Gouveris, P.; et al. Carboplatin and paclitaxel in advanced or metastatic endometrial cancer. Gynecol. Oncol. 2008, 109, 250–254. [Google Scholar] [PubMed]
- Arimoto, T.; Nakagawa, S.; Yasugi, T.; Yoshikawa, H.; Kawana, K.; Yano, T.; Taketani, Y. Treatment with paclitaxel plus carboplatin, alone or with irradiation, of advanced or recurrent endometrial carcinoma. Gynecol. Oncol. 2007, 104, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Lentz, S.S.; Brady, M.F.; Major, F.J.; Reid, G.C.; Soper, J.T. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. 1996, 14, 357–361. [Google Scholar] [CrossRef]
- Thigpen, J.T.; Brady, M.F.; Alvarez, R.D.; Adelson, M.D.; Homesley, H.D.; Manetta, A.; Soper, J.T.; Given, F.T. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: A dose-response study by the Gynecologic Oncology Group. J. Clin. Oncol. 1999, 17, 1736–1744. [Google Scholar] [CrossRef]
- Ethier, J.L.; Desautels, D.N.; Amir, E.; MacKay, H. Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol. Oncol. 2017, 147, 158–166. [Google Scholar] [CrossRef]
- Mahdi, H.; Ray-Coquard, I.; Lorusso, D.; Mirza, M.R.; Monk, B.J.; Slomovitz, B. Evolving treatment paradigms in metastatic or recurrent low-grade endometrial cancer: When is hormonal-based therapy the preferred option? Int. J. Gynecol. Cancer 2023, 33, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, W.J.; Massuger, L.F.A.G.; Pijnenborg, J.M.A.; Romano, A.; ENITEC. Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- van Weelden, W.J.; Lalisang, R.I.; Bulten, J.; Lindemann, K.; van Beekhuizen, H.J.; Trum, H.; Boll, D.; Werner, H.M.J.; van Lonkhuijzen, L.R.C.W.; Yigit, R.; et al. Impact of hormonal biomarkers on response to hormonal therapy in advanced and recurrent endometrial cancer. Am. J. Obstet. Gynecol. 2021, 225, 407.e1-407.e16. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, T.; Brady, M.F.; Homesley, H.D.; Soper, J.T.; Bell, J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J. Clin. Oncol. 2001, 19, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Decruze, S.B.; Green, J.A. Hormone therapy in advanced and recurrent endometrial cancer: A systematic review. Int. J. Gynecol. Cancer. 2007, 17, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Verhaegh, W.; van Ooijen, H.; Inda, M.A.; Hatzis, P.; Versteeg, R.; Smid, M.; Martens, J.; Foekens, J.; van de Wiel, P.; Clevers, H.; et al. Selection of personalized patient therapy through the use of knowledge-based computational models that identify tumor-driving signal transduction pathways. Cancer Res. 2014, 74, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, W.J.; van der Putten, L.J.M.; Inda, M.A.; van Brussel, A.; Snijders, M.; Schriever, L.M.M.; Bulten, J.; Massuger, L.F.A.G.; van de Stolpe, A.; Pijnenborg, J.M.A. Oestrogen receptor pathway activity is associated with outcome in endometrial cancer. Br. J. Cancer 2020, 123, 785–792. [Google Scholar] [PubMed]
- Singh, M.; Zaino, R.J.; Filiaci, V.J.; Leslie, K.K. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2007, 106, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Sommeijer, D.W.; Sjoquist, K.M.; Friedlander, M. Hormonal treatment in recurrent and metastatic gynaecological cancers: A review of the current literature. Curr. Oncol. Rep. 2013, 15, 541–548. [Google Scholar] [CrossRef]
- Kokka, F.; Brockbank, E.; Oram, D.; Gallagher, C.; Bryant, A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2010, 12, CD007926. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, J.M.A.; van Weelden, W.J.; Reijnen, C.; Xanthoulea, S.; Romano, A. Redefining the Position of Hormonal Therapy in Endometrial Cancer in the Era of Molecular Classification. J. Clin. Oncol. 2024, 42, 8–12. [Google Scholar] [CrossRef]
- Soslow, R.A.; Wethington, S.L.; Cesari, M.; Chiappetta, D.; Olvera, N.; Shia, J.; Levine, D.A. Clinicopathologic analysis of matched primary and recurrent endometrial carcinoma. Am. J. Surg. Pathol. 2012, 36, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Tangen, I.L.; Werner, H.M.; Berg, A.; Halle, M.K.; Kusonmano, K.; Trovik, J.; Hoivik, E.A.; Mills, G.B.; Krakstad, C.; Salvesen, H.B. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur. J. Cancer 2014, 50, 3003–3010. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, C.; Monteiro-Reis, S.; Vieira, R.; Pereira, A.; Rodrigues, M.; Jeronimo, C.; Lopes, J.M. Endometrial Endometrioid Carcinoma Metastases Show Decreased ER-Alpha and PR-A Expression Compared to Matched Primary Tumors. PLoS ONE 2015, 10, e0134969. [Google Scholar]
- Runowicz, C.D.; Nuchtern, L.M.; Braunstein, J.D.; Jones, J.G. Heterogeneity in hormone receptor status in primary and metastatic endometrial cancer. Gynecol. Oncol. 1990, 38, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.J.; Hoivik, E.A.; Halle, M.K.; Taylor-Weiner, A.; Cherniack, A.D.; Berg, A.; Holst, F.; Zack, T.I.; Werner, H.M.; Staby, K.M.; et al. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat. Genet. 2016, 48, 848–855. [Google Scholar] [PubMed]
- Slomovitz, B.M.; Filiaci, V.L.; Walker, J.L.; Taub, M.C.; Finkelstein, K.A.; Moroney, J.W.; Fleury, A.C.; Muller, C.Y.; Holman, L.L.; Copeland, L.J.; et al. A randomized phase II trial of everolimus and letrozole or hormonal therapy in women with advanced, persistent or recurrent endometrial carcinoma: A GOG Foundation study. Gynecol. Oncol. 2022, 164, 481–491. [Google Scholar]
- Kurra, V.; Krajewski, K.M.; Jagannathan, J.; Giardino, A.; Berlin, S.; Ramaiya, N. Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging 2013, 13, 113–122. [Google Scholar] [CrossRef]
- FEDERA. Human Tissue and Medical Research: Code of Conduct for Responsible Use 2011. Available online: https://www.coreon.org/wp-content/uploads/2020/04/coreon-code-of-conduct-english.pdf (accessed on 31 July 2020).
- Geels, Y.P.; van der Putten, L.J.M.; van Tilborg, A.A.G.; Nienhaus, B.E.C.; van den Berg-van Erp, S.H.; Snijders, M.P.L.M.; van der Wurff, A.; Massuger, L.F.A.G.; Bulten, J.; Pijnenborg, J.M.A. Immunohistochemical Profiles of Endometrioid Endometrial Carcinomas with and Without Metastatic Disease. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 173–179. [Google Scholar] [CrossRef]
- Van der Putten, L.J.M.; Visser, N.C.M.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. Added Value of Estrogen Receptor, Progesterone Receptor, and L1 Cell Adhesion Molecule Expression to Histology-Based Endometrial Carcinoma Recurrence Prediction Models: An ENITEC Collaboration Study. Int. J. Gynecol. Cancer 2018, 28, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Makker, A.; Goel, M.M. Tumor progression, metastasis, and modulators of epithelial-mesenchymal transition in endometrioid endometrial carcinoma: An update. Endocr. Relat. Cancer 2016, 23, R85–R111. [Google Scholar] [CrossRef]
- van der Horst, P.H.; Wang, Y.; Vandenput, I.; Kuhne, L.C.; Ewing, P.C.; van Ijcken, W.F.; van der Zee, M.; Amant, F.; Burger, C.W.; Blok, L.J. Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS ONE 2012, 7, e30840. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.A.; Blok, E.J.; Kuppen, P.J.K.; Charehbili, A.; den Biezen-Timmermans, E.C.; van Brussel, A.; Fruytier, S.E.; Meershoek-Klein Kranenbarg, E.; Kloet, S.; van der Burg, B.; et al. Estrogen Receptor Pathway Activity Score to Predict Clinical Response or Resistance to Neoadjuvant Endocrine Therapy in Primary Breast Cancer. Mol. Cancer Ther. 2020, 19, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van de Steen-Banasik, E.; et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000, 355, 1404–1411. [Google Scholar] [CrossRef]
- Nout, R.A.; Smit, V.T.; Putter, H.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Kroese, M.C.; et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- De Hullu, J.A.; Pras, E.; Hollema, H.; van der Zee, A.G.; Bogchelman, D.H.; Mourits, M.J. Presentations of endometrial activity after curative radiotherapy for cervical cancer. Maturitas 2005, 51, 172–176. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).