Cancer-Associated Thrombosis: Pathophysiology, Laboratory Assessment, and Current Guidelines
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Risk Factors
3.1. Patient-Specific Risk Factors
3.2. Treatment-Associated Factors
3.3. Cancer-Specific Risk Factors
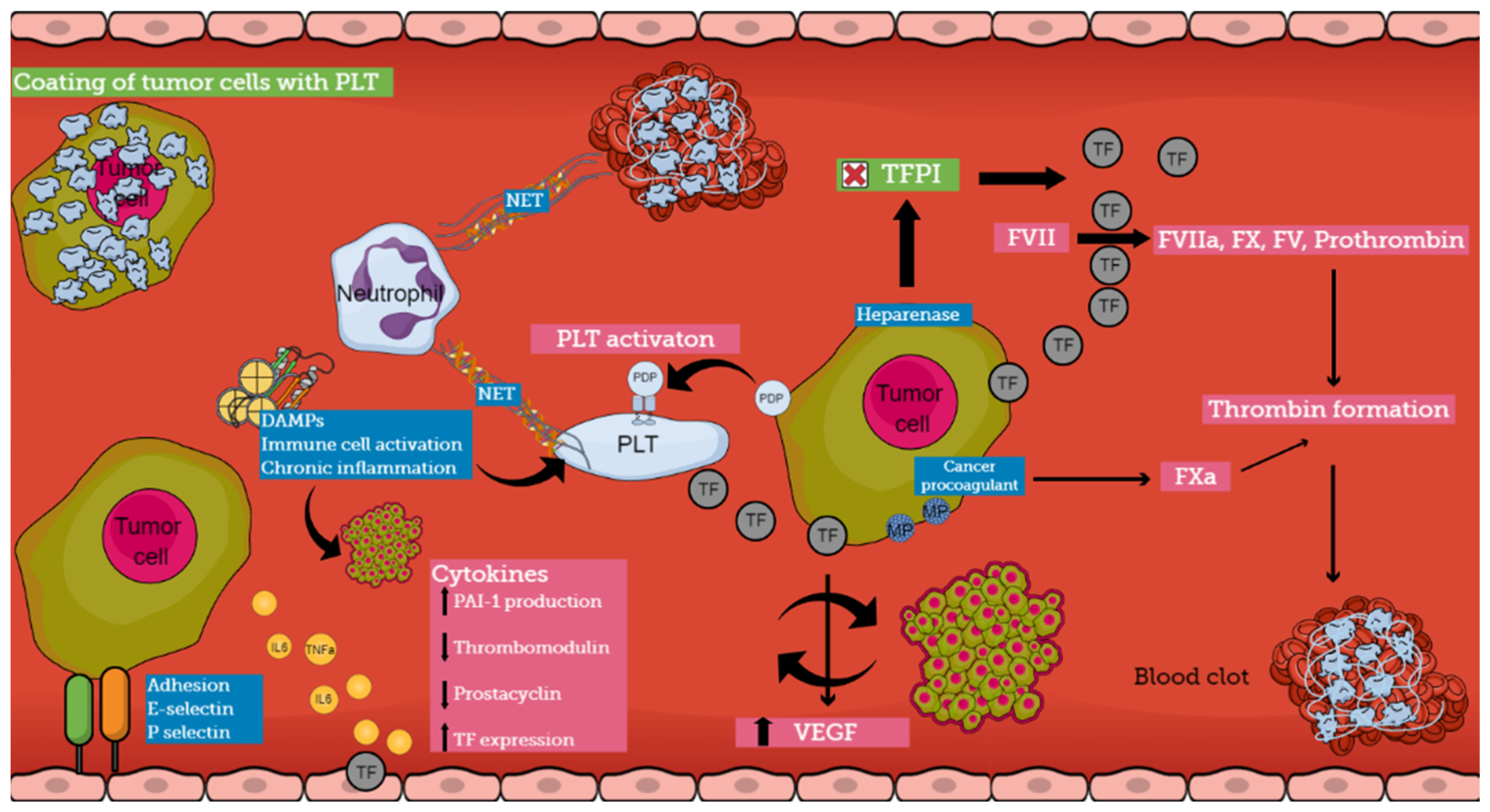
4. Pathogenesis
4.1. Direct Mechanisms of CAT
4.2. Indirect Mechanisms of CAT
5. Laboratory Assessment
6. Treatment of Cancer-Associated Thrombosis
7. Risk Assessment Models
8. Current Guidelines
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Levi, M. Cancer-related coagulopathies. Thromb. Res. 2014, 133, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Falanga, A.; Piccioli, A. Cancer and venous thromboembolism. Lancet Oncol. 2005, 6, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-associated thrombosis: An overview of mechanisms, risk factors and treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Akindo, D.B.; Ajayi, O.I. Thrombotic pathogenesis and laboratory diagnosis in cancer patients, an update. Int. J. Gen. Med. 2023, 16, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Schieppati, F.; Russo, D. Cancer tissue procoagulant mechanisms and the hypercoagulable state of patients with cancer. Semin. Thromb. Hemost. 2015, 41, 756–764. [Google Scholar] [PubMed]
- Guntupalli, S.R.; Spinosa, D.; Wethington, S.; Eskander, R.; Khorana, A.A. Prevention of venous thromboembolism in patients with cancer. BMJ 2023, 381, e072715. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S. Cancer associated thrombosis: Risk factors and outcomes. Thromb. Res. 2016, 140, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Moik, F.; Ay, C. Hemostasis and cancer: Impact of hemostatic biomarkers for the prediction of clinical outcomes in patients with cancer. J. Thromb. Haemost. 2022, 20, 2733–2745. [Google Scholar] [CrossRef]
- Mulder, F.I.; Horvāth-Puhộ, E.; van Es, N.; van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Büller, H.R.; Sørensen, H.T. Venous thromboembolism in cancer patients: A population-based cohort study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef]
- Connolly, G.C.; Khorana, A.A. Emerging risk stratification approaches to cancer-associated thrombosis: Risk factors, biomarkers and a risk score. Thromb. Res. 2010, 125, S1–S7. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Int. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef]
- Ay, C.; Vormittag, R.; Dunkler, D.; Simanek, R.; Chiriac, A.I.; Drach, J.; Quehenberger, P.; Wagner, O.; Zielinski, C.; Pabinger, I. D-dimer and prothrombin fragment 1,2 predict venous thromboembolism in patients with cancer: Results from Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2009, 27, 4124–4129. [Google Scholar] [CrossRef]
- Wun, T.; White, R.H. Venous thromboembolism (VTE) in patients with cancer: Epidemiology and risk factors. Cancer Investig. 2009, 27, 63–74. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Fischer, R.I.; Kudeter, N.M.; Lyman, G.H. Thromboembolism in hospitalized neutropenic cancer patients. J. Clin. Oncol. 2006, 24, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Mandalā, M.; Reni, M.; Cascinu, S.; Barni, S.; Florani, I.; Cereda, S.; Berardi, R.; Mosconi, S.; Torri, V.; Labianca, R. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann. Oncol. 2007, 18, 1660–1665. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Frequency risk factors and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007, 110, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Niciforovic, D.; Markovic, M.; Papic, D. Cancer-Associated Thrombosis: Epidemiology, Pathophysiological Mechanisms, Treatment, and Risk Assessment. Clin. Med. Insights Oncol. 2023, 17, 11795549231220297. [Google Scholar] [CrossRef]
- Albánez, S.; Ogiwara, K.; Micheis, A.; Hopman, W.; Grabell, J.; James, P.; Lillikrap, D. Aging and ABO blood type influence von Willebrand factor and factor VIII levels through interrelated mechanisms. J. Thromb. Haemost. 2016, 14, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Caprini, J.A. The prophylaxis of venous thrombosis in patients with cancer undergoing major abdominal surgery: Emerging options. J. Surg. Oncol. 2007, 96, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Secin, F.P.; Jiborn, T.; Bjartell, A.S.; Fournier, G.; Salomon, L.; Abbou, C.C.; Haber, G.P.; Gill, I.S.; Crocitto, L.E.; Nelson, R.A.; et al. Multi-institutional study of symptomatic deep venous thrombosis and pulmonary embolism in prostate cancer patients undergoing laparoscopic or robot-assisted laparoscopic radical prostatectomy. Eur. Urol. 2008, 53, 134–145. [Google Scholar] [CrossRef]
- Agnelli, G.; Bolis, G.; Capussoti, L.; Scarpa, R.M.; Tonelli, F.; Bonizzoni, E.; Moia, M.; Parazzini, F.; Rossi, R.; Sonaglia, F.; et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: The ®RISTOS project. Ann. Surg. 2006, 243, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; Card, T.R.; West, J.; Crooks, C.; Grainge, M.J. Incidence of venous thromboembolism in patients with cancer—A cohort study using linked United Kingdom databases. Eur. J. Cancer 2013, 49, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Beemath, A.; Mayers, F.A.; Skaf, E.; Sanchez, J.; Olson, R.E. Incidence of venous thromboembolism in patients hospitalized with cancer. Am. J. Med. 2006, 119, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sallah, S.; Wan, J.Y.; Nguyen, N.P. Venous thrombosis in patients with solid tumors: Determination of frequency and characteristics. Thromb. Haemost. 2002, 87, 575–579. [Google Scholar] [PubMed]
- Levitan, N.; Dowlati, A.; Remick, S.C.; Tahsildar, H.I.; Sivinski, L.D.; Beyth, R.; Rimm, A.A. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claim data. Medicine 1999, 78, 285–291. [Google Scholar] [CrossRef]
- Chew, H.K.; Davies, A.M.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. The incidence of venous thromboembolism among patients with primary lung cancer. J. Thromb. Haemost. 2008, 6, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, A.; Wun, T.; Khatri, V.; Chew, H.K.; Harvey, D.; Zhou, H.; Whote, R.H. Venous thromboembolism in patients with colorectal cancer:incidence and effect on survival. J. Clin. Oncol. 2006, 24, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Russo, L. The mechanisms of cancer-associated thrombosis. Thromb. Res. 2015, 135, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Rickles, F.R.; Falanga, A. Molecular basis for the relationship between thrombosis and cancer. Thromb. Res. 2001, 102, V215–V224. [Google Scholar] [CrossRef]
- Falanga, A.; Russo, L.; Milesi, V.; Vignoli, A. Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol. Hematol. 2017, 118, 79–83. [Google Scholar] [CrossRef]
- Tagnon, H.J.; Whitmore, W.F.; Shulman, N.R. Fibrinolysis in metastatic cancer of the prostate. Cancer 1952, 5, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Girardi, L.; Wang, T.F.; Ageno, W.; Carrier, M. Updates in the Incidence, Pathogenesis, and Management of Cancer and Venous Thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Riedl, J.; Preusser, M.; Nazari, P.M.; Posch, F.; Panzer, S.; Marosi, C.; Birner, P.; Thaler, J.; Brostjan, C.; Lötsch, D.; et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 2017, 129, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Kimura, I. Podoplanin as a marker for mesothelioma. Pathol. Int. 2005, 55, 83–86. [Google Scholar] [CrossRef]
- Kato, Y.; Sasagawa, I.; Kaneko, M.; Osawa, M.; Fujita, N.; Tsuruo, T. Aggrus: A diagnostic marker that distinguishes seminoma from embryonal carcinoma in testicular germ cell tumors. Oncogene 2004, 23, 8552–8556. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, B.; Xu, M.; Jiang, Y.; Zhou, J.; Yang, J.; Gu, H.; Ruan, C.; Wu, J.; Zhao, Y. Blocking podoplanin inhibits platelet activation and decreases cancer-associated venous thrombosis. Thromb. Res. 2021, 200, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.; Ay, C.; Mackman, N.; Bertina, R.M.; Kaider, A.; Marosi, C.; Key, N.S.; Barcel, D.A.; Scheithauer, W.; Kornek, G.; et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J. Thromb. Haemost. 2012, 10, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Stegner, D.; Dütting, S.; Nieswandt, B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb. Res. 2014, 133, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Tinholt, M.; Viken, M.K.; Dahm, A.E.; Vollan, H.K.; Sahlberg, K.K.; Garred, O.; Børresen-Dale, A.L.; Jacobsen, A.F.; Kristensen, V.; Bukholm, I.; et al. Increased coagulation activity and genetic polymorphisms in the F5, F10 and EPCR genes are associated with breast cancer: A case-control study. BMC Cancer 2014, 14, 845. [Google Scholar] [CrossRef]
- Paulsen, B.; Skille, H.; Smith, E.N.; Hveem, K.; Gabrielsen, M.E.; Brækkan, S.K.; Rosendaal, F.R.; Frazer, K.A.; Gran, O.V.; Hansen, J.B. Fibrinogen gamma gene rs2066865 and risk of cancer-related venous thromboembolism. Haematologica 2020, 105, 1963–1968. [Google Scholar] [CrossRef]
- Pabinger, I.; Thaler, J.; Ay, C. Biomarkers for prediction of venous thromboembolism in cancer. Blood 2013, 122, 12. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Moore, E.E.; Moore, H.; Thomas, S.; Lune, S.V.; Zimmer, D.; Dynako, J.; Hake, D.; Crowell, Z.; McCauley, R.; et al. Use of Viscoelastography in Malignancy-Associated Coagulopathy and Thrombosis: A Review. Semin. Thromb. Hemost. 2019, 45, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Salvagno, G.L.; Ippolito, L.; Franchini, M.; Favaloro, E.J. Shortened activated partial thromboplastin time: Causes and management. Blood Coagul. Fibrinolysis 2010, 21, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Vormittag, R.; Dunkler, D.; Simanek, R.; Chiriac, A.L.; Drach, J.; Quehenberger, P.; Wagner, O.; Zielinski, C.C.; Pabinger, I. Predictive value of D-dimer levels for venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis study (CATS). Blood 2008, 112, 3824. [Google Scholar] [CrossRef]
- Stender, M.T.; Frøkjaer, J.B.; Larsen, T.B.; Lundbye-Christensen, S.; Thoracious-Ussing, O. Preoperative plasma D-dimer is a predictor of postoperative deep venous thrombosis in colorectal cancer patients: A clinical prospective cohort study with one-year follow-up. Dis. Colon Rectum 2009, 52, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Kodama, J.; Seki, N.; Masahiro, S.; Kusumoto, T.; Nakamura, K.; Hongo, A.; Hiramatsou, Y. D-dimer level as a risk factor for postoperative venous thromboembolism in Japanese women with gynecologic cancer. Ann. Oncol. 2010, 21, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Martini, F.; Portanera, L.; Massimiani, G.; Riondino, S.; La Farina, F.; Mariotti, S.; Guadagni, F.; Roselli, M. Novel high-sensitive D-dimer level dissemination predicts chemotherapy-associated venous thromboembolism in intermediate risk lung cancer patients. Clin. Lung Cancer 2012, 13, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Loukopoulou, I.; Papadopoulos, D.V.; Trikoupis, I.G.; Sokou, R.; Tsante, K.A.; Mantzios, P.G.; Nikolopoulos, G.K.; Koulouvaris, P.; Houhoula, D.; et al. Fibrinolysis shutdown and elevated D-dimer levels have high prognostic capacity for postoperative thromboembolic complications in patients with bone tumors. J. Thromb. Thrombolysis 2023, 55, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, B.; Legnani, C.; Cini, M.; Guazzaloca, G.; Paraleti, G. The role of D-dimer and residual venous obstruction in recurrence of venous thromboembolism after anticoagulation withdrawal in cancer patients. Haematologica 2005, 90, 713–715. [Google Scholar]
- Trujillo-Santos, J.; Di Micco, P.; Iannuzzo, M.; Lecumberri, R.; Guijarro, R.; Madridano, O.; Monreal, M.; RIETE Investigators. Elevated white blood cell count and outcome in cancer patients with venous thromboembolism. Findings from the RIETE Registry. Thromb. Haemost. 2008, 100, 905–911. [Google Scholar] [CrossRef]
- Starlinh, N.; Rao, S.; Cunningham, D.; Ivenson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R. Thromboembolism in patients with advanced gastroesophageal cancer treated with anthracycline, platinum and fluoropyrimidine combination chemotherapy: A report from the UK National Cancer Research Institute Upper Gastrointestinal Clinical Studies Group. J. Clin. Oncol. 2009, 27, 3786–3793. [Google Scholar] [CrossRef]
- Simanek, R.; Vormittag, R.; Ay, C.; Alguel, G.; Dunkler, D.; Schwarzinger, I.; Steger, G.; Jaeger, U.; Zielinski, C.; Pabinger, I. High platelet count associated with venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). J. Thromb. Haemost. 2010, 8, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Westrick, R.J.; Eitzman, D.T. Plasminogen activator inhibitor-1 in vascular thrombosis. Curr. Drug Targets 2007, 8, 966–1002. [Google Scholar] [CrossRef] [PubMed]
- Andrén-Sandberg, A.; Lecander, I.; Martinsson, G.; Astedt, B. Peaks in plasma plasminogen activator inhibitor-1 concentration may explain thrombotic events in cases of pancreatic carcinoma. Cancer 1992, 69, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, F.L.; Ciusani, E.; Silvani, A.; Corsini, E.; Frigerio, S.; Pogliani, S.; Parati, E.; Croci, D.; Boiardi, A.; Salmaggi, A. Genetic and plasma markers of venous thromboembolism in patients with high grade glioma. Clin. Cancer Res. 2004, 10, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Parastatidou, S.; Tsantes, E.A.; Bonova, E.; Tsante, K.A.; Mantzios, P.G.; Vaiopoulos, A.G.; Tsalas, S.; Konstantinidi, A.; Houhoula, D.; et al. Sepsis-Induced Coagulopathy: An Update on Pathophysiology, Biomarkers, and Current Guidelines. Life 2023, 13, 350. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Minei, K.M.; Scerbo, M.L.; Radwan, Z.A.; Wade, C.E.; Kozar, R.A.; Gill, B.S.; Albarado, R.; McNutt, M.K.; Khan, S.; et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: Experience with 1974 consecutive trauma patients. Ann. Surg. 2012, 256, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Piovani, D.; Kriebardis, A.G.; Gial-eraki, A.; Nikolopoulos, G.K.; et al. Rotational Thromboelastometry Findings Are Associated with Symptomatic Venous Thromboembolic Complications after Hip Fracture Surgery. Clin. Orthop. Relat. Res. 2021, 479, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Blasi, A.; Molina, V.; Sanchez-Cabús, S.; Balust, J.; Garcia-Valdecasas, J.C.; Taura, P. Prediction of thromboembolic complications after liver resection for cholangiocarcinoma: Is there a place for thromboelastometry? Blood Coagul. Fibrinolysis 2018, 29, 61–66. [Google Scholar] [CrossRef]
- Moore, H.B.; Paniccia, A.; Lawson, P.J.; Torphy, R.J.; Nydam, T.L.; Moore, E.E.; McCarter, M.D.; Schulick, R.D.; Edil, B.H. Utility of Viscoe-lastic Assays Beyond Coagulation: Can Preoperative Thrombelastography Indices Predict Tumor Histology, Nodal Disease, and Resectability in Patients Undergoing Pancreatectomy? J. Am. Coll. Surg. 2018, 227, 55–62. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Loukopoulou, I.; Papadopoulos, D.V.; Trikoupis, I.G.; Roustemis, A.G.; Goumenos, S.; Sokou, R.; Tsante, K.A.; Kriebardis, A.G.; Koulouvaris, P.; et al. The Hypercoagulable Profile of Patients with Bone Tumors: A Pilot Observational Study Using Rotational Thromboelastometry. Cancers 2022, 14, 3930. [Google Scholar] [CrossRef] [PubMed]
- Thorson, C.M.; Van Haren, R.M.; Ryan, M.L.; Curia, E.; Sleeman, D.; Levi, J.U.; Livingstone, A.S.; Proctor, K.G. Persistence of hypercoagulable state after resection of intra-abdominal malignancies. J. Am. Coll. Surg. 2013, 216, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Trikoupis, I.G.; Papadopoulos, D.V.; Goumenos, S.; Piovani, D.; Nikolopoulos, G.K.; Gialeraki, A.; Bonovas, S.; Papagel-opoulos, P.J.; Kontogeorgakos, V.A.; et al. The Safety and Efficacy of Tranexamic Acid in Oncology Patients Undergoing Endoprosthetic Reconstruction and a ROTEM-Based Evaluation of Their Hemostatic Profile: A Pilot Study. Cancers 2021, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-W.; Ye, P.-J. Clinical analysis of acute cerebral infarction accompanied with lung cancer. J. Acute Dis. 2016, 5, 307–310. [Google Scholar] [CrossRef]
- Attaran, S.; Somov, P.; Awad, W.I. Randomised high- and low-dose heparin prophylaxis in patients undergoing thoracotomy for benign and malignant disease: Effect on thrombo-elastography. Eur. J. Cardiothorac. Surg. 2010, 37, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, Ι.; Nowak, K.; Koett, J.; Mess, C.; Wagner, L.; Gaiser, T.; Mayer, F.T.; Goertz, L.; Schneider, S.W.; Bauer, A.T. Von Willebrand factor in the plasma and in the tumor tissue predicts cancer-associated thrombosis and mortality. Haematologica 2023, 108, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; McCrae, K.R.; Milentijevic, D.; Fortier, J.; Nelson, W.W.; Laliberté, F.; Crivera, C.; Lefebvre, P.; Yannicelli, D.; Schein, J. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res. Pract. Thromb. Haemost. 2017, 1, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hokusai VTEInvestigators Büller, H.R.; Décousus, H.; Grosso, M.A.; Mercuri, M.; Middeldorp, S.; Prins, M.H.; Raskob, G.E.; Schellong, S.M.; Schwocho, L.; Segers, A.; et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N. Engl. J. Med. 2013, 369, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- EINSTEIN Investigators; Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Raskob, G.E.; et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar]
- Frere, C.; Farge, D.; Schrag, D.; Prata, P.H.; Connors, J.M. Direct oral anticoagulant versus low molecular weight heparin for the treatment of cancer-associated venous thromboembolism: 2022 updated systematic review and meta-analysis of randomized controlled trials. J. Hematol. Oncol. 2022, 15, 69. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor with Low Molecular Weight Heparin in Patients with Cancer with Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- McBane, R.D.; Wysokinski, W.E.; Le-Rademacher, J.G.; Zemla, T.; Ashrani, A.; Tafur, A.; Perepu, U.; Anderson, D.; Gundabolu, K.; Kuzma, C.; et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J. Thromb. Haemost. 2020, 18, 411–421. [Google Scholar] [CrossRef]
- Planquette, B.; Bertoletti, L.; Charles-Nelson, A.; Laporte, S.; Grange, C.; Mahé, I.; Pernod, G.; Elias, A.; Couturaud, F.; Falvo, N.; et al. Rivaroxaban vs dalteparin in cancer-associated thromboembolism. Chest 2022, 161, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Gates, L.E.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoo, C.; Seo, S.; Jeong, J.H.; Ryoo, B.Y.; Kim, K.P.; Lee, J.B.; Lee, K.W.; Kim, J.W.; Kim, I.H.; et al. A Phase II study to compare the safety and efficacy of direct oral anticoagulants versus subcutaneous dalteparin for cancer-associated venous thromboembolism in patients with advanced upper gastrointestinal, hepatobiliary and pancreatic cancer: PRIORITY. Cancers 2022, 14, 559. [Google Scholar] [CrossRef]
- Dictato, M. Thrombosis in GI cancer. In Proceedings of the ESMO, World Congress on Gastrointestinal Cancer, Barcelona, Spain, 28 June–1 July 2023. [Google Scholar]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef]
- Bazarbashi, S.; El Zawahry, H.M.; Owaidah, T.; AlBader, M.A.; Warsi, A.; Marashi, M.; Dawoud, E.; Jaafar, H.; Sholkamy, S.M.; Haddad, F.; et al. The Role of Direct Oral Anticoagulants in the Treatment of Cancer-Associated Venous Thromboembolism: Review by Middle East and North African Experts. J. Blood Med. 2024, 15, 171–189. [Google Scholar] [CrossRef]
- Alikhan, R.; Cohen, A.T.; Combe, S.; Samana, M.M.; Desjardins, L.; Eldor, A.; Janbon, C.; Leizorovicz, A.; Olsson, C.G.; Turpie, A.G. Pre-vention of venous thromboembolism in medical patients with enoxaparin: A subgroup analysis of the MEDENOX study. Blood Coagul. Fibrinolysis 2003, 14, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Dunkler, D.; Marosi, C.; Chiriac, A.L.; Vormittag, R.; Simanek, R.; Quehenberger, P.; Zielinski, C.; Pabinger, I. Prediction of venous thromboembolism in cancer patients. Blood 2010, 116, 5377–5382. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G.; Barni, S.; Gasparini, G.; LaBianca, R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Intern. Emerg. Med. 2012, 7, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Louzada, M.L.; Carrier, M.; Lazo-Langner, A.; Dao, V.; Kovacs, M.J.; Ramsay, T.O.; Rodger, M.A.; Zhang, J.; Lee, A.Y.; Meyer, G.; et al. Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism. Circulation 2012, 126, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.S.; Talmage, K.E.; Massari, J.V.; La Jeunesse, C.M.; Flick, M.J.; Kombrinck, K.W.; Jirousková, M.; Degen, J.L. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005, 105, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Bohlke, K.; Khorana, A.A.; Kuderer, N.M.; Lee, A.Y.; Arcelus, J.I.; Balaban, E.P.; Clarke, J.M.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J. Clin. Oncol. 2015, 33, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z.; et al. Cancer-Associated Venous Thromboembolic Disease, Version 2. 2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1181–1201. [Google Scholar]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Muñoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022, 23, 334–347. [Google Scholar] [CrossRef]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Jara-Palomares, L.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Khorana, A.A.; Moretto, P.; Le Gal, G.; Karp, R.; Zwicker, J.I. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am. J. Med. 2014, 127, 82–86. [Google Scholar] [CrossRef]
- Leizorovicz, A.; Cohen, A.T.; Turpie, A.G.; Olsson, C.G.; Vaitkus, P.T.; Goldhaber, S.Z.; PREVENT Medical Thromboprophylaxis Study Group. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004, 110, 874–879. [Google Scholar] [CrossRef]
- Samama, M.M.; Cohen, A.T.; Darmon, J.Y.; Desjardins, L.; Eldor, A.; Janbon, C.; Leizorovicz, A.; Nguyen, H.; Olsson, C.G.; Turpie, A.G.; et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N. Engl. J. Med. 1999, 341, 793–800. [Google Scholar] [CrossRef]
- Belch, J.J.; Lowe, G.D.; Ward, A.G.; Forbes, C.D.; Prentice, C.R. Prevention of deep vein thrombosis in medical patients by low-dose heparin. Scott. Med. J. 1981, 26, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Douketis, J.D.; Gianni, M.; Lim, W.; Crowther, M.A. Meta-analysis: Anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann. Intern. Med. 2007, 146, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Connors, J.M. Prophylaxis against venous thromboembolism in ambulatory patients with cancer. N. Engl. J. Med. 2014, 370, 2515–2519. [Google Scholar] [CrossRef]
- Matar, C.F.; Kahale, L.A.; Hakoum, M.B.; Tsolakian, I.G.; Etxeandia-Ikobaltzeta, I.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Barba, M.; Schü-nemann, H.; et al. Anticoagulation for perioperative thromboprophylaxis in people with cancer. Cochrane Database Syst. Rev. 2018, 7, CD009447. [Google Scholar] [CrossRef]

| Conventional Tests | |
| PT/INR | Normal |
| aPTT | Normal or shorter * (* not established) |
| D-Dimers | Elevated (CATS score) |
| White blood count | Normal or elevated (not established) |
| Markers of hypercoagulability | |
| F1 + 2 prothrombin fragment | Elevated (not used as routine screening) |
| Thrombin generation test | Elevated (not used as routine screening) |
| Thrombin antithrombin complex | Elevated (not used as routine screening) |
| Markers of impaired fibrinolysis | |
| Plasminogen activator inhibitor-1 | Elevated (not used as routine screening) |
| Novel biomarkers | |
| Soluble CD62P | Elevated (CATS score) |
| Microparticles | Elevated (not standardized) |
| Platelet Factor-4 | Elevated (not established) |
| CD40 Ligand | Elevated (not established) |
| VWF/ADAMTS 13 ratio | Elevated (not established) |
| Molecular tests | Uncertain |
| Viscoelastic methods (POC) | |
| ROTEM | Shorter CT, increased MCF, increased a-angle |
| TEG | Shorter R and K time, increased MA |
| Therapeutic Agent | Advantages | Disadvantages |
|---|---|---|
| Vitamin K antagonists |
| |
| LMWHs | Compared to Vit K antagonists
|
|
| DOACs | Compared to LMWH
| Compared to LMWH
|
| Parameters | Points | |
|---|---|---|
| Tumor type | Very high-risk tumor (pancreatic, stomach) | 2 |
| High-risk tumor (bladder, gynecological, lung, lymphoma) | 1 | |
| Other type of tumor | 0 | |
| Thrombocytosis (≥350 × 109/L) | 1 | |
| Elevated pre-chemotherapy white blood count (>11 × 109/L) | 1 | |
| High Body Mass Index (≥35 kg/m2) | 1 | |
| Low hemoglobin level (<10 g/Dl) | 1 | |
| Use of erythropoietin stimulating agent | 1 | |
| Low Risk (0–1 points): no prophylaxis is recommended. Intermediate risk (2 points): consider prophylaxis. High risk (≥3 points): prophylaxis indicated. | ||
| Scientific Society | Initial Treatment Recommendations | Long Term Treatment Recommendations |
|---|---|---|
| ASCO [79] | Recommended agents:
| Recommended agents:
|
| ASCO [76] | Recommended agents:
| Recommended agents:
|
| NCCN [92] | Recommended agents:
| |
| ASH [89] | Recommended agents:
| Recommended agents:
|
| ESMO [93] | Recommended agents:
|
|
| ITAC [94] | Recommended agents:
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsantes, A.G.; Petrou, E.; Tsante, K.A.; Sokou, R.; Frantzeskaki, F.; Domouchtsidou, A.; Chaldoupis, A.E.; Fortis, S.P.; Piovani, D.; Nikolopoulos, G.K.; et al. Cancer-Associated Thrombosis: Pathophysiology, Laboratory Assessment, and Current Guidelines. Cancers 2024, 16, 2082. https://doi.org/10.3390/cancers16112082
Tsantes AG, Petrou E, Tsante KA, Sokou R, Frantzeskaki F, Domouchtsidou A, Chaldoupis AE, Fortis SP, Piovani D, Nikolopoulos GK, et al. Cancer-Associated Thrombosis: Pathophysiology, Laboratory Assessment, and Current Guidelines. Cancers. 2024; 16(11):2082. https://doi.org/10.3390/cancers16112082
Chicago/Turabian StyleTsantes, Andreas G., Eleni Petrou, Konstantina A. Tsante, Rozeta Sokou, Frantzeska Frantzeskaki, Aglaia Domouchtsidou, Anastasios E. Chaldoupis, Sotirios P. Fortis, Daniele Piovani, Georgios K. Nikolopoulos, and et al. 2024. "Cancer-Associated Thrombosis: Pathophysiology, Laboratory Assessment, and Current Guidelines" Cancers 16, no. 11: 2082. https://doi.org/10.3390/cancers16112082
APA StyleTsantes, A. G., Petrou, E., Tsante, K. A., Sokou, R., Frantzeskaki, F., Domouchtsidou, A., Chaldoupis, A. E., Fortis, S. P., Piovani, D., Nikolopoulos, G. K., Iacovidou, N., Bonovas, S., Samonis, G., & Tsantes, A. E. (2024). Cancer-Associated Thrombosis: Pathophysiology, Laboratory Assessment, and Current Guidelines. Cancers, 16(11), 2082. https://doi.org/10.3390/cancers16112082












