Use of Bladder-Related Medication in Non-Muscle Invasive Bladder Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Population
2.3. Definitions
2.4. Statistical Analysis
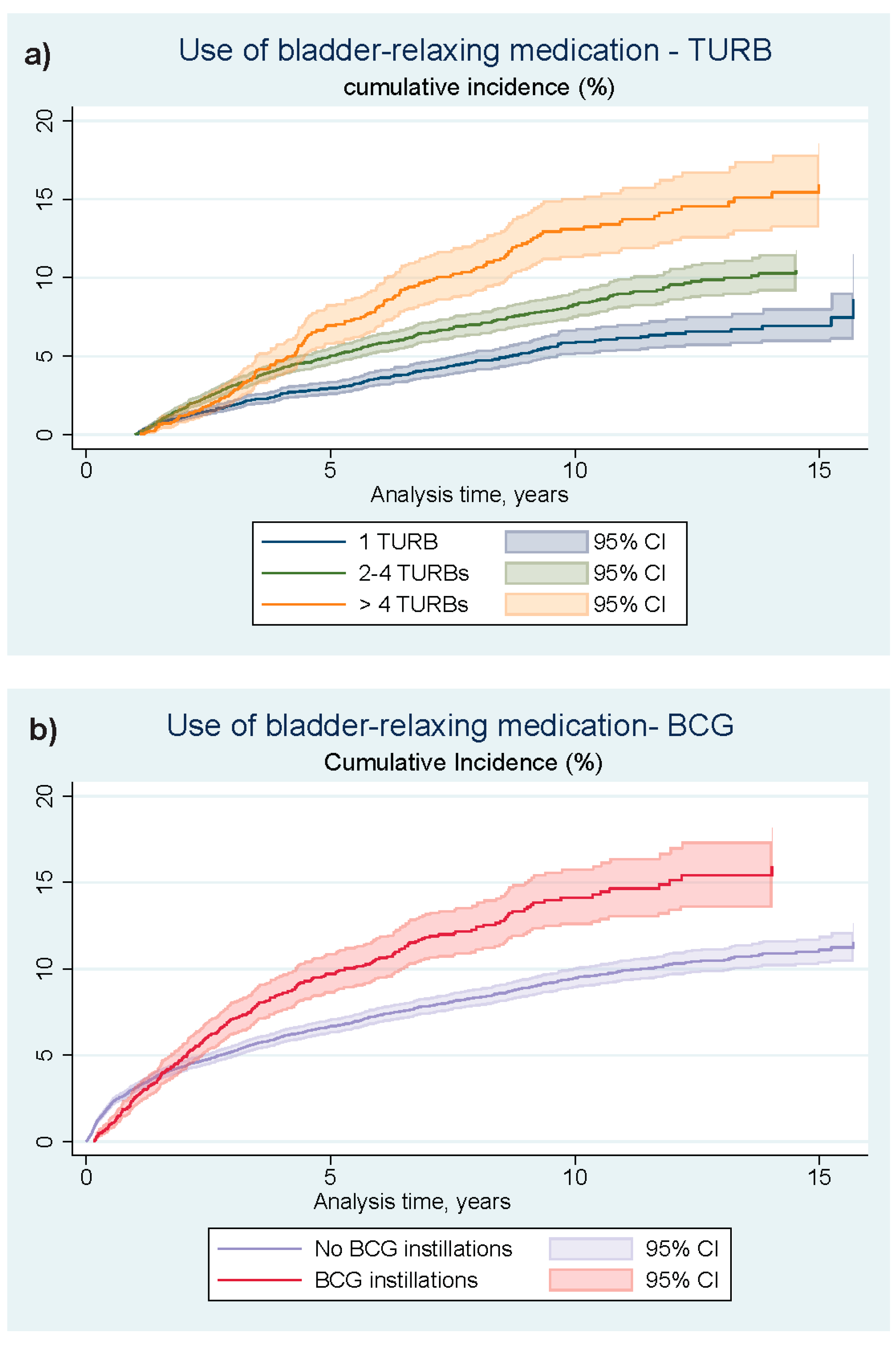
3. Results
Sub Analyses: BCG-Treated Patients and MMC-Treated Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- DaBlaCa-Data Annual Report 2020, Bladder Cancer, Denmark. Available online: https://www.sundhed.dk/content/cms/86/15686_dablaca_aarsrapport_2020_offentlig_260221.pdf (accessed on 22 April 2022).
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–477; discussion 475–477. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- DaBlaCa [National Guidelines, Bladder Cancer]. Available online: http://www.skejby.net/DaBlaCa-web/DaBlaCaWEB.htm (accessed on 5 May 2022).
- Tan, W.S.; Rodney, S.; Lamb, B.; Feneley, M.; Kelly, J. Management of non-muscle invasive bladder cancer: A comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer Treat. Rev. 2016, 47, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ibarrola, R.; Soria, F.; Abufaraj, M.; D’Andrea, D.; Preto, M.; Gust, K.M.; Briganti, A.; Shariat, S.F.; Gontero, P. Surgical checklist impact on recurrence-free survival of patients with non-muscle-invasive bladder cancer undergoing transurethral resection of bladder tumour. BJU Int. 2019, 123, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, P.; Finney, S.M.; Head, E.; Somani, B.K.; Zachou, A.; Smith, G.; Mishriki, S.F.; N’Dow, J.; Grigor, K.M. Good quality white-light transurethral resection of bladder tumours (GQ-WLTURBT) with experienced surgeons performing complete resections and obtaining detrusor muscle reduces early recurrence in new non-muscle-invasive bladder cancer: Validation across time and place and recommendation for benchmarking. BJU Int. 2012, 109, 1666–1673. [Google Scholar] [PubMed]
- Bradshaw, H.D.; Radley, S.C.; Rosario, D.J.; Chapple, C.R. Towards a better understanding of involuntary detrusor activity. BJU Int. 2005, 95, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Shang, P.F.; Kwong, J.; Wang, Z.P.; Tian, J.; Jiang, L.; Yang, K.; Yue, Z.J.; Tian, J.Q. Intravesical Bacillus Calmette-Guerin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst. Rev. 2011, Cd006885. [Google Scholar] [CrossRef] [PubMed]
- Brausi, M.; Oddens, J.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Turkeri, L.; Marreaud, S.; Collette, S.; et al. Side Effects of Bacillus Calmette-Guerin (BCG) in the Treatment of Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Bladder: Results of the EORTC Genito-Urinary Cancers Group Randomised Phase 3 Study Comparing One-third Dose with Full Dose and 1 Year with 3 Years of Maintenance BCG. Eur.Urol. 2014, 65, 69–76. [Google Scholar]
- Frank, L. Epidemiology. When an entire country is a cohort. Science 2000, 287, 2398–2399. [Google Scholar] [CrossRef]
- DanmarksStatestik Statestikbanken. Available online: http://extranet.dst.dk/pyramide/pyramide.htm#!y=2017&a=16,65&v=2&g (accessed on 31 July 2022).
- Lynge, E.; Sandegaard, J.L.; Rebolj, M. The Danish National Patient Register. Scand. J. Public Health 2011, 39 (Suppl. S7), 30–33. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schmidt, S.A.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sorensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Blichert-Refsgaard, L.; Nørgaard, M.; Bengtsen, M.B.; Jensen, J.B. Positive Predictive Values of Procedure Codes on the Treatment of Non-Muscle Invasive Bladder Cancer in the Danish National Patient Registry. Clin. Epidemiol. 2022, 14, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Sundhedsdatastyrelsen Landsregistret for Patologi. Available online: https://www.esundhed.dk/Dokumentation/DocumentationExtended?id=18 (accessed on 3 July 2022).
- Petersen, A. SNOMED-Kodning Af Neoplastiske Blærelæsioner. Available online: https://www.patobank.dk/wp-content/uploads/2019/10/B._Blaerecancer-2.pdf (accessed on 3 July 2022).
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bergkvist, A.; Ljungqvist, A.; Moberger, G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir. Scand. 1965, 130, 371–378. [Google Scholar] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. International Language for Drug Utilization Research. Available online: https://www.whocc.no/ (accessed on 3 July 2022).
- Pottegard, A.; Schmidt, S.A.J.; Wallach-Kildemoes, H.; Sorensen, H.T.; Hallas, J.; Schmidt, M. Data Resource Profile: The Danish National Prescription Registry. Int. J. Epidemiol. 2017, 46, 798–798f. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, R.; Lash, T.L.; Hamilton-Dutoit, S.J.; Bjerregaard, B.; Vyberg, M.; Pedersen, L. Existing data sources for clinical epidemiology: The Danish National Pathology Registry and Data Bank. Clin. Epidemiol. 2010, 2, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, S.; Silvestri, T.; Bassi, S.; Porcaro, A.B.; Cerruto, M.A.; Talamini, R.; Artibani, W. Health-related quality of life after BCG or MMC induction for non-muscle invasive bladder cancer. Can. J. Urol. 2018, 25, 9480–9485. [Google Scholar] [PubMed]
- Bohle, A.; Bock, P.R. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: Formal meta-analysis of comparative studies on tumor progression. Urology 2004, 63, 682–686; discussion 686–687. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Miura, N.; Babjuk, M.; Karakiewicz, P.I.; Mostafaei, H.; Laukhtina, E.; Quhal, F.; Motlagh, R.S.; Pradere, B.; Kimura, S.; et al. Low compliance to guidelines in nonmuscle-invasive bladder carcinoma: A systematic review. Urol. Oncol. 2020, 38, 774–782. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.G.; Burger, M. Low adherence to guidelines in non-muscle-invasive disease. Nat. Rev. Urol. 2016, 13, 570–571. [Google Scholar] [CrossRef] [PubMed]
- WHO Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 16 April 2022).
- Sanchez, A.; Wszolek, M.F. Quality of life in patients with non-muscle-invasive bladder cancer. Nat. Rev. Urol. 2015, 12, 186–188. [Google Scholar] [CrossRef]
- Catto, J.W.F.; Downing, A.; Mason, S.; Wright, P.; Absolom, K.; Bottomley, S.; Hounsome, L.; Hussain, S.; Varughese, M.; Raw, C.; et al. Quality of Life After Bladder Cancer: A Cross-sectional Survey of Patient-reported Outcomes. Eur. Urol. 2021, 79, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.; Frick, J. Quality of life in patients undergoing bacille Calmette-Guérin therapy for superficial bladder cancer. Br. J. Urol. 1996, 78, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Connors, J.N.; Ladd, I.G.; Bogaczyk, T.L.; Larson, S.L. Defining Priorities to Improve Patient Experience in Non-Muscle Invasive Bladder Cancer. Bladder Cancer 2018, 4, 121–128. [Google Scholar] [CrossRef] [PubMed]
- González-Padilla, D.A.; González-Díaz, A.; Guerrero-Ramos, F.; Rodríguez-Serrano, A.; García-Jarabo, E.; Corona-laPuerta, M.; Rodríguez-Antolín, A.; Villacampa-Aubá, F. Quality of life and adverse events in patients with nonmuscle invasive bladder cancer receiving adjuvant treatment with BCG, MMC, or chemohyperthermia. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 76.e9–76.e14. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, M.S.; Bue, P.; Azawi, N.; Blichert-Refsgaard, L.; Sundelin, M.O.; Dyrskjøt, L.; Jensen, J.B. The DaBlaCa-13 Study: Short-term, Intensive Chemoresection Versus Standard Adjuvant Intravesical Instillations in Non-muscle-invasive Bladder Cancer-A Randomised Controlled Trial. Eur. Urol. 2020, 78, 856–862. [Google Scholar] [CrossRef]
- Lindgren, M.S.; Hansen, E.; Azawi, N.; Nielsen, A.M.; Dyrskjøt, L.; Jensen, J.B. DaBlaCa-13 Study: Oncological Outcome of Short-Term, Intensive Chemoresection With Mitomycin in Nonmuscle Invasive Bladder Cancer: Primary Outcome of a Randomized Controlled Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 206–211. [Google Scholar] [CrossRef]


| N | 17,774 |
| Sex: female | 24.24% |
| Male | 75.76% |
| Baseline age, median (IQR) *: | 70 (63, 77) |
| Primary histology, freq. (%) | |
| PUNLMP | 886 (4.98%) |
| pTa LG | 8032 (45.19%) |
| pTa (grade unknown) | 785 (4.42%) |
| pTa HG | 2470 (13.90%) |
| CIS | 1085 (6.10%) |
| pTa, concomitant CIS | 570 (3.21%) |
| pT1a | 1485 (8.35%) |
| pT1 (sub-division unknown) | 1491 (8.39%) |
| pT1b | 970 (5.46%) |
| Any collection of prescriptions before NMIBC ** diagnosis, freq. (%) | |
| Bladder relaxing agents | 1203 (6.77%) |
| Cystitis-relevant antibiotics | 9050 (50.92%) |
| Bladder-Relaxing Agents (Anticholinergics and β3-Agonists) * | Cystitis-Relevant Antibiotics * | |
|---|---|---|
| TURB exposure ** | HR [95% conf. interval] | HR [95% conf. interval] |
| One TURB | 1 (reference group) | 1 (reference group) |
| 2–4 TURBs | 1.98 [1.75; 2.23] | 1.77 [1.69; 1.86] |
| ≥five TURBs | 4.01 [3.33; 4.83] | 2.27 [2.05; 2.51] |
| Adjuvant BCG treatment *** | HR [95% conf. interval] | HR [95% conf. interval] |
| No installations | 1 (reference group) | 1 (reference group) |
| Yes | 1.92 [1.69; 2.18] | 1.39 [1.31; 1.48] |
| Adjuvant MMC treatment **** | HR [95% conf. interval] | HR [95% conf. interval] |
| No installations | 1 (reference group) | 1 (reference group) |
| Yes | 2.26 [1.73; 2.95] | 1.36 [1.17; 1.59] |
| Multivariate analysis ***** | HR [95% conf. interval] | HR [95% conf. interval] |
| TURB exposure | ||
| one TURB | 1 (reference group) | 1 (reference group) |
| 2–4 TURBs | 1.86 [1.64; 2.10] | 1.74 [1.66; 1.82] |
| >five TURBs | 3.42 [2.82; 4.15] | 2.16 [1.95; 2.39] |
| Adjuvant BCG treatment: | ||
| No | 1 (reference group) | 1 (reference group) |
| yes | 1.51 [1.33; 1.73] | 1.19 [1.12; 1.27] |
| Adjuvant MMC treatment: | ||
| No | 1 (reference group) | 1 (reference group) |
| yes | 1.66 [1.27; 2.18] | 1.16 [1.00; 1.35] |
| More than Six Instillations within the First Two Years from NMIBC-Diagnose | Bladder-Relaxing Medication (Anticholinergics and β3-Agonists) HR [95% Conf. Interval] | Cystitis-Relevant Antibiotics HR [95% Conf. Interval] |
|---|---|---|
| BCG N = 3365 | ||
| No | 1 (reference group) | 1 (reference group) |
| Yes | 1.34 [0.97; 1.86] | 0.80 [0.63; 1.02] |
| MMC N = 521 | ||
| No | 1 (reference group) | 1 (reference group) |
| yes | 0.52 [0.21; 1.30] | 0.95 [0.54; 1.66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blichert-Refsgaard, L.; Graugaard-Jensen, C.; Nørgaard, M.; Jensen, J.B. Use of Bladder-Related Medication in Non-Muscle Invasive Bladder Cancer Patients. Cancers 2024, 16, 1936. https://doi.org/10.3390/cancers16101936
Blichert-Refsgaard L, Graugaard-Jensen C, Nørgaard M, Jensen JB. Use of Bladder-Related Medication in Non-Muscle Invasive Bladder Cancer Patients. Cancers. 2024; 16(10):1936. https://doi.org/10.3390/cancers16101936
Chicago/Turabian StyleBlichert-Refsgaard, Linea, Charlotte Graugaard-Jensen, Mette Nørgaard, and Jørgen Bjerggaard Jensen. 2024. "Use of Bladder-Related Medication in Non-Muscle Invasive Bladder Cancer Patients" Cancers 16, no. 10: 1936. https://doi.org/10.3390/cancers16101936
APA StyleBlichert-Refsgaard, L., Graugaard-Jensen, C., Nørgaard, M., & Jensen, J. B. (2024). Use of Bladder-Related Medication in Non-Muscle Invasive Bladder Cancer Patients. Cancers, 16(10), 1936. https://doi.org/10.3390/cancers16101936







