Simple Summary
This research investigates the effects of South Korea’s national insurance coverage (NIC) expansion and the inclusion of genetic counselors on BRCA1/2 mutation testing rates in breast cancer patients. By analyzing data from the Samsung Medical Center, the study reveals a notable increase in testing rates following NIC expansion and the addition of genetic counselors. Particularly noteworthy is the rise in testing rates among triple-negative breast cancer (TNBC) patients under 60. Additionally, the involvement of genetic counselors led to a significant increase in follow-up patients undergoing testing. The NIC expansion also broadened insurance coverage for TNBC patients, thereby enhancing testing accessibility. These findings underscore the positive impact of NIC expansion and genetic counselor involvement on improving patient management. Addressing financial barriers to testing and integrating genetic counseling into healthcare practices present promising strategies for advancing early detection and tailored treatment approaches, thereby contributing to global efforts in cancer care and management.
Abstract
Purpose: This study aims to evaluate the impact of South Korea’s national insurance coverage (NIC) expansion and the addition of genetic counselors on BRCA1/2 mutation testing rates in breast cancer patients. Materials and Methods: A retrospective review was conducted at the Samsung Medical Center (SMC), dividing patients into three groups: pre-NIC expansion, post-NIC expansion, and post-extra genetic counselor involvement. The number of BRCA1/2 tests performed and the detection rates among newly diagnosed and follow-up patients, particularly focusing on triple-negative breast cancer (TNBC) cases, were analyzed. Results: Post-NIC expansion, there was a significant increase in BRCA1/2 testing rates, with a gradual rise in detection rates while maintaining statistical significance. TNBC patients under 60 experienced substantial increases in testing rates. The number of follow-up patients recalled for testing also rose significantly after the extra genetic counselor involvement. Additionally, NIC expansion increased insurance coverage for TNBC patients, enhancing accessibility to testing. Conclusion: The study highlights the positive impact of NIC expansion and genetic counselor involvement on BRCA1/2 mutation testing rates and subsequent patient management. Addressing financial barriers to testing and incorporating genetic counseling significantly improve patient outcomes. This model provides a potential strategy for enhancing early detection and personalized treatment for breast cancer patients with BRCA1/2 mutations, contributing to global cancer management efforts.
1. Introduction
Breast cancer remains one of the most prevalent and concerning diseases affecting women worldwide [1,2]. The risk of breast cancer in the general population is about 12%; however, when a BRCA1/2 mutation is present, the risk increases dramatically. BRCA1 mutations appear in 50–80% and BRCA2 mutations in 40–70% of breast cancer risks. BRCA1 and BRCA2 mutations account for about 40–50% of hereditary breast cancers and about 5–10% of all breast cancers. Even though many are not yet diagnosed with breast cancer, approximately 46% of BRCA1 mutation carriers and 52% of BRCA2 mutation carriers are diagnosed with breast cancer at some point in their lifetime. Thus, the early detection of BRCA1/2 mutations comes to the forefront nowadays [3,4,5,6]. However, amidst the growing awareness and demand for BRCA1/2 mutation testing, the issue of its high cost has emerged as a substantial barrier, limiting access to this crucial tool for many individuals. Therefore, the public insurance coverage of BRCA1/2 mutation testing becomes an important issue.
In a previous study, the high prevalence of BRCA1/2 mutations in Korean TNBC patients diagnosed under the age of 60 was analyzed, and this led to the Korean NIC expansion in July 2020 [7,8,9]. Along with the insurance coverage issue, genetic counseling before and after a genetic test is also important. To explain and recommend BRCA1/2 mutation testing to patients who meet the criteria, as well as to provide quality counseling after BRCA1/2 mutation testing, the need for genetic counselors is inevitable [10]. Since genetic counseling is an important but time-consuming process, it is difficult to provide counseling to a sufficient number of patients, especially when a limited number of counselors are present.
The purpose of this study is to evaluate the effect of the Korean NIC expansion and the additional recruitment of genetic counselors on the increase in BRCA1/2 mutation tests and BRCA1/2 mutation rates.
2. Materials and Methods
2.1. Patient Selection
This is a single-institution retrospective review. All patients who received breast cancer surgery from August 2019 to December 2021 at Samsung Medical Center (SMC) were divided into three groups according to the time marks. Group 1 was patients who underwent primary breast cancer surgery at our center before the Korean NIC expansion (August 2019–June 2020), group 2 consisted of patients after the Korean NIC expansion but before additional genetic counselor involvement (July 2020–November 2020), and group 3 consisted of patients after additional genetic counselor involvement (December 2020–December 2021). Among the total number of patients who received primary breast cancer surgery in each time period, patients who underwent BRCA1/2 mutation tests were selected and assessed according to the Korean NIC criteria on the BRCA1/2 mutation test. Moreover, follow-up patients who were recalled for BRCA1/2 mutation tests were selected during two different time periods: after the Korean NIC expansion (July 2020–November 2020) and after additional genetic counselor involvement (December 2020–December 2021). We also went through the numbers of new TNBC patients tested for the BRCA1/2 mutation who were within and out of insurance coverage before and after the Korean NIC expansion.
2.2. Rationale for Patient Selection and Criteria
In a previous clinical trial at our center, we analyzed the higher prevalence of BRCA1/2 mutations in Korean TNBC patients diagnosed under the age of 60, and this led to the Korean NIC expansion [7]. After the Korean NIC expansion, the 3 major changes in BRCA1/2 mutation test criteria became the following (Table 1) [11,12]:
Breast cancer diagnosed ≤40 years old;
Breast cancer patient with family history of breast cancer, ovarian cancer, metastatic prostate cancer, or pancreatic cancer (within 3rd degree);
TNBC diagnosed ≤60 years old.

Table 1.
The effect of national insurance coverage changes on BRCA1/2 gene mutation testing in Korea.
Table 1.
The effect of national insurance coverage changes on BRCA1/2 gene mutation testing in Korea.
| April 2012–July 2020 | After July 2020 |
|---|---|
| BC diagnosed <40 years old | BC diagnosed ≤40 years old |
| BC patients with FHx of BC or OC (within 2nd degree) | BC patients with FHx of BC, OC, metastatic prostate cancer, or pancreatic cancer (within 3rd degree) |
| Personal history of BC and OC | Personal history of BC and/or OC |
| Male BC | Male BC |
| Bilateral BC | Bilateral BC |
| TNBC diagnosed ≤60 years old | |
| Serous OC patient | Serous OC patient |
BC, breast cancer; FHx, family history; OC, ovarian cancer; TNBC, triple-negative breast cancer.
2.3. Group Selection and Comparison
To obtain statistical significance in the comparison of each of the group, which has different patient number pools due to the different time periods set, the proportion of patients instead of the exact number of patients was compared. Only newly diagnosed breast cancer patients were selected to analyze the increase in the BRCA1/2 mutation testing number in each group, and old patients undergoing regular OPD follow-ups who met the new insurance criteria were selected to analyze the recall rate after the NIC expansion as well as after additional genetic counselor involvement. Since the age expansion in TNBC patients was one of the major changes in the Korean NIC expansion, the number of TNBC patients was counted separately to see how effective the NIC expansion was on BRCA1/2 mutation testing in new TNBC patients.
2.4. Statistics
The BRCA1/2 mutation testing numbers in each group were assessed with Pearson’s chi-squared test. All statistical analyses were performed using R Statistical Software (version 3.6.3; Foundation for Statistical Computing, Vienna, Austria). Statistical significance was accepted for p-values of <0.05.
2.5. Ethics
This study adhered to the ethical tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of SMC (IRB number: 2023-01-103-001). The need for informed consent was waived because of the retrospective nature of this study.
3. Results
A total of 7299 patients received breast cancer surgery from August 2019 to December 2021 in the SMC, and they were divided into three groups according to the time marks. Group 1 consists of 2539 patients who underwent primary breast cancer surgery at our center before the Korean NIC expansion (August 2019–June 2020), group 2 consists of 1164 patients after the NIC expansion but before additional genetic counselor involvement (July 2020–November 2020), and group 3 consists of 3596 patients after additional genetic counselor involvement (December 2020–December 2021).
3.1. Major Changes in BRCA1/2 Mutation Testing Numbers in Newly Diagnosed Breast Cancer Patients after the Korean NIC Expansion and Additional Genetic Counselor Involvement
The total number of patients who underwent BRCA1/2 mutation tests at the SMC dramatically increased during 2020–2021, which reflects the time period after the Korean NIC expansion (Figure 1). Before the Korean NIC expansion, 32.8% of new patients who underwent primary breast cancer surgery were tested for the BRCA1/2 mutation. Then, 37.8% were tested after the Korean NIC expansion, and 42% were tested after additional genetic counselor involvement, respectively. The increase in the number of BRCA1/2 mutation tests in each group was statistically significant (p-value: 0.003 (group 1 vs. 2), 0.011 (group 2 vs. 3), <0.001 (group 1, 2 vs. 3). Even though the BRCA1/2 mutation testing numbers have increased, the BRCA1/2 mutation detection rate has remained similar (9.6%, 9.3%, and 9.5% in each group; p-value: 0.92 (group 1 vs. 2), 1.00 (group 2 vs. 3), 1.00 (group 1, 2 vs. 3)) (Table 2). Criteria-wise, breast cancer patients diagnosed under the age of 40, bilateral breast cancer patients, and patients with a personal history of ovarian/pancreatic cancer did not show a statistically significant increase in the number of BRCA1/2 mutation tests. However, there was a significant increase in the BRCA1/2 mutation testing numbers among TNBC patients diagnosed under the age of 60: 0.8% before the Korean NIC expansion, 5.2% after the Korean NIC expansion, and 15.3% after additional genetic counselor involvement (Table 2). To examine statistical differences across the three groups, Pearson’s chi-squared test followed by a pairwise comparison was conducted, and the results showed statistically significant differences across the three groups for TNBC patients aged 60 or younger (p < 0.001). According to our data on insurance coverage in TNBC patients tested for the BRCA1/2 mutation since 2016, there has been a dramatic reversal in the number of patients who were covered with insurance or not at the time of the Korean NIC expansion. Interestingly, as patients with a family history (within the third degree) of metastatic prostatic or pancreatic cancer were included in the expanded insurance criteria, the number of BRCA1/2 mutation tests carried out on these patients increased dramatically from 0.4% before the Korean NIC expansion to 8.9% after the Korean NIC expansion and remained about the same (7.4%) after additional genetic counselor involvement.
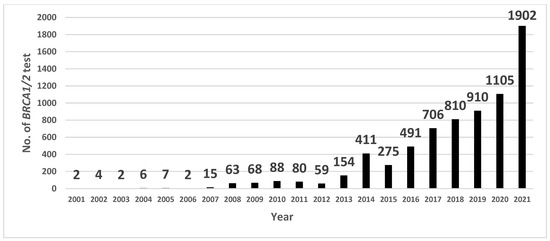
Figure 1.
Number of BRCA1/2 gene mutation tests on breast cancer patients at Samsung Medical Center (SMC).

Table 2.
Changes in BRCA1/2 gene mutation testing numbers before and after NIC expansion and after genetic counselor involvement (among new patients who underwent primary breast cancer surgery).
3.2. Follow-Up Patients Recalled for BRCA1/2 Mutation Testing
Among the total of 2536 patients who underwent BRCA1/2 mutation tests, which includes both new and old patients, 584 follow-up patients were recalled for BRCA1/2 mutation testing under new insurance criteria, with a mean BRCA1/2 mutation detection rate of 7.7%. Among all of the recalled patients, 538 (92.8%) of follow-up patients were recalled and tested for the BRCA1/2 mutation after additional genetic counselor involvement (Table 3).

Table 3.
Follow-up patients recalled for BRCA1/2 gene mutation testing after NIC expansion.
3.3. Effect of Insurance Coverage Expansion on BRCA1/2 Mutation Testing in New TNBC Patients
Before the Korean NIC expansion, when only TNBC patients under the age of 40 were included in the BRCA1/2 mutation testing criteria, only 34.1% of TNBC patients were within the insurance coverage range and could benefit from BRCA1/2 mutation tests. However, there was a dramatic increase in this patient percentage to 82.1% being covered by insurance after the Korean NIC criteria, which included TNBC patients between the ages of 40 and 60 (Table 4).

Table 4.
Insurance coverage in TNBC for new patients.
4. Discussion
The NIC expansion had a significant role in increasing the total number of patients under the criteria to be tested for the BRCA1/2 mutation while maintaining a similar detection rate (about 9%, Table 2), which clearly shows that the proper tests were being carried out under the proper criteria [11,12]. This increase was even more significant in TNBC patients and in breast cancer patients with a family history of metastatic or pancreatic cancer [12,13]. Moreover, the additional recruitment of genetic counselors led to a dramatic increase in recalling follow-up patients to be tested for the BRCA1/2 mutation.
Our results show that the Korean NIC expansion and additional genetic counselor involvement together brought about a significant increase in the number of effective BRCA1/2 mutation tests being provided to newly diagnosed breast cancer patients. The observed gradual increase in the total number of BRCA1/2 mutation tests, alongside a proportional rise in the testing frequency, while maintaining a stable BRCA1/2 mutation detection rate, serves as evidence that appropriate BRCA1/2 mutation tests are being conducted for eligible patients according to established criteria. It is noteworthy that the presence or absence of NIC for the BRCA1/2 mutation tests varies globally. Taiwan lacks NIC for genetic tests (including for the BRCA1/2 mutation). This shared characteristic highlights the financial burden on patients. Therefore, the strength of gene mutation test recommendations remains low, and BRCA1/2 mutation tests are not recommended to all patients meeting the NCCN criteria but only to those who can afford them financially. An absence of NIC is also observed in Vietnam and Singapore, where patients may face similar financial challenges [14]. Conversely, Japan, akin to Korea, provides NIC for BRCA1/2 mutation tests. This shared characteristic sets Japan and Korea apart from the other countries in this comparison. Even though the availability and structure of NIC for BRCA1/2 mutation tests may vary among these nations, professional genetic counseling and the referral of BRCA1/2-unaffected carriers and BRCA1/2-positive patients to specialized departments are available in all of the countries mentioned above. This diverse landscape of insurance coverage and genetic counseling practices emphasizes the need to consider the global context. The adoption of genetic tests and counseling for cancer patients still remains low in Asia due to several difficulties: financial reasons, lack of time and resources, and limited access to genetic counselors [15]. If NIC expansion lowered the financial barriers to genetic testing for cancer patients and more professional genetic counselors were available for proper genetic counseling in Asia, more patients would be able to benefit from it.
In terms of genetic counseling, the framework of counseling itself has not changed dramatically. However, since preliminary counseling (before the patient visits a doctor’s office) was added to the counseling flow, triple checks and counseling have become possible with the additional recruitment of genetic counselors. Also, more thorough pre- and post-genetic counseling is now possible by reinforcing the manpower of specialists (Figure 2). It is also clear from the results that the Korean NIC expansion and genetic counseling’s role together have important effects not only on new patients but also on follow-up patients who can be recalled for testing under the new insurance criteria with the support of professional counseling [16,17]. A great increase in the recall of follow-up patients at our center was possible after recruiting additional genetic counselors. The recall of follow-up patients for BRCA1/2 mutation tests is meaningful since about 8% of newly tested patients could be detected as BRCA-positive, which would lead to prophylactic treatments and an increase in the overall survival rate [18]. The international comparison of insurance coverage and genetic counseling practices further highlights the importance of addressing the financial aspect of BRCA1/2 mutation testing, as access to such tests is a critical factor in early cancer detection and proper management. The differences in NIC and the availability of counseling services across countries underline the need for a global perspective in the management of breast cancer patients. Our positive results regarding the Korean NIC expansion could serve as a potential model for other nations aiming to improve early detection and personalized treatment for breast cancer patients with BRCA1/2 mutations.
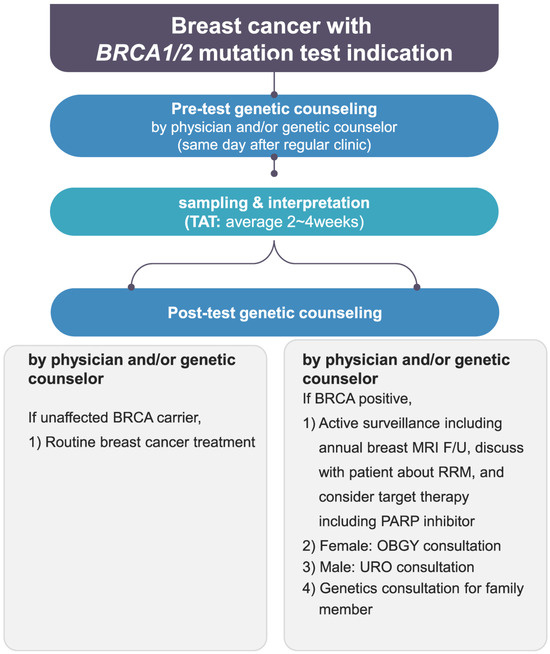
Figure 2.
Genetic counseling flowchart at the Breast Center of Samsung Medical Center. TAT, turn around time; MRI, magnetic resonance imaging; F/U, follow-up; RRM, risk-reducing mastectomy; URO, urology; OBGY, obstetrics and gynecology.
The proper detection of the BRCA1/2 mutation status of patients helps clinicians to make quick but informed and timely decisions for patients [19,20], which gradually leads to appropriate treatments. Also, the identification of the BRCA1/2 mutation status can not only inform family members of BRCA1/2 mutation carriers about their potential cancer risks but also help identify those who could benefit from active surveillance or risk reduction strategies, such as risk-reducing surgeries for breasts and ovaries [5,21,22,23]. For the early detection of the BRCA1/2 mutation status to be possible, financial aspects should be addressed, foremost since a BRCA1/2 mutation test is a costly process. If the Korean NIC could be expanded even more within reasonable standards, more patients would benefit from it. The effectiveness of surveillance in detecting breast cancer in BRCA1/2 mutation carriers was evaluated in 2017. The study included 2482 BRCA1/2 mutation carriers and found that enhanced surveillance with annual breast MRI scans and mammography resulted in a higher likelihood of detecting early-stage breast cancers. Specifically, the study showed that surveillance with breast MRI detected 56% of breast cancers in BRCA1 mutation carriers and 73% in BRCA2 mutation carriers. Additionally, mammography detected 33% of breast cancers in BRCA1 mutation carriers and 43% in BRCA2 mutation carriers [24].
BRCA1/2 mutation testing is of significant importance to breast cancer patients for several reasons, as it can impact their treatment decisions and overall management [17,18]. Gentile et al. reported that the rate of preoperative genetic diagnosis turned out to be only 21.8% in a large series of BRCA1/2 mutation carriers, with a great impact on the subsequent management of these patients [25]. The ability to ascertain the BRCA1/2 mutation status prior to surgery allows for informed genetic counseling and discussion of surgical options. This further underscores the importance of BRCA1/2 mutation testing in guiding clinical decision-making processes [26]. Hartmann et al. investigated the risk of subsequent breast cancer in BRCA1/2 mutation carriers who underwent a risk-reducing mastectomy [27]. The study found that among the BRCA1 mutation carriers, a risk-reducing mastectomy was associated with a 90% reduction in the risk of developing subsequent breast cancer (incidental cancer) during a median follow-up of 3.6 years [28]. For BRCA2 mutation carriers, a risk-reducing mastectomy was associated with an 86% reduction in the risk of developing subsequent breast cancer during the same follow-up period [29]. However, there remains ongoing debate regarding the optimal surgical management for BRCA1/2 mutation carriers, as evidence suggests that different surgical approaches (breast conserving surgery vs. bilateral mastectomy) may not significantly impact the oncological outcomes [25]. Therefore, the ability to ascertain the BRCA1/2 mutation status before surgery and engage in appropriate genetic counseling for surgical decision making would indeed confer significant advantages in patient management. Furthermore, recent findings by Apostolova et al. underscore the impact of preoperative genetic testing on surgical decision making. Their conclusion that genetic testing results delivered prior to index breast surgery increase the uptake of bilateral risk-reducing mastectomy (RRM) in affected BRCA1/2 and PALB2 carriers [30] aligns closely with our discussion on the importance of ascertaining the BRCA1/2 mutation status before surgery. This highlights the critical role of genetic testing in optimizing surgical decision making and underscores the need for efforts to make genetic testing more mainstream in clinical practice. In terms of treatment, the OlympiA trial investigated the effectiveness of adding olaparib, a targeted therapy, to standard therapy in breast cancer patients with BRCA1/2 mutations. The trial results showed that the addition of olaparib reduced the risk of invasive disease recurrence or death by 42% in patients with high-risk, early-stage breast cancer and BRCA1/2 mutations. According to the study, the invasive disease-free survival rate was 87.5% in the olaparib group compared to 77.1% in the placebo group after a 3-year follow-up. Also, the absolute improvement in invasive disease-free survival at three years with olaparib was 9.4%. These results suggest that olaparib benefited both groups of BRCA1/2 mutation carriers [31].
These findings demonstrate the importance of BRCA1/2 mutation testing in breast cancer patients, as identifying BRCA1/2 mutations can lead to personalized treatment decisions, such as the use of targeted therapies like olaparib, which have shown significant benefits in reducing the risk of recurrence and improving survival rates in patients with BRCA1/2 mutations.
5. Conclusions
In conclusion, the expansion of the Korean NIC and the concurrent recruitment of additional genetic counselors have had a significant and beneficial effect on the number of BRCA1/2 mutation tests conducted at the SMC. The results clearly indicate a gradual increase in the number of tests performed, all while maintaining a sufficient positive detection rate. This outcome underscores the positive impact of these measures, eventually leading to more tailored treatments for affected patients.
Author Contributions
Conceptualization: J.M.R.; data curation: M.K., B.Y.J., B.J.C., J.Y., J.E.L., S.W.K. and S.J.N.; investigation: Y.K. and J.Y.C.; methodology: H.L. and D.S.S.; supervision: J.M.R.; writing—original draft: S.Y.J.; writing—review and editing: J.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National R&D Program for Cancer Control through the National Cancer Center (NCC), funded by the Ministry of Health and Welfare, Republic of Korea (HA23C0144).
Institutional Review Board Statement
This study adhered to the ethical tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of SMC (IRB number: 2023-01-103-001, 7 February 2023).
Informed Consent Statement
The need for informed consent was waived because of the retrospective nature of this study.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silva, F.C.; Lisboa, B.C.; Figueiredo, M.C.; Torrezan, G.T.; Santos, É.M.; Krepischi, A.C.; Rossi, B.M.; Achatz, M.I.; Carraro, D.M. Hereditary breast and ovarian cancer: Assessment of point mutations and copy number variations in Brazilian patients. BMC Med. Genet. 2014, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Fackenthal, J.D.; Olopade, O.I. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat. Rev. Cancer 2007, 7, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Foulkes, W.D. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer 2004, 4, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Mitra, N.; Wan, F.; Sinilnikova, O.M.; Healey, S.; McGuffog, L.; Mazoyer, S.; Chenevix-Trench, G.; Easton, D.F.; Antoniou, A.C.; et al. Association of Type and Location of BRCA1 and BRCA2 Mutations With Risk of Breast and Ovarian Cancer. JAMA 2015, 313, 1347–1361. [Google Scholar] [CrossRef]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.C.; Rosen, B.; Bradley, L.; Fan, I.; Tang, J.; Li, S.; Zhang, S.; Shaw, P.A.; et al. Population BRCA1 and BRCA2 Mutation Frequencies and Cancer Penetrances: A Kin–Cohort Study in Ontario, Canada. JNCI J. Natl. Cancer Inst. 2006, 98, 1694–1706. [Google Scholar] [CrossRef]
- van der Kolk, D.M.; de Bock, G.H.; Leegte, B.K.; Schaapveld, M.; Mourits, M.J.E.; de Vries, J.; van der Hout, A.H.; Oosterwijk, J.C. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: High cancer incidence at older age. Breast Cancer Res. Treat. 2010, 124, 643–651. [Google Scholar] [CrossRef]
- Ryu, J.M.; Korean Hereditary Breast Cancer Study Group; Choi, H.J.; Kim, I.; Nam, S.J.; Kim, S.W.; Yu, J.; Lee, S.K.; Choi, D.H.; Park, Y.H.; et al. Prevalence and oncologic outcomes of BRCA1/2 mutations in unselected triple-negative breast cancer patients in Korea. Breast Cancer Res. Treat. 2019, 173, 385–395. [Google Scholar] [CrossRef]
- Ahn, S.H.; Hwang, U.K.; Kwak, B.S.; Yoon, H.S.; Ku, B.K.; Kang, H.J.; Kim, J.S.; Ko, B.K.; Ko, C.D.; Yoon, K.S.; et al. Prevalence of BRCA1 and BRCA2 Mutations in Korean Breast Cancer Patients. J. Korean Med. Sci. 2004, 19, 269–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stavropoulou, A.V.; Fostira, F.; Pertesi, M.; Tsitlaidou, M.; Voutsinas, G.E.; Triantafyllidou, O.; Bamias, A.; Dimopoulos, M.A.; Timotheadou, E.; Pectasides, D.; et al. Prevalence of BRCA1 Mutations in Familial and Sporadic Greek Ovarian Cancer Cases. PLoS ONE 2013, 8, e58182. [Google Scholar] [CrossRef]
- Wang, G.; Beattie, M.S.; Ponce, N.A.; Phillips, K.A. Eligibility criteria in private and public coverage policies for BRCA genetic testing and genetic counseling. Genet Med. 2011, 13, 1045–1050. [Google Scholar] [CrossRef]
- Korean Breast Cancer Society. The 10th Korean Clinical Practice Guideline for Breast Cancer [Internet]; Korean Breast Cancer Society: Seoul, Republic of Korea, 2023; Available online: https://www.kbcs.or.kr/sub02/sub02.html (accessed on 1 December 2023).
- Health Insurance Review & Assessment Service. Korean Health Insurance Review and Assessment Service Announcement [Internet]; Health Insurance Review & Assessment Service: Wonju, Republic of Korea, 2020. [Google Scholar]
- Health Insurance Review & Assessment Service. Korean Health Insurance Review and Assessment Service. Scope of Accreditation on BRCA (BRCA1, BRCA2) Gene Mutation Test [Internet]; Health Insurance Review & Assessment Service: Wonju, Republic of Korea, 2012. [Google Scholar]
- Hayashi, S.; Kubo, M.; Kaneshiro, K.; Kai, M.; Yamada, M.; Morisaki, T.; Takao, Y.; Shimazaki, A.; Shikada, S.; Nakamura, M. Genetic medicine is accelerating in Japan. Breast Cancer 2022, 29, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.; Tan, D.S.P.; Ryu, J.M. Genetic counselling and testing for breast and ovarian cancer in Asia: A multinational survey of unmet needs. J. Clin. Oncol. 2022, 40 (Suppl. S16), 10586. [Google Scholar] [CrossRef]
- Gleeson, M.; Meiser, B.; Barlow-Stewart, K.; Trainer, A.H.; Tucker, K.; Watts, K.J.; Friedlander, M.; Kasparian, N. Communication and Information Needs of Women Diagnosed With Ovarian Cancer Regarding Treatment-Focused Genetic Testing. Oncol. Nurs. Forum 2013, 40, 275–283. [Google Scholar] [CrossRef] [PubMed]
- American Society of Breast Surgeons. Consensus Guideline on Genetic Testiing for Hereditary Breast Cancer [Internet]. American Society of Breast Surgeons; 2019. Available online: https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-Genetic-Testing-for-Hereditary-Breast-Cancer.pdf (accessed on 1 December 2023).
- National Comprehensive Cancer Network. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Genetic/Familial High-Risk Assessment: Breast and Ovarian. V.1.2020. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419 (accessed on 1 December 2023).
- Pruthi, S.; Gostout, B.S.; Lindor, N.M. Identification and Management of Women With BRCA Mutations or Hereditary Predisposition for Breast and Ovarian Cancer. Mayo Clin. Proc. 2010, 85, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. BRCA1 and BRCA2: Cancer Risk and Genetic Testing [Internet]. National Cancer Institute; 2020. Available online: http://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet (accessed on 1 December 2023).
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average Risks of Breast and Ovarian Cancer Associated with BRCA1 or BRCA2 Mutations Detected in Case Series Unselected for Family History: A Combined Analysis of 22 Studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Iversen, E.S.; Friebel, T.; Finkelstein, D.; Weber, B.L.; Eisen, A.; Peterson, L.E.; Schildkraut, J.M.; Isaacs, C.; Peshkin, B.N.; et al. Characterization of BRCA1 and BRCA2 Mutations in a Large United States Sample. J. Clin. Oncol. 2006, 24, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.J.; Kruse, T.A.; Tan, Q.; Lænkholm, A.-V.; Bak, M.; Lykkesfeldt, A.E.; Sørensen, K.P.; Hansen, T.V.O.; Ejlertsen, B.; Gerdes, A.-M.; et al. Classifications within Molecular Subtypes Enables Identification of BRCA1/BRCA2 Mutation Carriers by RNA Tumor Profiling. PLoS ONE 2013, 8, e64268. [Google Scholar] [CrossRef] [PubMed]
- van Zelst, J.C.M.; Mus, R.D.M.; Woldringh, G.; Rutten, M.J.C.M.; Bult, P.; Vreemann, S.; de Jong, M.; Karssemeijer, N.; Hoogerbrugge, N.; Mann, R.M. Surveillance of Women with the BRCA1 or BRCA2 Mutation by Using Biannual Automated Breast US, MR Imaging, and Mammography. Radiology 2017, 285, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Losurdo, A.; Sagona, A.; Zuradelli, M.; Gatzemeier, W.; Barbieri, E.; Testori, A.; Errico, V.; Bianchi, P.; Biondi, E.; et al. Surgical management of BRCA-mutation carriers: A single institution experience. Eur. J. Surg. Oncol. (EJSO) 2022, 48, 1706–1712. [Google Scholar] [CrossRef]
- Woo, J.; Gwak, G.; Park, I.; Bae, B.N.; Lee, S.K.; Chae, B.J.; Yu, J.; Lee, J.E.; Kim, S.W.; Nam, S.J.; et al. Preoperative diagnosis of BRCA1/2 mutation impacts decision-making for risk-reducing mastectomy in breast cancer patients. Sci. Rep. 2021, 11, 14747. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Sellers, T.A.; Schaid, D.J.; Frank, T.S.; Soderberg, C.L.; Sitta, D.L.; Frost, M.H.; Grant, C.S.; Donohue, J.H.; Woods, J.E.; et al. Efficacy of Bilateral Prophylactic Mastectomy in BRCA1 and BRCA2 Gene Mutation Carriers. JNCI J. Natl. Cancer Inst. 2001, 93, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.; Tmg, B.T.T.; Hanson, H.; Seal, S.; Warren-Perry, M.; Hughes, D.; Howell, I.; Turnbull, C.; Houlston, R.; Shanley, S.; et al. BRCA1 testing should be offered to individuals with triple-negative breast cancer diagnosed below 50 years. Br. J. Cancer 2012, 106, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- van Asperen, C.J.; Brohet, R.M.; Meijers-Heijboer, E.J.; Hoogerbrugge, N.; Verhoef, S.; Vasen, H.F.A.; Ausems, M.G.E.M.; Menko, F.H.; Garcia, E.B.G.; Klijn, J.G.M.; et al. Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J. Med. Genet. 2005, 42, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, C.; Ferroum, A.; Alhassan, B.; Prakash, I.; Basik, M.; Boileau, J.F.; Martel, K.; Meterissian, S.; Corpuz, V.V.; Wong, N.; et al. Timing of Genetic Testing in BRCA1/2 and PALB2-Associated Breast Cancer: Preoperative Result Disclosure Increases Uptake of Risk-Reducing Mastectomy and Reduces Unnecessary Exposure to Radiotherapy. Eur. J. Surg. Oncol. (EJSO) 2024, 50, 108324. [Google Scholar] [CrossRef]
- Tutt, A.N.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).