Impact of Nutritional Status on Neutrophil-to-Lymphocyte Ratio as a Predictor of Efficacy and Adverse Events of Immune Check-Point Inhibitors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. ICIs Treatment Regimens
2.3. Follow-up
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
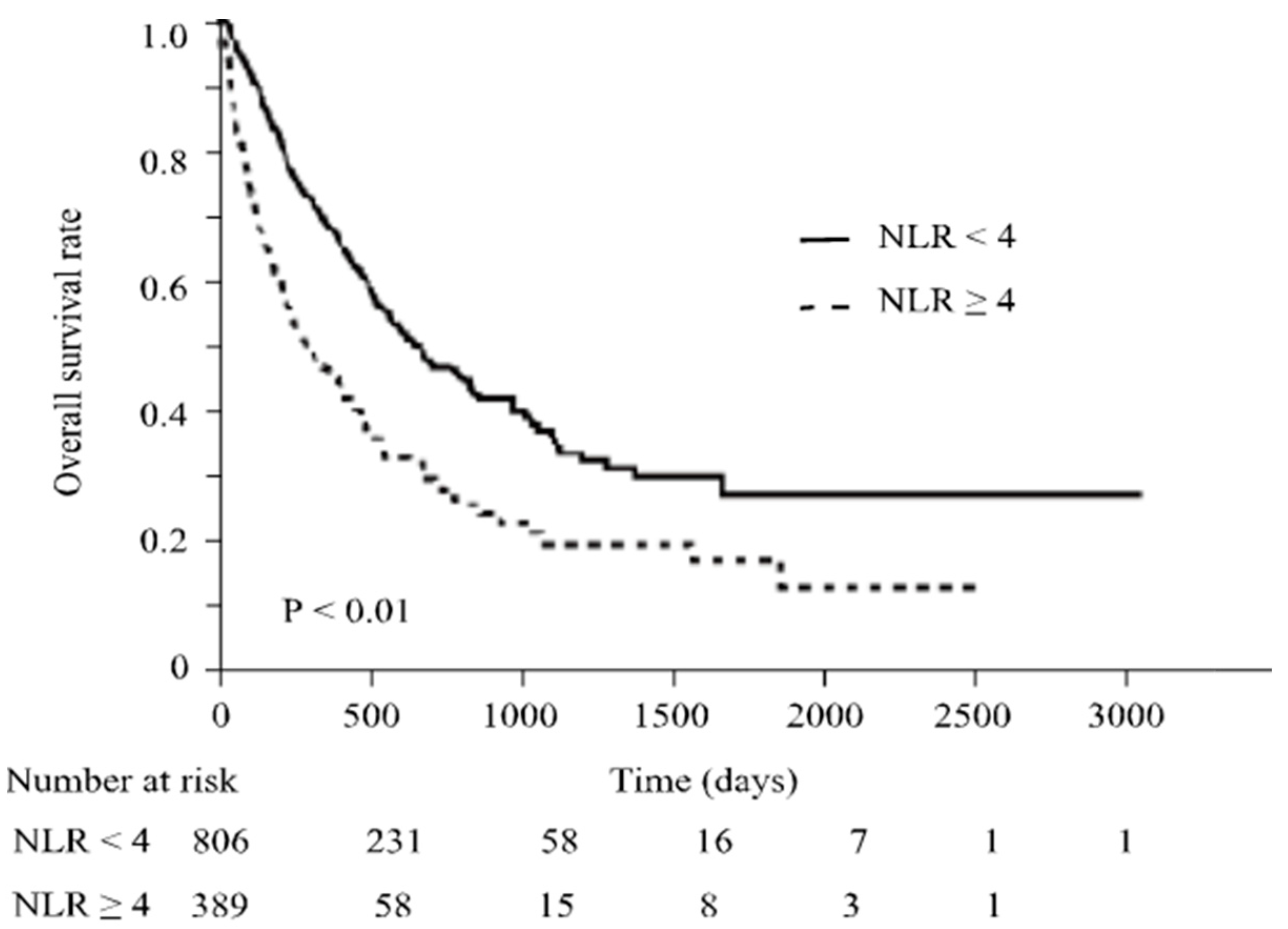
3.2. Factors Associated with ICI Response and irAE Occurrence
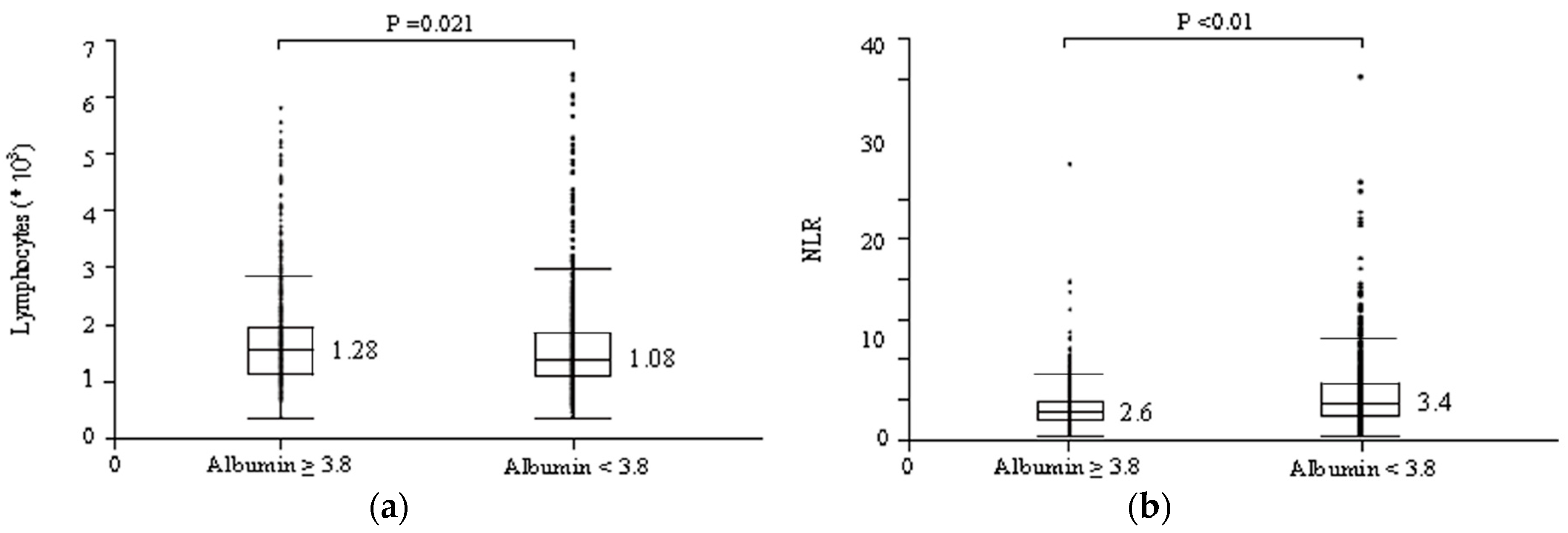
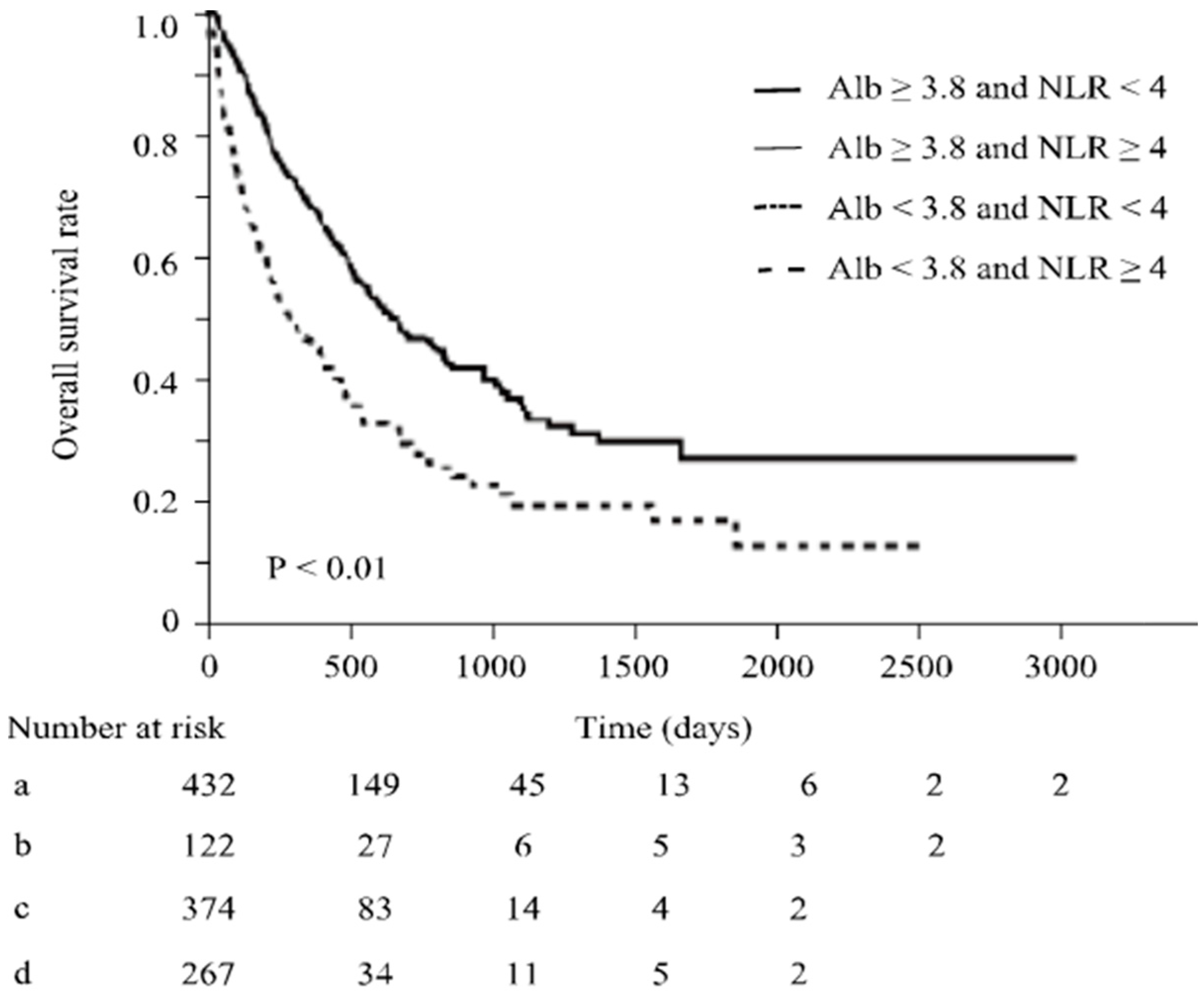
3.3. Serum Albumin Levels May Affect the Relationships between NLR and irAE Occurrence and between NLR and Therapeutic Effects of ICIs
3.4. Effect of Albumin Levels on Overall Survival by in ICI Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Shikha, S.; Bellur, P. Cancer immunotherapy with checkpoint inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin. Cancer Biol. 2020, 64, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Fessas, P.; Possamai, L.A.; Clark, J.; Daniels, E.; Gudd, C.; Mullish, B.H.; Alexander, J.L.; Pinato, D.J. Immunotoxicity from checkpoint inhibitor therapy: Clinical features and underlying mechanisms. Immunology 2020, 159, 167–177. [Google Scholar] [CrossRef]
- Myers, G. Immune-related adverse events of immune checkpoint inhibitors: A brief review. Curr. Oncol. 2018, 25, 342–347. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors. A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Hoffner, B.; Rubin, K.M. Meeting the challenge of immune-related adverse events with optimized telephone triage and dedicated oncology acute care. J. Adv. Pract. Oncol. 2019, 10 (Suppl. S1), 9–20. [Google Scholar]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Rowland, A.; Kichenadasse, G.; Wiese, M.D.; Gurney, H.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br. J. Cancer 2017, 117, 913–920. [Google Scholar] [CrossRef]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.J.; Kothari, S.; Aggarwal, C.; Bauml, J.M.; Alley, E.W.; Evans, T.L.; Kosteva, J.A.; Ciunci, C.A.; Gabriel, P.E.; Thompson, J.C.; et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Daly, L.E.; Power, D.G.; O’Reilly, Á.; Donnellan, P.; Cushen, S.J.; O’Sullivan, K.; Twomey, M.; Woodlock, D.P.; Redmond, H.P.; Ryan, A.M. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer 2017, 116, 310–317. [Google Scholar] [CrossRef]
- Diehl, A.; Yarchoan, M.; Hopkins, A.; Jaffee, E.; Grossman, S.A. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 2017, 8, 114268–114280. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, M.; Strong, C.J.; Minturn, D.; Gray, D.K.; Kopple, J.D. Nutritional status and lymphocyte function in maintenance hemodialysis patients. Am. J. Clin. Nutr. 1984, 39, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Fock, R.A.; Blatt, S.L.; Beutler, B.; Pereira, J.; Tsujita, M.; de Barros, F.E.; Borelli, P. Study of lymphocyte subpopulations in bone marrow in a model of protein–energy malnutrition. Nutrition 2010, 26, 1021–1028. [Google Scholar] [CrossRef]
- Veldhoen, M.; Ferreira, C. Influence of nutrient-derived metabolites on lymphocyte immunity. Nat. Med. 2015, 21, 709–718. [Google Scholar] [CrossRef]
- Björkholm, M.; Bark, S.; Backman, L.; Jarstrand, C.; Holm, G. Lymphocyte and granulocyte function in nutritionally depleted patients. The effect of 2 weeks of total parenteral nutrition. Clin. Nutr. 1993, 12, 350–354. [Google Scholar] [CrossRef]
- Chandra, R.K. Lymphocyte Subpopulations in Human Malnutrition: Cytotoxic and Suppressor Cells. Pediatrics 1977, 59, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.V. The Role of Nutrition in Cancer Patients. Nutr. Cancer 2021, 73, 2849–2850. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Lin, P.T.; Lin, C.C.; Wu, T.H.; Liu, L.T.; Su, C.W.; Teng, W.; Tsai, C.Y.; Huang, C.H.; Chen, W.T.; et al. Adding nutritional status to the original BCLC stage improves mortality prediction for hepatocellular carcinoma patients in HBV-endemic regions. Am. J. Cancer Res. 2023, 13, 3618–3628. [Google Scholar] [PubMed]
- Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int. Suppl. 2012, 2, 139–274. [Google Scholar]
- Bernardi, M.; Angeli, P.; Claria, J.; Moreau, R.; Gines, P.; Jalan, R.; Caraceni, P.; Fernandez, J.; Gerbes, A.L.; O’Brien, A.J.; et al. Albumin in decompensated cirrhosis: New concepts and perspectives. Gut 2020, 69, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Numerical and functional deficiency in T helper cells in protein energy malnutrition. Clin. Exp. Immunol. 1983, 51, 126–132. [Google Scholar] [PubMed]
- Malas, S.; Harrasser, M.; Lacy, K.E.; Karagiannis, S.N. Antibody therapies for melanoma: New and emerging opportunities to activate immunity (Review). Oncol. Rep. 2014, 32, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Vasaturo, A.; Di Blasio, S.; Peeters, D.G.; de Koning, C.C.; de Vries, J.M.; Figdor, C.G.; Hato, S.V. Clinical Implications of Co-Inhibitory Molecule Expression in the Tumor Microenvironment for DC Vaccination: A Game of Stop and Go. Front. Immunol. 2013, 4, 417. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Lee, P.Y.; Oen KQ, X.; Lim GR, S.; Hartono, J.L.; Muthiah, M.; Huang, D.Q.; Teo FS, W.; Li, A.Y.; Mak, A.; Chandran, N.S.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers 2021, 13, 1308. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, D.; Khaki, A.R.; Morelli, M.P.; Diamantopoulos, L.; Singh, N. Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Sci. Rep. 2021, 11, 9029. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, O.; Yumiko, S.; McMillan, D.C.; Miki, C. Clinical burden of nutrition assessment in treatment for gastrointestinal cancer. J. Jpn. Soc. Parenter. Enter. Nutr. 2017, 32, 829–840. [Google Scholar]

| Patient Characteristics | Study Patients (n = 1234) |
|---|---|
| Age (years) | 69 (60–95) + |
| Gender | |
| Male (%) | 892 (72.3) |
| Female (%) | 342 (27.7) |
| White blood cells (/μL) | 5325 (4150–6960) + |
| Neutrophils (/μL) | 3286 (2097–4610) + |
| Lymphocytes (/μL) | 1172 (813–1713) + |
| NLR | 2.9 (1.8–4.6) + |
| Albumin (g/dL) | 3.8 (3.3–4.0) + |
| Total Bilirubin (mg/dL) | 0.5 (0.4–0.7) + |
| Aspartate aminotransferase (U/L) | 20 (17–27) + |
| Alanine aminotransferase (U/L) | 15 (11–23) + |
| Creatinine (mg/dL) | 0.8 (0.7–1.0) + |
| Cholinesterase (U/L) | 246 (185–305) + |
| Total cholesterol (mg/dL) | 178 (151–210) + |
| Immunoglobulin G (mg/dL) | 1144 (953–1403) + |
| Drug | |
| Nivolumab (%) | 526 (42.6) |
| Pembrolizumab (%) | 369 (29.9) |
| Atezolizumab (%) | 187 (12.2) |
| Nivolumab + Ipilimumab (%) | 76 (6.2) |
| Durvalumab (%) | 47 (3.8) |
| Ipilimumab (%) | 16 (1.3) |
| Avelumab (%) | 13 (1.0) |
| irAE occurrence (%) | 333 (27.0) |
| irAE grade (1 /2 /3 /4) | 55 /150 /108 /19 |
| Time to irAE occurrence (days) | 68 (26–167) + |
| Observation period (days) | 280 (138–489) + |
| Type of irAEs | All Events (n = 413) |
|---|---|
| Liver injury (%) | 84 (20.8) |
| Pneumonia (%) | 71 (17.2) |
| Thyroid dysfunction (%) | 63 (15.3) |
| Rash (%) | 54 (13.1) |
| Adrenocortical insufficiency (%) | 28 (6.8) |
| Colitis (%) | 29 (7.0) |
| Renal dysfunction (%) | 16 (3.9) |
| Arthritis (%) | 9 (2.2) |
| Cytopenia (%) | 8 (1.9) |
| Pituitary dysfunction (%) | 7 (1.7) |
| Infusion reaction (%) | 7 (1.7) |
| Gastritis (%) | 5 (1.2) |
| Type 1 diabetes (%) | 5 (1.2) |
| Muscle pain (%) | 3 (0.7) |
| Cholangitis (%) | 3 (0.7) |
| Pancreatitis (%) | 3 (0.7) |
| Others (%) | 18 (4.4) |
| Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (Range) + | p-Value | Hazard Ratio (Range) + | p-Value | |
| Age (≥70 years) | 0.9 (0.83–1.1) + | NS | ||
| Sex (male) | 0.9 (0.79–1.1) + | NS | ||
| NLR < 4 | 1.3 (1.1–1.5) + | <0.01 | 1.2 (1.1–1.4) + | 0.033 |
| Total Bilirubin (≥1.0 mg/dL) | 1.0 (0.77–1.3) + | NS | ||
| Creatinine (<0.8 mg/dL) | 1.2 (0.99–1.3) + | 0.056 | 1.1 (0.98–1.3) + | NS |
| Immunoglobulin G (≥1200 mg/dL) | 0.93 (0.74–1.2) + | NS | ||
| Fever within 24 h (present) | 1.1 (0.79–1.3) + | NS | ||
| irAEs (present) | 1.9 (1.6–2.2) + | <0.01 | 1.9 (1.6–2.2) + | <0.01 |
| Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odds Ratio (Range) + | p-Value | Odds Ratio (Range) + | p-Value | |
| Age (≥70years) | 1.1 (0.85-1.4) + | NS | ||
| Sex (male) | 1.0 (0.78–1.3) + | NS | ||
| NLR < 4 | 1.4 (1.0–1.8) + | 0.042 | 1.4 (1.0–1.9) + | 0.022 |
| Total Bilirubin (≥1.0 mg/dL) | 0.8 (0.49–1.3) + | NS | ||
| Creatinine (≥0.8 mg/dL) | 1.2 (0.90–1.5) + | NS | ||
| Immunoglobulin G (≥1200 mg/dL) | 0.88 (0.58–1.3) + | NS | ||
| Ipilimumab (present) | 3.7 (2.3–5.9) + | <0.01 | 3.6 (2.2–5.9) + | <0.01 |
| Albumin < 3.8 g/dL | Albumin ≥ 3.8 g/dL | |||
|---|---|---|---|---|
| Parameters | Multivariate Analysis | Multivariate Analysis | ||
| Odds Ratio (Range) + | p-Value | Odds Ratio (Range) + | p-Value | |
| NLR < 4 | 1.2 (0.74–1.8) + | NS | 1.7 (1.1–2.6) + | 0.030 |
| Ipilimumab (present) | 3.3 (1.7–6.4) + | <0.01 | 4.0 (1.9–8.7) + | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sue, M.; Takeuchi, Y.; Hirata, S.; Takaki, A.; Otsuka, M. Impact of Nutritional Status on Neutrophil-to-Lymphocyte Ratio as a Predictor of Efficacy and Adverse Events of Immune Check-Point Inhibitors. Cancers 2024, 16, 1811. https://doi.org/10.3390/cancers16101811
Sue M, Takeuchi Y, Hirata S, Takaki A, Otsuka M. Impact of Nutritional Status on Neutrophil-to-Lymphocyte Ratio as a Predictor of Efficacy and Adverse Events of Immune Check-Point Inhibitors. Cancers. 2024; 16(10):1811. https://doi.org/10.3390/cancers16101811
Chicago/Turabian StyleSue, Masahiko, Yasuto Takeuchi, Shoichiro Hirata, Akinobu Takaki, and Motoyuki Otsuka. 2024. "Impact of Nutritional Status on Neutrophil-to-Lymphocyte Ratio as a Predictor of Efficacy and Adverse Events of Immune Check-Point Inhibitors" Cancers 16, no. 10: 1811. https://doi.org/10.3390/cancers16101811
APA StyleSue, M., Takeuchi, Y., Hirata, S., Takaki, A., & Otsuka, M. (2024). Impact of Nutritional Status on Neutrophil-to-Lymphocyte Ratio as a Predictor of Efficacy and Adverse Events of Immune Check-Point Inhibitors. Cancers, 16(10), 1811. https://doi.org/10.3390/cancers16101811




