Unlocking Bevacizumab’s Potential: rCBVmax as a Predictive Biomarker for Enhanced Survival in Glioblastoma IDH-Wildtype Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cohort Description
- Inclusion in one of two cohorts: the BVZ cohort (comprising patients treated with bevacizumab after tumor progression) or the control cohort (consisting of patients who did not receive additional treatment after tumor progression);
- A minimum survival period of 30 days;
- Compliance with the standard Stupp treatment protocol.
2.2. Magnetic Resonance Imaging (MRI)
2.3. MRI Processing and rCBV Calculation
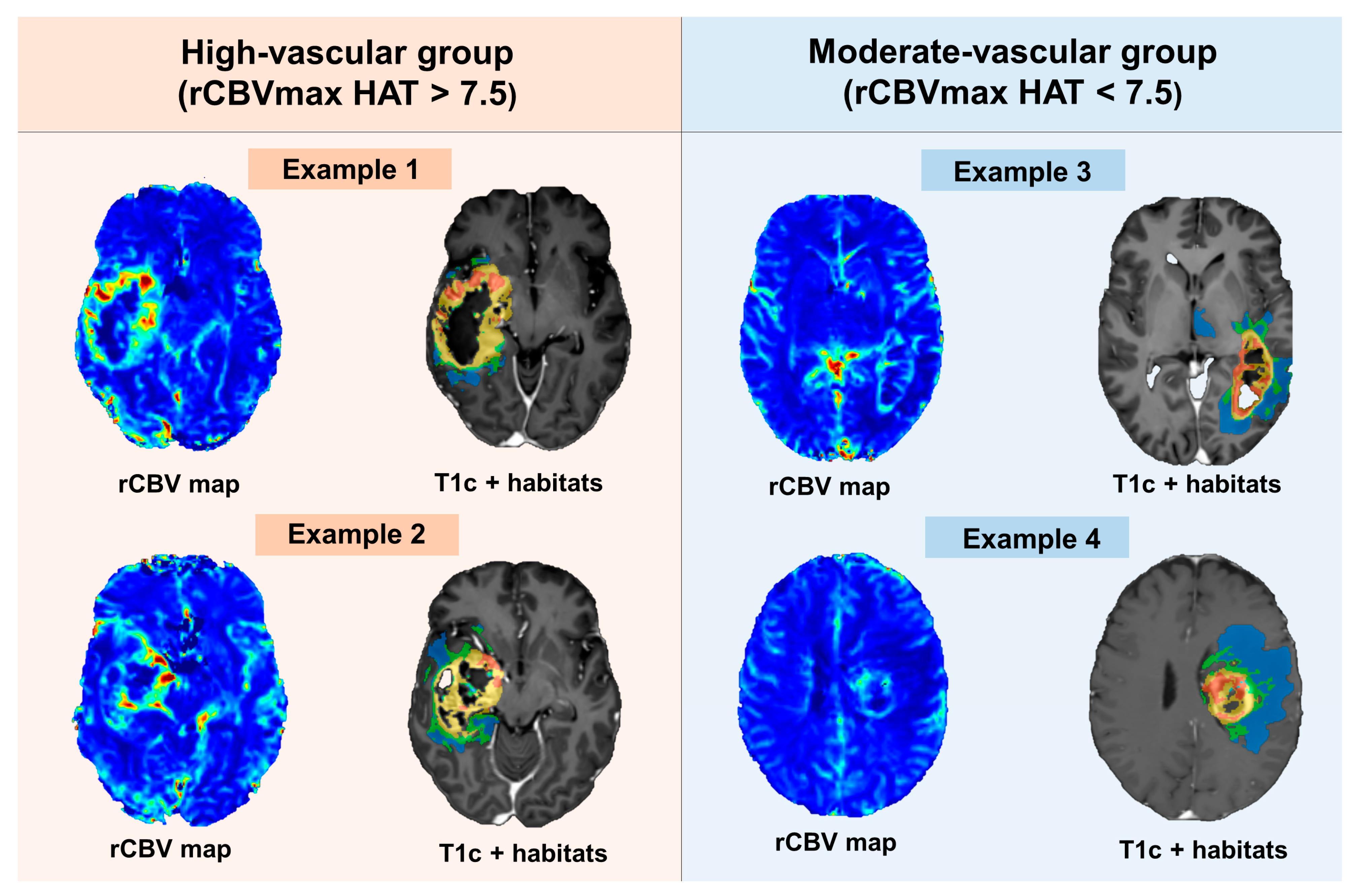
2.4. rCBV Threshold to Define Vascular Groups
2.5. Statistical Analyses
2.5.1. Dataset Description: Distinctions between Moderate- and High-Vascularity Groups
2.5.2. Analysis of Survival Differences among Groups
2.5.3. Comparison of Responses to BVZ between Vascular Groups
3. Results
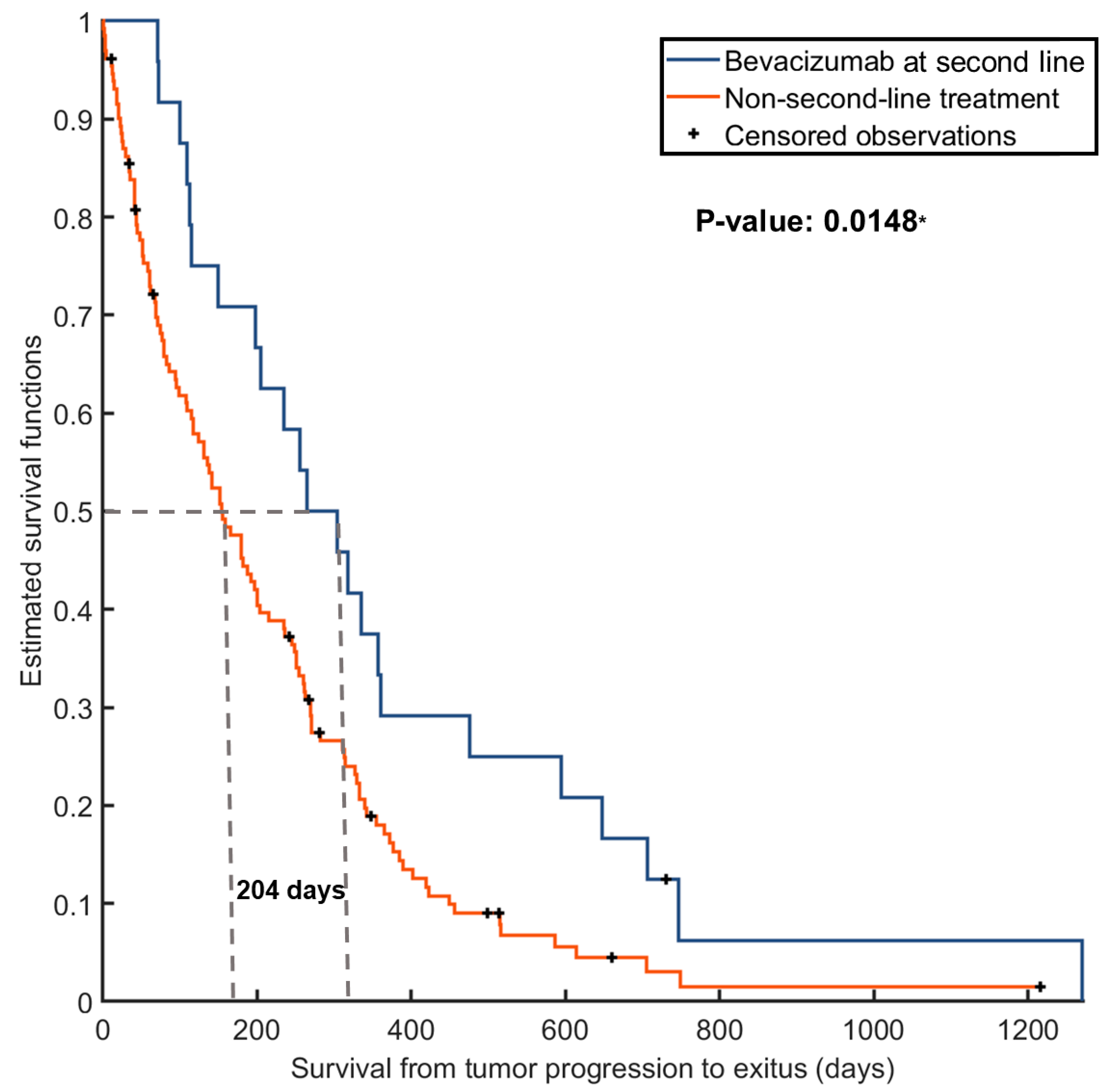
3.1. Benefit of Providing BVZ for the Entire Cohort
3.2. Cohort and Group Description
3.3. Survival Differences between Vascular Groups
3.4. Enhanced Responses to BVZ in Patients with Moderate-Vascular Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGahan, B.G.; Neilsen, B.K.; Kelly, D.L.; McComb, R.D.; Kazmi, S.A.J.; White, M.L.; Zhang, Y.; Aizenberg, M.R. Assessment of vascularity in glioblastoma and its implications on patient outcomes. J. Neuro-Oncol. 2017, 132, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Birner, P.; Piribauer, M.; Fischer, I.; Gatterbauer, B.; Marosi, C.; Ambros, P.F.; Ambros, I.M.; Bredel, M.; Oberhuber, G.; Rössler, K.; et al. Vascular Patterns in Glioblastoma Influence Clinical Outcome and Associate with Variable Expression of Angiogenic Proteins: Evidence for Distinct Angiogenic Subtypes. Brain Pathol. 2003, 13, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Marsden, P.A. Angiogenesis in glioblastoma. N. Engl. J. Med. 2013, 369, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Folkerth, R.D. Histologic measures of angiogenesis in human primary brain tumors. In Angiogenesis in Brain Tumors; Cancer Treatment and Research Series; Springer: Berlin/Heidelberg, Germany, 2004; Volume 117, pp. 79–95. [Google Scholar]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Juan-Albarracín, J.; Fuster-Garcia, E.; Pérez-Girbés, A.; Aparici-Robles, F.; Alberich-Bayarri, Á.; Revert-Ventura, A.; Martí-Bonmatí, L.; García-Gómez, J.M. Glioblastoma: Vascular Habitats Detected at Preoperative Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging Predict Survival. Radiology 2018, 287, 944–954. [Google Scholar] [CrossRef]
- lvarez-Torres, M.; Juan-Albarracín, J.; Fuster-Garcia, E.; Bellvís-Bataller, F.; Lorente, D.; Reynés, G.; Font de Mora, J.; Aparici-Robles, F.; Botella, C.; Muñoz-Langa, J.; et al. Robust association between vascular habitats and patient prognosis in glioblastoma: An international multi-center study. J. Magn. Reson. Imaging 2020, 51, 1478–1486. [Google Scholar] [CrossRef]
- Malik, D.G.; Rath, T.J.; Urcuyo Acevedo, J.C.; Canoll, P.D.; Swanson, K.R.; Boxerman, J.L.; Quarles, C.C.; Schmainda, K.M.; Burns, T.C.; Hu, L.S. Advanced MRI Protocols to Discriminate Glioma from Treatment Effects: State of the Art and Future Directions. Front. Radiol. 2022, 2, 809373. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: Don’t forget the peritumoral brain zone. Neuro-Oncology 2015, 17, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Hara, A.; Kunisada, T.; Yoshimura, S.; Iwama, T.; Park, D.M. The evidence of glioblastoma heterogeneity. Sci. Rep. 2015, 5, 7979. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Torres, M.d.M.; Fuster-García, E.; Juan-Albarracín, J.; Reynés, G.; Aparici-Robles, F.; Ferrer-Lozano, J.; García-Gómez, J.M. Local detection of microvessels in IDH-wildtype glioblastoma using relative cerebral blood volume: An imaging marker useful for astrocytoma grade 4 classification. BMC Cancer 2022, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, O.M.; Del Mar Álvarez-Torres, M.; Figueiredo, P.; Hangel, G.; Keil, V.C.; Nechifor, R.E.; Riemer, F.; Schmainda, K.M.; Warnert, E.A.H.; Wiegers, E.C.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 1: Perfusion and Diffusion Techniques. Front. Oncol. 2022, 12, 810263. [Google Scholar] [CrossRef] [PubMed]
- Stumpo, V.; Guida, L.; Bellomo, J.; Van Niftrik, C.H.B.; Sebök, M.; Berhouma, M.; Bink, A.; Weller, M.; Kulcsar, Z.; Regli, L.; et al. Hemodynamic Imaging in Cerebral Diffuse Glioma-Part B: Molecular Correlates, Treatment Effect Monitoring, Prognosis, and Future Directions. Cancers 2022, 14, 1342. [Google Scholar] [CrossRef] [PubMed]
- Guida, L.; Stumpo, V.; Bellomo, J.; Van Niftrik, C.H.B.; Sebök, M.; Berhouma, M.; Bink, A.; Weller, M.; Kulcsar, Z.; Regli, L.; et al. Hemodynamic Imaging in Cerebral Diffuse Glioma-Part A: Concept, Differential Diagnosis and Tumor Grading. Cancers 2022, 14, 1432. [Google Scholar] [CrossRef] [PubMed]
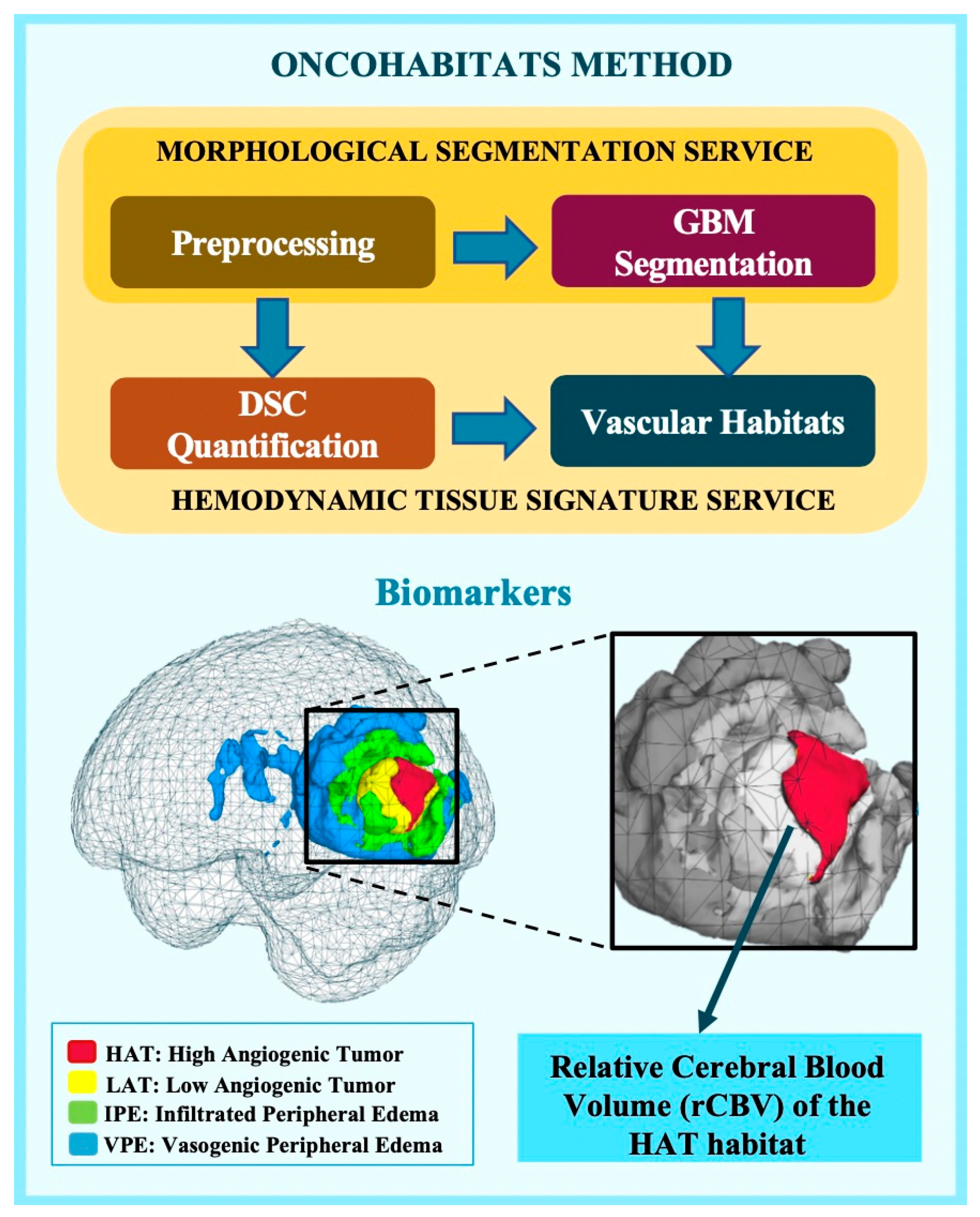
- Juan-Albarracín, J.; Fuster-Garcia, E.; García-Ferrando, G.A.; García-Gómez, J.M. ONCOhabitats: A system for glioblastoma heter-ogeneity assessment through MRI. Int. J. Med. Inform. 2019, 128, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Despotović, I.; Goossens, B.; Philips, W. MRI segmentation of the human brain: Challenges, methods, and applications. Comput. Math. Methods Med. 2015, 2015, 450341. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Clavreul, A.; Aubry, M.; Com, E.; De Tayrac, M.; Eliat, P.-A.; Henry, C.; Rousseau, A.; Mosser, J.; Menei, P. Characterizing the peritumoral brain zone in glioblastoma: A multidisciplinary analysis. J. Neuro-Oncol. 2015, 122, 53–61. [Google Scholar] [CrossRef]
- Schoenegger, K.; Oberndorfer, S.; Wuschitz, B.; Struhal, W.; Hainfellner, J.; Prayer, D.; Heinzl, H.; Lahrmann, H.; Marosi, C.; Grisold, W. Peritumoral edema on MRI at initial diagnosis: An independent prognostic factor for glioblastoma? Eur. J. Neurol. 2009, 16, 874–878. [Google Scholar] [CrossRef]
- Akbari, H.; Macyszyn, L.; Da, X.; Wolf, R.L.; Bilello, M.; Verma, R.; O’Rourke, D.M.; Davatzikos, C. Pattern analysis of dynamic suscep-tibility contrast-enhanced MR imaging demonstrates peritumoral tissue heterogeneity. Radiology 2014, 273, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Torres, M.d.M.; Fuster-García, E.; Balaña, C.; Puig, J.; García-Gómez, J.M. Lack of Benefit of Extending Temozolomide Treatment in Patients with High Vascular Glioblastoma with Methylated MGMT. Cancers 2021, 13, 5420. [Google Scholar] [CrossRef]
- Jensen, R.L.; Mumert, M.L.; Gillespie, D.L.; Kinney, A.Y.; Schabel, M.C.; Salzman, K.L. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is pre-dictive of patient outcome. Neuro-Oncology 2014, 16, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.S.; Eschbacher, J.M.; Dueck, A.C.; Heiserman, J.E.; Liu, S.; Karis, J.P.; Smith, K.A.; Shapiro, W.R.; Pinnaduwage, D.S.; Coons, S.W.; et al. Correlations between Perfusion MR Imaging Cerebral Blood Volume, Microvessel Quantification, and Clinical Outcome Using Stereotactic Analysis in Recurrent High-Grade Glioma. Am. J. Neuroradiol. 2012, 33, 69–76. [Google Scholar] [CrossRef]
- Lev, M.H.; Ozsunar, Y.; Henson, J.W.; Rasheed, A.A.; Barest, G.D.; Harsh, G.R., 4th; Fitzek, M.M.; Chiocca, E.A.; Rabinov, J.D.; Csavoy, A.N.; et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: Confounding effect of elevated rCBV of oligodendrogliomas [corrected]. Am. J. Neuroradiol. 2004, 25, 214–221. [Google Scholar] [PubMed]
- Reardon, D.A.; Desjardins, A.; Peters, K.B.; Gururangan, S.; Sampson, J.H.; McLendon, R.E.; Herndon, J.E., 2nd; Bulusu, A.; Threatt, S.; Friedman, A.H.; et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naïve, recurrent glioblastoma. J. Neurooncol. 2012, 107, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E., 2nd; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.A.; Camphausen, K.; et al. Phase II Trial of Single-Agent Bevacizumab Followed by Bevacizumab Plus Irinotecan at Tumor Progression in Recurrent Glioblastoma. J. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; Vredenburgh, J.; Huang, J.; Zheng, M.; Cloughesy, T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef]
- Ali, S.A.; McHayleh, W.M.; Ahmad, A.; Sehgal, R.; Braffet, M.; Rahman, M.; Bejjani, G.; Friedland, D.M.; Neil, D.; Ajlan, A.; et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: A series of 13 cases. J. Neurosurg. 2008, 109, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Bokstein, F.; Shpigel, S.; Blumenthal, D.T. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 2008, 112, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Gutin, P.H.; Iwamoto, F.M.; Beal, K.; Mohile, N.A.; Karimi, S.; Hou, B.L.; Lymberis, S.; Yamada, Y.; Chang, J.; Abrey, L.E. Safety and Efficacy of Bevacizumab with Hypofractionated Stereotactic Irradiation for Recurrent Malignant Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Sathornsumetee, S.; Desjardins, A.; Vredenburgh, J.J.; McLendon, R.E.; Marcello, J.; Herndon, J.E.; Mathe, A.; Hamilton, M.; Rich, J.N.; Norfleet, J.A.; et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro-Oncology 2010, 12, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Lee, E.Q.; Hammond, S.; Grimm, S.A.; Norden, A.D.; Beroukhim, R.; Gerard, M.; Schiff, D.; Chi, A.S.; Batchelor, T.T.; et al. Phase I study of panobinostat in combination with bevacizumab for recurrent high-grade glioma. J. Neuro-Oncol. 2012, 107, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Sampson, J.H.; Sathornsumetee, S.; McLendon, R.E.; Herndon, J.E., 2nd; Marcello, J.E.; Norfleet, J.; et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: A phase II study. Br. J. Cancer. 2009, 101, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Anderson, S.K.; Lafky, J.M.; Uhm, J.H.; Giannini, C.; Kumar, S.K.; Kimlinger, T.K.; Northfelt, D.W.; Flynn, P.J.; Jaeckle, K.A.; et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): A north central cancer treatment group trial. Clin. Cancer Res. 2013, 19, 4816–4823. [Google Scholar] [CrossRef]
- Cabrera, A.R.; Cuneo, K.C.; Vredenburgh, J.J.; Sampson, J.H.; Kirkpatrick, J.P. Stereotactic radiosurgery and bevacizumab for recurrent glioblastoma multiforme. J. Natl. Compr. Cancer Netw. 2012, 10, 695–699. [Google Scholar] [CrossRef]
- Zuniga, R.M.; Torcuator, R.; Jain, R.; Anderson, J.; Doyle, T.; Ellika, S.; Schultz, L.; Mikkelsen, T. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J. Neuro-Oncol. 2009, 91, 329–336. [Google Scholar] [CrossRef]
- Balaña, C.; Estival, A.; Pineda, E.; Sepúlveda, J.; Mesía, C.; del Barco, S.; Gil-Gil, M.; Hardy, M.; Indacoechea, A.; Cardona, A.F. Prolonged survival after bevacizumab rechallenge in glioblastoma patients with previous response to bevacizumab. Neuro-Oncol. Pract. 2017, 4, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, A. The glioblastoma vasculature as a target for cancer therapy. Biochem. Soc. Trans. 2014, 42, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Wiestler, B.; Burth, S.; Wick, A.; Nowosielski, M.; Heiland, S.; Schlemmer, H.-P.; Wick, W.; Bendszus, M.; Radbruch, A. Relative cerebral blood volume is a potential predictive imaging biomarker of bevacizumab efficacy in recurrent glioblastoma. Neuro-Oncology 2015, 17, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.; Esteve-Codina, A.; Martinez-Garcia, M.; Alameda, F.; Carrato, C.; Arpi, O.; Balana, C. Glioblastoma gene expression subtypes and correlation with clinical, molecular and immunohistochemical characteristics in a homogenously treated cohort: GLIOCAT project. J. Clin. Oncol. 2019, 37, 2029. [Google Scholar] [CrossRef]
- Boxerman, J.L.; Snyder, B.S.; Barboriak, D.P.; Schmainda, K.M. Early post-bevacizumab change in rCBV from DSC-MRI identifies pseudore-sponse in recurrent glioblastoma: Results from ACRIN 6677/RTOG 0625. Front. Oncol. 2023, 26, 1061502. [Google Scholar] [CrossRef] [PubMed]


| Variables | Entire Cohort | Moderate-Vascular Group | High-Vascular Group | Mann–Whitney Results (p-Value) |
|---|---|---|---|---|
| Number of patients | 106 | 25 | 81 | - |
| Proportions per group | 100% | 23.5% | 76.4% | |
| Gender | ||||
| -Number of patients | 44 | 17 | 27 | |
| -Proportion of females | 41.5% | 68.0% | 33.3% | 0.0022 * |
| Age at diagnosis (years) | ||||
| -Mean | 59 | 58 | 59 | 0.6470 |
| -Range (min, max) | (17,77) | (25,76) | (17,77) | - |
| Overall survival (months) | ||||
| -Median | 13.4 | 14.8 | 13.2 | 0.5246 |
| Extent of resection. No. of patients (%) | ||||
| -Complete | 20 (18.9%) | 6 (24.0%) | 14 (17.3%) | 0.4586 |
| -Partial maximum | 21 (19.8%) | 4 (16.0%) | 17 (21.0%) | 0.5899 |
| -Partial | 41 (38.7%) | 9 (36.0%) | 32 (39.5%) | 0.7575 |
| -Biopsy | 24 (22.6%) | 6 (24.0%) | 18 (22.2%) | 0.9198 |
| MGMT methylation status. No. of patients (%) | ||||
| -Methylated | 38 (35.8%) | 8 (32.0%) | 30 (37.0%) | 0.6510 |
| -Unmethylated | 48 (45.3%) | 8 (32.0%) | 40 (49.4%) | 0.1298 |
| -Unknown info | 20 (18.9%) | 9 (36.0%) | 11 (13.6%) | - |
| Preoperative KPS | ||||
| -Patients with info | 89 (84.0%) | 20 (80.0%) | 69 (85.2%) | - |
| -Median KPS | 80 | 80 | 80 | 0.8383 |
| Postoperative KPS | ||||
| -Patients with info | 99 (93.4%) | 25 (100%) | 74 (91.4%) | - |
| -Median KPS | 70 | 70 | 70 | 0.6711 |
| Treatment. No. of patients (%) | ||||
| -Complications | 15 (14.1%) | 3 (12.0%) | 12 (14.8%) | 0.7300 |
| -Complete CT | 92 (86.8%) | 21 (84.0%) | 71 (87.6%) | 0.6432 |
| -Complete RT | 99 (93.4%) | 23 (92.0%) | 76 (93.8%) | 0.7555 |
| Variables | Bevacizumab Group | Control Group | Mann–Whitney Results (p-Value) |
|---|---|---|---|
| Number of patients | 39 | 67 | - |
| Proportions per group | 36.8% | 63.2% | |
| Gender | |||
| -Number of females | 15 | 29 | |
| -Proportion of females | 38.4% | 43.2% | 0.6314 |
| Age at diagnosis (years) | |||
| -Mean | 54 | 61 | 0.0019 * |
| -Range (min, max) | (17,72) | (25,77) | - |
| Overall survival (months) | |||
| -Median | 18.3 | 9.6 | <0.0001 * |
| Extent of resection. No. of patients (%) | |||
| -Complete | 7 (17.9%) | 13 (19.4%) | 0.8581 |
| -Partial maximum | 6 (15.4%) | 15 (22.4%) | 0.3878 |
| -Partial | 19 (48.7%) | 22 (32.8%) | 0.1079 |
| -Biopsy | 8 (20.5%) | 16 (23.9%) | 0.3028 |
| MGMT methylation status. No. of patients (%) | |||
| -Methylated | 13 (33.3%) | 25 (37.3%) | 0.6848 |
| -Unmethylated | 22 (56.4%) | 26 (38.8%) | 0.0812 |
| -Unknown info | 4 (10.3%) | 16 (23.9%) | 0.0862 |
| Preoperative KPS | |||
| -Patients with info | 37 (94.5%) | 52 (77.6%) | - |
| -Median KPS | 80 | 80 | 0.2600 |
| Postoperative KPS | |||
| -Patients with info | 38 (97.4%) | 61 (91.0%) | - |
| -Postoperative | 80 | 80 | 0.8893 |
| Treatment. No. of patients (%) | |||
| -Complications | 4 (10.3%) | 11 (16.4%) | 0.3853 |
| -Complete CT | 37 (94.9%) | 55 (82.1%) | 0.0629 |
| -Complete RT | 37 (94.9%) | 62 (92.5%) | 0.6478 |
| Survival Time from Progression | Moderate-Vascular Group | High-Vascular Group |
|---|---|---|
| Absolute numbers (percentage) | ||
| 3 months | 11 (100%) | 24 (85.7%) |
| 6 months | 9 (81.8%) | 19 (67.8%) |
| 12 months | 4 (36.4%) | 8 (28.6%) |
| 18 months | 4 (36.4%) | 3 (10.7%) |
| 24 months | 2 (18.2%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Torres, M.d.M.; Balaña, C.; Fuster-García, E.; Puig, J.; García-Gómez, J.M. Unlocking Bevacizumab’s Potential: rCBVmax as a Predictive Biomarker for Enhanced Survival in Glioblastoma IDH-Wildtype Patients. Cancers 2024, 16, 161. https://doi.org/10.3390/cancers16010161
Álvarez-Torres MdM, Balaña C, Fuster-García E, Puig J, García-Gómez JM. Unlocking Bevacizumab’s Potential: rCBVmax as a Predictive Biomarker for Enhanced Survival in Glioblastoma IDH-Wildtype Patients. Cancers. 2024; 16(1):161. https://doi.org/10.3390/cancers16010161
Chicago/Turabian StyleÁlvarez-Torres, María del Mar, Carmen Balaña, Elies Fuster-García, Josep Puig, and Juan Miguel García-Gómez. 2024. "Unlocking Bevacizumab’s Potential: rCBVmax as a Predictive Biomarker for Enhanced Survival in Glioblastoma IDH-Wildtype Patients" Cancers 16, no. 1: 161. https://doi.org/10.3390/cancers16010161
APA StyleÁlvarez-Torres, M. d. M., Balaña, C., Fuster-García, E., Puig, J., & García-Gómez, J. M. (2024). Unlocking Bevacizumab’s Potential: rCBVmax as a Predictive Biomarker for Enhanced Survival in Glioblastoma IDH-Wildtype Patients. Cancers, 16(1), 161. https://doi.org/10.3390/cancers16010161








