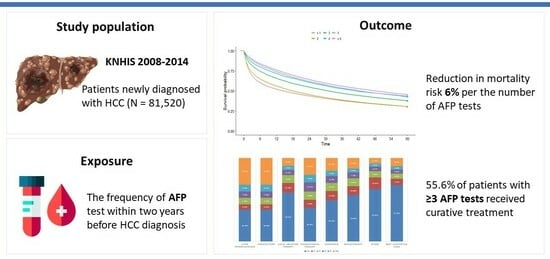
Regular Alpha-Fetoprotein Tests Boost Curative Treatment and Survival for Hepatocellular Carcinoma Patients in an Endemic Area
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Source: National Health Insurance Service
2.3. Study Population
2.4. Clinical Variables
2.5. Operational Definitions
2.6. Definition of Outcomes
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Baseline Characteristics
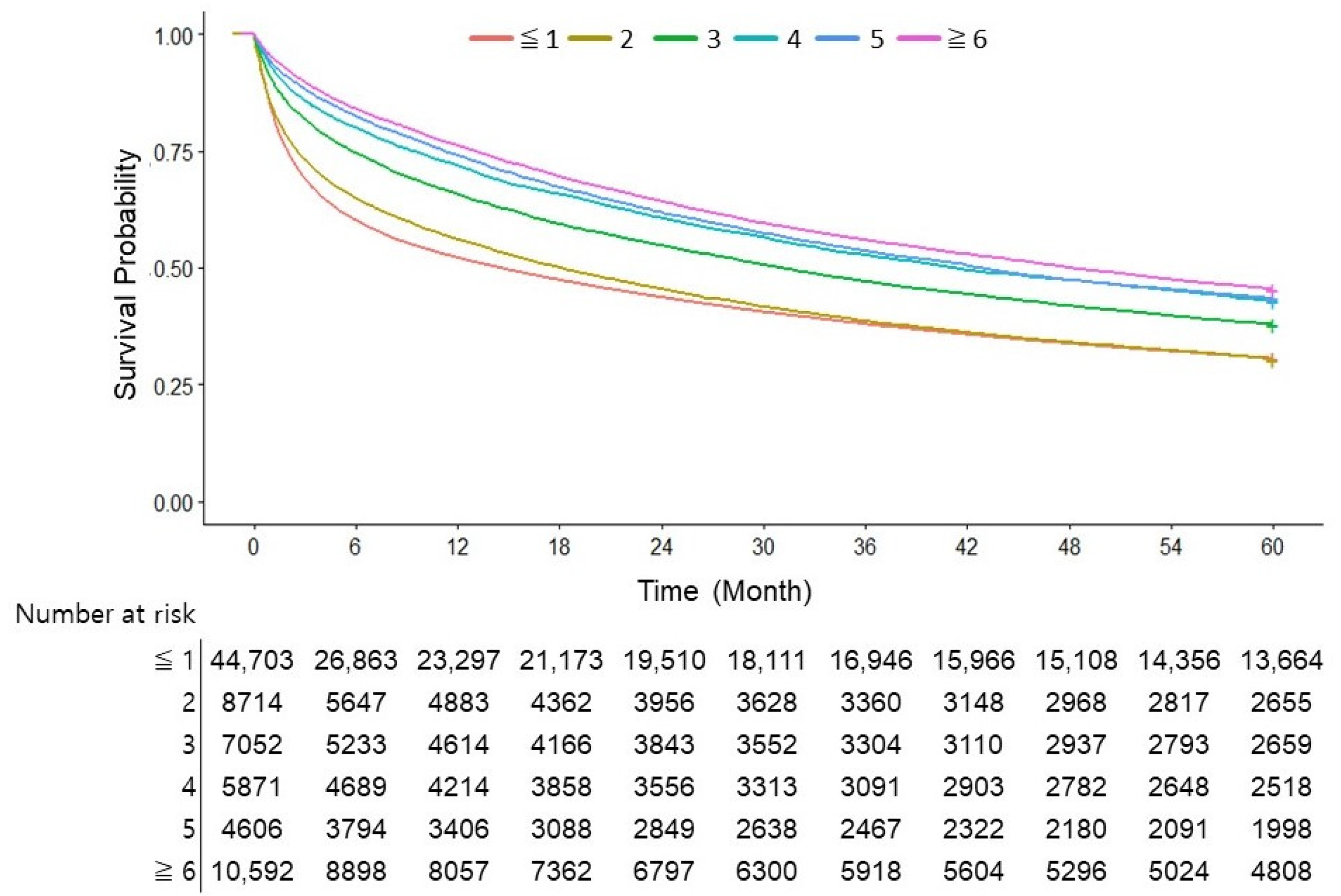
3.3. Number of AFP Tests Was an Independent Risk Factor for Overall Survival in Patients with HCC
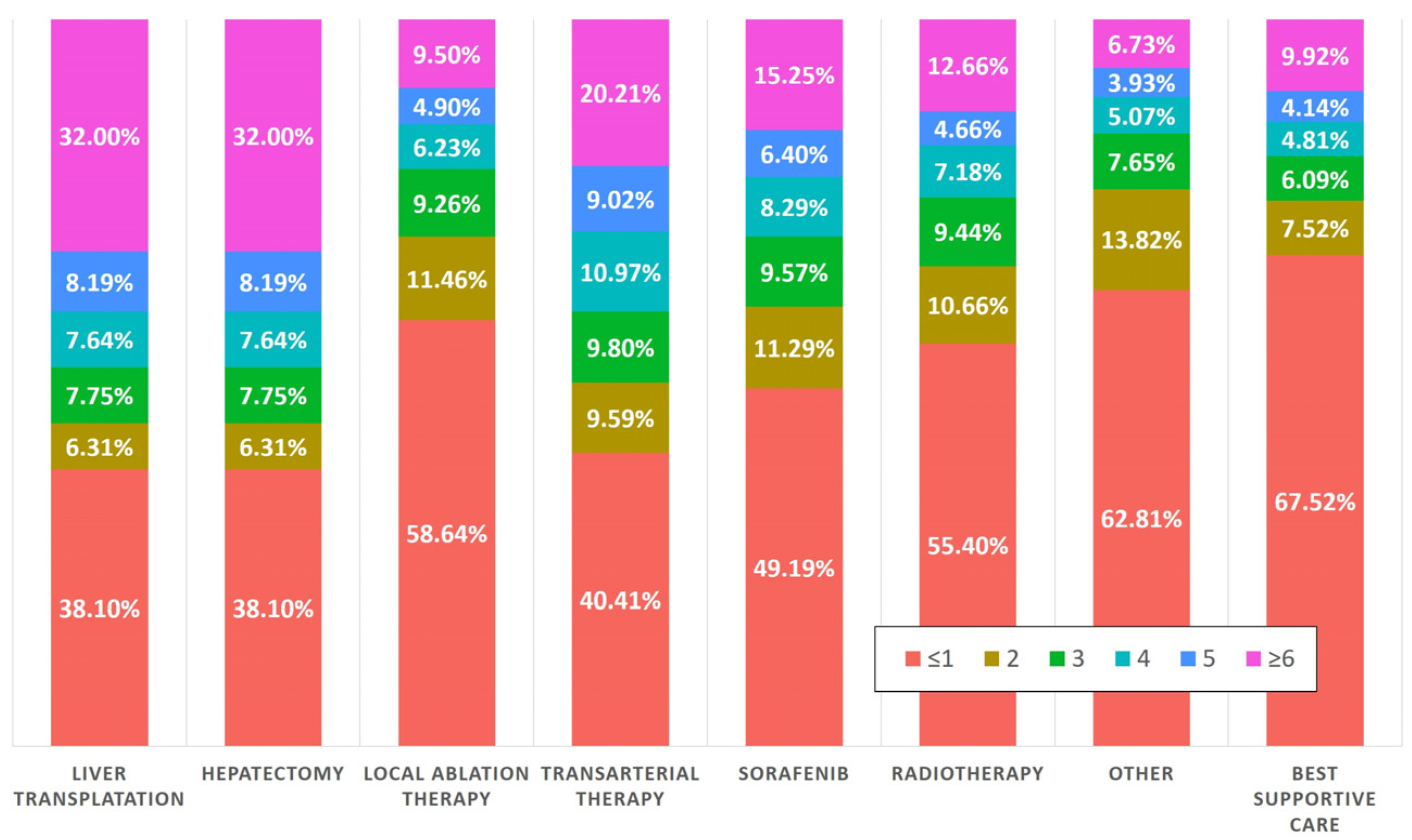
3.4. Nearly Half of the Patients Who Had Undergone AFP Testing Underwent Curative Treatment
3.5. Three or More AFP Tests in Two Years Were Associated with Decreased Overall Survival
3.6. AFP Testing in Viral Hepatitis-Associated HCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korean Liver Cancer Association; National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J. Liver Cancer 2023, 23, 1–120. [Google Scholar] [CrossRef]
- Kim, B.H.; Lim, Y.S.; Kim, E.Y.; Kong, H.J.; Won, Y.J.; Han, S.; Park, S.; Hwang, J.S. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J. Gastroenterol. Hepatol. 2018, 33, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Singal, A.G.; Zhang, E.; Narasimman, M.; Rich, N.E.; Waljee, A.K.; Hoshida, Y.; Yang, J.D.; Reig, M.; Cabibbo, G.; Nahon, P.; et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 77, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Cho, Y.; Park, J.W. Surveillance for hepatocellular carcinoma: It is time to move forward. Clin. Mol. Hepatol. 2022, 28, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Toan, B.N.; Tan, C.K.; Hasan, I.; Setiawan, L.; Yu, M.L.; Izumi, N.; Huyen, N.N.; Chow, P.K.; Mohamed, R.; et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin. Mol. Hepatol. 2023, 29, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Biselli, M.; Conti, F.; Gramenzi, A.; Frigerio, M.; Cucchetti, A.; Fatti, G.; D’Angelo, M.; Dall’Agata, M.; Giannini, E.G.; Farinati, F.; et al. A new approach to the use of α-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br. J. Cancer 2015, 112, 69–76. [Google Scholar] [CrossRef]
- Kim, K.A.; Lee, J.S.; Jung, E.S.; Kim, J.Y.; Bae, W.K.; Kim, N.H.; Moon, Y.S. Usefulness of serum alpha-fetoprotein (AFP) as a marker for hepatocellular carcinoma (HCC) in hepatitis C virus related cirrhosis: Analysis of the factors influencing AFP elevation without HCC development. Korean J. Gastroenterol. 2006, 48, 321–326. [Google Scholar]
- Gebo, K.A.; Chander, G.; Jenckes, M.W.; Ghanem, K.G.; Herlong, H.F.; Torbenson, M.S.; El-Kamary, S.S.; Bass, E.B. Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C: A systematic review. Hepatology 2002, 36, S84–S92. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Parkin, D.M.; Chen, Q.G.; Lu, J.H.; Shen, Q.J.; Zhang, B.C.; Zhu, Y.R. Screening for liver cancer: Results of a randomised controlled trial in Qidong, China. J. Med. Screen. 2003, 10, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; da Fonseca, L.G.; Faivre, S. New trials and results in systemic treatment of HCC. J. Hepatol. 2018, 69, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar] [PubMed]
- Almeida, P.H.; Matielo, C.E.L.; Curvelo, L.A.; Rocco, R.A.; Felga, G.; Della Guardia, B.; Boteon, Y.L. Update on the management and treatment of viral hepatitis. World J. Gastroenterol. 2021, 27, 3249–3261. [Google Scholar] [CrossRef]
- World Health Organization. Republic of Korea Health System Review; World Health Organization, Regional Office for the Western: Geneva, Switzerland, 2015. [Google Scholar]
- Singal, A.G.; Conjeevaram, H.S.; Volk, M.L.; Fu, S.; Fontana, R.J.; Askari, F.; Su, G.L.; Lok, A.S.; Marrero, J.A. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 793–799. [Google Scholar] [CrossRef]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Gopal, P.; Yopp, A.C.; Waljee, A.K.; Chiang, J.; Nehra, M.; Kandunoori, P.; Singal, A.G. Factors that affect accuracy of α-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 870–877. [Google Scholar] [CrossRef]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef]
- Ahn, S.B.; Choi, J.; Jun, D.W.; Oh, H.; Yoon, E.L.; Kim, H.S.; Jeong, S.W.; Kim, S.E.; Shim, J.J.; Cho, Y.K.; et al. Twelve-month post-treatment parameters are superior in predicting hepatocellular carcinoma in patients with chronic hepatitis B. Liver Int. Off. J. Int. Assoc. Study Liver 2021, 41, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.G.; Dimitropoulou, P.; Turner, R.M.; Jenks, S.J.; Cusack, P.; Hey, S.; Blunsum, A.; Kelly, S.; Sturgeon, C.; Hayes, P.C.; et al. Alpha-Fetoprotein Detection of Hepatocellular Carcinoma Leads to a Standardized Analysis of Dynamic AFP to Improve Screening Based Detection. PLoS ONE 2016, 11, e0156801. [Google Scholar] [CrossRef] [PubMed]
- Su, T.H.; Chang, S.H.; Chen, C.L.; Liao, S.H.; Tseng, T.C.; Hsu, S.J.; Hong, C.M.; Liu, C.H.; Yang, H.C.; Liu, C.J.; et al. Serial increase and high alpha-fetoprotein levels predict the development of hepatocellular carcinoma in 6 months. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2023, 53, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.; Ott, P.; Andersen, P.K.; Sørensen, H.T.; Vilstrup, H. Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis: A Danish nationwide cohort study. Ann. Intern. Med. 2012, 156, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Tsai, H.I.; Lee, W.C.; Huang, S.W.; Lin, C.Y.; Hsieh, Y.C.; Kuo, T.; Chen, C.W.; Yu, M.C. Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal? J. Clin. Med. 2019, 8, 1736. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Wang, J.; Chenivesse, X.; Henglein, B.; Bréchot, C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature 1990, 343, 555–557. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Kajiyama, Y.; Tian, J.; Locker, J. Characterization of distant enhancers and promoters in the albumin-alpha-fetoprotein locus during active and silenced expression. J. Biol. Chem. 2006, 281, 30122–30131. [Google Scholar] [CrossRef]
- Shen, H.; Luan, F.; Liu, H.; Gao, L.; Liang, X.; Zhang, L.; Sun, W.; Ma, C. ZHX2 is a repressor of alpha-fetoprotein expression in human hepatoma cell lines. J. Cell. Mol. Med. 2008, 12, 2772–2780. [Google Scholar] [CrossRef]
- Ramai, D.; Singh, J.; Lester, J.; Khan, S.R.; Chandan, S.; Tartaglia, N.; Ambrosi, A.; Serviddio, G.; Facciorusso, A. Systematic review with meta-analysis: Bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 53, 977–984. [Google Scholar] [CrossRef] [PubMed]
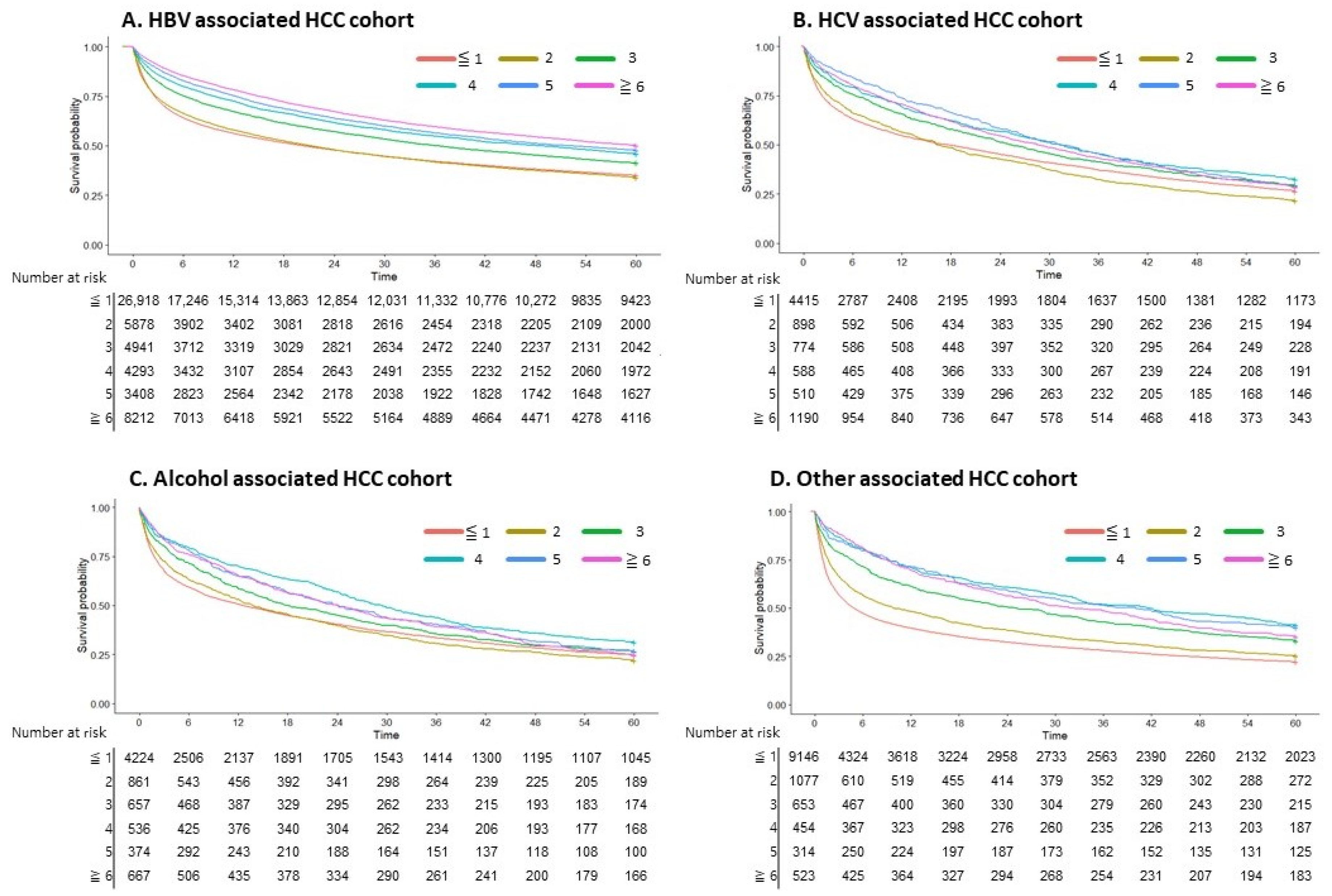
| HBV n = 53,656 | HCV n = 8376 | Alcohol n = 7319 | Other n = 12,169 | p-Value | ||
|---|---|---|---|---|---|---|
| Male, n (%) | 42,744 (79.66) | 5830 (69.60) | 6837 (93.41) | 8522 (70.03) | <0.001 | |
| Age, n (%) | <0.001 | |||||
| 10~19 | 13 (0.02) | 22 (0.18) | ||||
| 20~29 | 152 (0.28) | 6 (0.07) | 4 (0.05) | 86 (0.71) | ||
| 30~39 | 1680 (3.13) | 35 (0.42) | 72 (0.98) | 163 (1.34) | ||
| 40~49 | 10,042 (18.72) | 366 (4.37) | 480 (6.56) | 647 (5.32) | ||
| 50~59 | 21,274 (39.65) | 1346 (16.07) | 1611 (22.01) | 1677 (13.78) | ||
| 60~69 | 13,680 (25.50) | 2487 (29.69) | 2552 (34.87) | 3055 (25.10) | ||
| 70+ | 6815 (12.70) | 4136 (49.38) | 2600 (35.52) | 6519 (53.57) | ||
| Index date year, n (%) | <0.001 | |||||
| 2008 | 7885 (14.70) | 1202 (14.35) | 1052 (14.37) | 2131 (17.51) | ||
| 2009 | 7715 (14.38) | 1197 (14.29) | 951 (12.99) | 1730 (14.22) | ||
| 2010 | 7557 (14.08) | 1166 (13.92) | 989 (13.51) | 1773 (14.57) | ||
| 2011 | 7909 (14.74) | 1238 (14.78) | 1047 (14.31) | 1586 (13.03) | ||
| 2012 | 7532 (14.04) | 1248 (14.90) | 1113 (15.21) | 1624 (13.35) | ||
| 2013 | 7480 (13.94) | 1169 (13.96) | 1106 (15.11) | 1661 (13.65) | ||
| 2014 | 7578 (14.12) | 1156 (13.80) | 1061 (14.5) | 1664 (13.67) | ||
| Cirrhosis, n (%) | 44,767 (83.43) | 6858 (81.88) | 6571 (89.78) | 5753 (47.28) | <0.001 | |
| Diabetes mellitus, n (%) | 24,890 (46.39) | 5040 (60.17) | 4733 (64.67) | 6444 (52.95) | <0.001 | |
| Hypertension, n (%) | 25,935 (48.34) | 5752 (68.67) | 4645 (63.46) | 7596 (62.42) | <0.001 | |
| Dyslipidemia, n (%) | 20,697 (38.57) | 3639 (43.45) | 3209 (43.84) | 4725 (38.83) | <0.001 | |
| CCI, n (%) | 6.76 (2.41) | 7.42 (2.47) | 7.54 (2.42) | 7.02 (2.79) | <0.001 | |
| low (≤6) | 29,029 (54.10) | 3402 (40.62) | 2764 (37.76) | 5596 (45.99) | <0.001 | |
| high (>6) | 24,627 (45.90) | 4974 (59.38) | 4555 (62.24) | 6573 (54.01) | ||
| HCC treatment, n (%) | <0.001 | |||||
| Liver transplantation | 782 (1.46) | 45 (0.54) | 51 (0.70) | 25 (0.21) | ||
| Hepatectomy | 7474 (13.93) | 580 (6.92) | 477 (6.54) | 1153 (9.47) | ||
| Local ablation therapy a | 5297 (9.87) | 1060 (12.66) | 660 (9.02) | 523 (4.30) | ||
| Transarterial therapy b | 24,649 (45.94) | 3861 (46.10) | 3114 (42.55) | 3207 (26.35) | ||
| Sorafenib | 2022 (3.77) | 216 (2.58) | 206 (2.81) | 379 (3.11) | ||
| Radiotherapy | 1545 (2.88) | 190 (2.27) | 144 (1.97) | 419 (3.44) | ||
| Other c | 733 (1.37) | 98 (1.17) | 108 (1.48) | 391 (3.21) | ||
| Best supportive care | 11,154 (20.79) | 2326 (27.77) | 2559 (34.96) | 6072 (49.90) | ||
| HBV n = 53,656 | HCV n = 8376 | Alcohol n = 7319 | Other n = 12,169 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Healthcare utilization | |||||||
| Hospital admission, mean (SD) | 2.83 (2.89) | 3.41 (3.84) | 3.66 (3.87) | 3.0 (3.69) | <0.001 | ||
| Clinic visits, mean (SD) | 35.90 (37.65) | 59.22 (53.67) | 46.13 (44.81) | 49.40 (50.98) | <0.001 | ||
| Blood tests, number | |||||||
| ALT, n (%) | 5.91 (5.25) | 7.06 (5.90) | 6.36 (6.08) | 3.85 (4.42) | <0.001 | ||
| 0 | 7776 (14.49) | 809 (9.66) | 851 (24.11) | 2839 (23.33) | <0.001 | ||
| 1 | 4481 (8.35) | 639 (7.63) | 646 (18.30) | 1773 (14.57) | |||
| 2 | 4296 (8.01) | 619 (7.39) | 635 (17.99) | 1464 (12.03) | |||
| 3 | 4209 (7.87) | 597 (7.13) | 646 (18.30) | 1206 (9.91) | |||
| ≥4 | 32,894 (61.31) | 5712 (68.19) | 752 (21.30) | 4887 (40.16) | |||
| AFP, n (%) | 2.42 (2.81) | 2.32 (2.82) | 1.89 (2.41) | 1.09 (1.91) | <0.001 | ||
| ≤1 | 26,920 (50.17) | 4416 (52.72) | 4224 (57.71) | 9147 (75.17) | <0.001 | ||
| 2 | 5881 (10.96) | 898 (10.72) | 861 (11.76) | 1078 (8.86) | |||
| 3 | 4941 (9.21) | 774 (9.24) | 657 (8.98) | 653 (5.37) | |||
| 4 | 4293 (8.00) | 588 (7.02) | 536 (7.32) | 454 (3.73) | |||
| 5 | 3408 (6.35) | 510 (6.09) | 374 (5.11) | 314 (2.58) | |||
| ≥6 | 8213 (15.31) | 1190 (14.21) | 667 (9.11) | 523 (4.30) | |||
| HBV DNA, n (%) | 1.09 (1.81) | 0.03 (0.19) | 0.03 (0.25) | 0.06 (0.33) | <0.001 | ||
| 0 | 30,590 (57.01) | 8190 (97.78) | 7119 (97.27) | 11,608 (95.39) | <0.001 | ||
| 1 | 10,091 (18.81) | 156 (1.86) | 167 (2.28) | 468 (3.85) | |||
| 2 | 4629 (8.63) | 24 (0.29) | 24 (0.33) | 53 (0.44) | |||
| 3 | 2730 (5.09) | 4 (0.05) | 4 (0.05) | 20 (0.16) | |||
| ≥4 | 5616 (10.47) | 2 (0.02) | 5 (0.07) | 20 (0.16) | |||
| Radiologic studies, number | |||||||
| abdominal CT, n (%) | 2.11 (2.20) | 2.01 (2.06) | 1.89 (2.02) | 1.24 (1.71) | <0.001 | ||
| 0 | 22,644 (42.20) | 3600 (42.98) | 3271 (44.69) | 7054 (57.97) | <0.001 | ||
| 1 | 7741 (14.43) | 1389 (16.58) | 1186 (16.20) | 1954 (16.06) | |||
| ≥2 | 23,271 (43.37) | 3387 (40.44) | 2862 (39.10) | 3161 (25.98) | |||
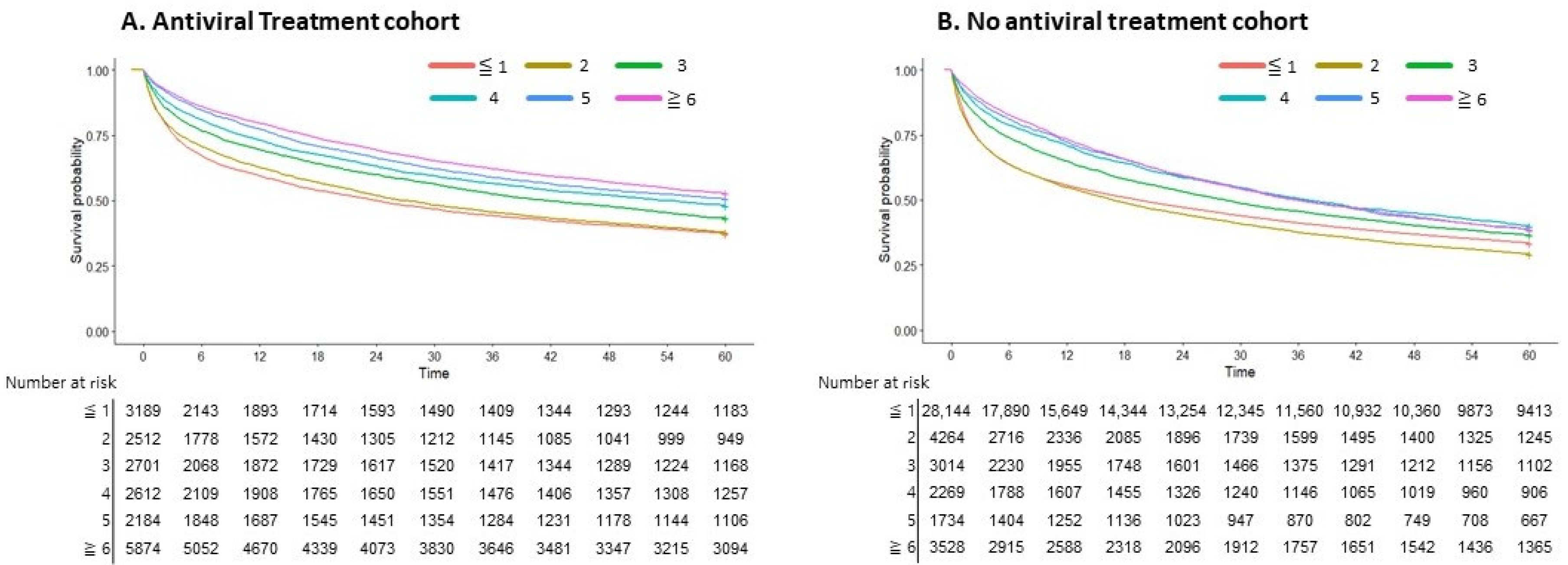
| Antiviral treatment | |||||||
| Yes | 17,992 (33.53) | 1082 (12.92) | NA | NA | <0.001 | ||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| All Cohort | HBV, HCV Cohort | |||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Whole Model | NA | <0.001 | <0.001 | |||
| Male gender | 1.13 (1.109–1.156) | <0.001 | 1.17 (1.143–1.193) | <0.001 | 1.19 (1.156–1.217) | <0.001 |
| Age | 1.02 (1.019–1.021) | <0.001 | 1.01 (1.012–1.013) | <0.001 | 1.00 (1.003–1.005) | <0.001 |
| Cirrhosis | 1.07 (1.044–1.089) | <0.001 | 1.00 (0.980–1.023) | 0.891 | 1.13 (1.102–1.168) | <0.001 |
| Diabetes mellitus | 1.20 (1.175–1.216) | <0.001 | 1.03 (1.009–1.046) | 0.004 | 1.04 (1.014–1.058) | 0.001 |
| Hypertension | 1.15 (1.128–1.167) | <0.001 | 0.77 (0.759–0.789) | <0.001 | 0.77 (0.752–0.787) | <0.001 |
| Dyslipidemia | 0.86 (0.841–0.871) | <0.001 | 0.76 (0.749–0.777) | <0.001 | 0.77 (0.754–0.787) | <0.001 |
| CCI | 1.21 (1.202–1.210) | <0.001 | 1.120 (1.192–1.200) | <0.001 | 1.22 (1.213–1.224) | <0.001 |
| HCC treatment, curative vs. non-curative | 0.23 (0.223–0.236) | <0.001 | 0.26 (0.254–0.269) | <0.001 | 0.26 (0.254–0.271) | <0.001 |
| AFP number | 0.93 (0.930–0.936) | <0.001 | 0.94 (0.940–0.947) | <0.001 | 0.96 (0.952–0.960) | <0.001 |
| Antiviral treatment | 0.71 (0.692–0.724) | <0.001 | NA | 0.88 (0.860–0.905) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.H.; Lee, J.; Yoon, E.L.; Jeong, S.W.; Kim, S.S.; Chon, Y.E.; Ahn, S.B.; Jun, D.W. Regular Alpha-Fetoprotein Tests Boost Curative Treatment and Survival for Hepatocellular Carcinoma Patients in an Endemic Area. Cancers 2024, 16, 150. https://doi.org/10.3390/cancers16010150
Oh JH, Lee J, Yoon EL, Jeong SW, Kim SS, Chon YE, Ahn SB, Jun DW. Regular Alpha-Fetoprotein Tests Boost Curative Treatment and Survival for Hepatocellular Carcinoma Patients in an Endemic Area. Cancers. 2024; 16(1):150. https://doi.org/10.3390/cancers16010150
Chicago/Turabian StyleOh, Joo Hyun, Jonghyun Lee, Eileen L. Yoon, Soung Won Jeong, Soon Sun Kim, Young Eun Chon, Sang Bong Ahn, and Dae Won Jun. 2024. "Regular Alpha-Fetoprotein Tests Boost Curative Treatment and Survival for Hepatocellular Carcinoma Patients in an Endemic Area" Cancers 16, no. 1: 150. https://doi.org/10.3390/cancers16010150
APA StyleOh, J. H., Lee, J., Yoon, E. L., Jeong, S. W., Kim, S. S., Chon, Y. E., Ahn, S. B., & Jun, D. W. (2024). Regular Alpha-Fetoprotein Tests Boost Curative Treatment and Survival for Hepatocellular Carcinoma Patients in an Endemic Area. Cancers, 16(1), 150. https://doi.org/10.3390/cancers16010150