Mogamulizumab Combined with Extracorporeal Photopheresis as a Novel Therapy in Erythrodermic Cutaneous T-cell Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Evaluations
2.2. Statistics
3. Results
3.1. Baseline Patient Characteristics
3.2. Clinical Efficacy
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dippel, E.; Assaf, C.; Becker, J.C.; von Bergwelt-Baildon, M.; Bernreiter, S.; Cozzio, A.; Eich, H.T.; Elsayad, K.; Follmann, M.; Grabbe, S.; et al. S2k-Leitlinie—Kutane Lymphome (ICD10 C82-C86): Update 2021. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2022, 20, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Willemze, R.; Pimpinelli, N.; Whittaker, S.; Olsen, E.A.; Ranki, A.; Dummer, R.; Hoppe, R.T. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007, 110, 479–484. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Pulitzer, M. Cutaneous T-cell Lymphoma. Clin. Lab. Med. 2017, 37, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Scarisbrick, J.J.; Prince, H.M.; Vermeer, M.H.; Quaglino, P.; Horwitz, S.; Porcu, P.; Stadler, R.; Wood, G.S.; Beylot-Barry, M.; Pham-Ledard, A.; et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Iżykowska, K.; Przybylski, G.K.; Gand, C.; Braun, F.C.; Grabarczyk, P.; Kuss, A.W.; Olek-Hrab, K.; Bastidas Torres, A.N.; Vermeer, M.H.; Zoutman, W.H.; et al. Genetic rearrangements result in altered gene expression and novel fusion transcripts in Sézary syndrome. Oncotarget 2017, 8, 39627–39639. [Google Scholar] [CrossRef]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet. Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Nicolay, J.P.; Albrecht, J.D.; Alberti-Violetti, S.; Berti, E. CCR4 in cutaneous T-cell lymphoma: Therapeutic targeting of a pathogenic driver. Eur. J. Immunol. 2021, 51, 1660–1671. [Google Scholar] [CrossRef]
- Beylot-Barry, M.; Quereux, G.; Nardin, C.; Duval-Modeste, A.-B.; Dereure, O.; Dalac-Rat, S.; Dobos, G.; Pham-Ledard, A.; Ram-Wolff, C.; D’Incan, M.; et al. Effectiveness of mogamulizumab in patients with Mycosis Fungoides or Sézary syndrome: A multicentre, retrospective, real-world French study. J. Eur. Acad. Dermatol. Venereol. JEADV 2023, 37, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Knobler, R.; Arenberger, P.; Arun, A.; Assaf, C.; Bagot, M.; Berlin, G.; Bohbot, A.; Calzavara-Pinton, P.; Child, F.; Cho, A.; et al. European dermatology forum—Updated guidelines on the use of extracorporeal photopheresis 2020—Part 1. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 2693–2716. [Google Scholar] [CrossRef] [PubMed]
- Knobler, R.; Duvic, M.; Querfeld, C.; Straus, D.; Horwitz, S.; Zain, J.; Foss, F.; Kuzel, T.; Campbell, K.; Geskin, L. Long-term follow-up and survival of cutaneous T-cell lymphoma patients treated with extracorporeal photopheresis. Photodermatol. Photoimmunol. Photomed. 2012, 28, 250–257. [Google Scholar] [CrossRef]
- Edelson, R.; Berger, C.; Gasparro, F.; Jegasothy, B.; Heald, P.; Wintroub, B.; Vonderheid, E.; Knobler, R.; Wolff, K.; Plewig, G. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N. Engl. J. Med. 1987, 316, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Knobler, R.; Fierro, M.T.; Savoia, P.; Marra, E.; Fava, P.; Bernengo, M.G. Extracorporeal photopheresis for the treatment of erythrodermic cutaneous T-cell lymphoma: A single center clinical experience with long-term follow-up data and a brief overview of the literature. Int. J. Dermatol. 2013, 52, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, J.P.; Melchers, S.; Albrecht, J.D.; Assaf, C.; Dippel, E.; Stadler, R.; Wehkamp, U.; Wobser, M.; Zhao, J.; Burghaus, I.; et al. Dimethyl fumarate treatment in relapsed and refractory cutaneous T cell lymphoma—A multicenter phase II study. Blood 2023, 142, 794–805. [Google Scholar] [CrossRef]
- Campbell, B.A.; Dobos, G.; Haider, Z.; Prince, H.M.; Bagot, M.; Evison, F.; van der Weyden, C.; McCormack, C.J.; Ram-Wolff, C.; Miladi, M.; et al. International Study of SS Shows Superiority of Combination Therapy & Heterogeneity of Treatment Strategies. Blood Adv. 2023, 7, 6639–6647. [Google Scholar] [CrossRef]
- Gao, C.; McCormack, C.; van der Weyden, C.; Goh, M.S.; Campbell, B.A.; Twigger, R.; Buelens, O.; Harrison, S.J.; Khoo, C.; Lade, S.; et al. Prolonged survival with the early use of a novel extracorporeal photopheresis regimen in patients with Sézary syndrome. Blood 2019, 134, 1346–1350. [Google Scholar] [CrossRef]
- Zic, J.A. Extracorporeal Photopheresis in the Treatment of Mycosis Fungoides and Sézary Syndrome. Dermatol. Clin. 2015, 33, 765–776. [Google Scholar] [CrossRef]
- Olsen, E.A.; Whittaker, S.; Kim, Y.H.; Duvic, M.; Prince, H.M.; Lessin, S.R.; Wood, G.S.; Willemze, R.; Demierre, M.-F.; Pimpinelli, N.; et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2598–2607. [Google Scholar] [CrossRef]
- Olsen, E.A.; Whittaker, S.; Willemze, R.; Pinter-Brown, L.; Foss, F.; Geskin, L.; Schwartz, L.; Horwitz, S.; Guitart, J.; Zic, J.; et al. Primary cutaneous lymphoma: Recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood 2022, 140, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Scarisbrick, J.J.; Bagot, M.; Ortiz-Romero, P.L. The changing therapeutic landscape, burden of disease, and unmet needs in patients with cutaneous T-cell lymphoma. Br. J. Haematol. 2021, 192, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, M.H.; Nicolay, J.P.; Scarisbrick, J.J.; Zinzani, P.L. The importance of assessing blood tumour burden in cutaneous T-cell lymphoma. Br. J. Dermatol. 2021, 185, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sanford, K.W.; Anderson, J.; Roseff, S.; McPherson, R.A. Iron Deficiency Anemia in Patients Undergoing Extracorporeal Photopheresis for Cutaneous T-Cell Lymphoma. Lab. Med. 2019, 50, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, J.; Li, J.; Zhao, Y.; Zhang, T.; Yang, R.; Ma, X. Safety and efficacy profile of mogamulizumab (Poteligeo) in the treatment of cancers: An update evidence from 14 studies. BMC Cancer 2021, 21, 618. [Google Scholar] [CrossRef] [PubMed]
- Gilson, D.; Whittaker, S.J.; Child, F.J.; Scarisbrick, J.J.; Illidge, T.M.; Parry, E.J.; Mohd Mustapa, M.F.; Exton, L.S.; Kanfer, E.; Rezvani, K.; et al. British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018. Br. J. Dermatol. 2019, 180, 496–526. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.; Scarisbrick, J.J. Maintenance therapy in patients with mycosis fungoides or Sézary syndrome: A neglected topic. Eur. J. Cancer 2021, 142, 38–47. [Google Scholar] [CrossRef]
- Prince, H.M.; Kim, Y.H.; Horwitz, S.M.; Dummer, R.; Scarisbrick, J.; Quaglino, P.; Zinzani, P.L.; Wolter, P.; Sanches, J.A.; Ortiz-Romero, P.L.; et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An international, open-label, randomised, phase 3, multicentre trial. Lancet 2017, 390, 555–566. [Google Scholar] [CrossRef]
- Huen, A.; Haverkos, B.M.; Zain, J.; Radhakrishnan, R.; Lechowicz, M.J.; Devata, S.; Korman, N.J.; Pinter-Brown, L.; Oki, Y.; Barde, P.J.; et al. Phase I/Ib Study of Tenalisib (RP6530), a Dual PI3K δ/γ Inhibitor in Patients with Relapsed/Refractory T-Cell Lymphoma. Cancers 2020, 12, 2293. [Google Scholar] [CrossRef]
- Khodadoust, M.S.; Rook, A.H.; Porcu, P.; Foss, F.; Moskowitz, A.J.; Shustov, A.; Shanbhag, S.; Sokol, L.; Fling, S.P.; Ramchurren, N.; et al. Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sézary Syndrome: A Multicenter Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 20–28. [Google Scholar] [CrossRef]
- Duvic, M.; Chiao, N.; Talpur, R. Extracorporeal photopheresis for the treatment of cutaneous T-cell lymphoma. J. Cutan. Med. Surg. 2003, 7, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Muniz, C.A.; Sánchez-Velázquez, A.; Arroyo-Andrés, J.; Agud-de Dios, M.; Tarín-Vicente, E.J.; Falkenhain-López, D.; Ortiz-Romero, P.L. Mogamulizumab combined with extracorporeal photopheresis for the treatment of refractory mycosis fungoides and Sézary syndrome. Report of seven cases. J. Eur. Acad. Dermatol. Venereol. JEADV 2023, 38, e102–e105. [Google Scholar] [CrossRef] [PubMed]
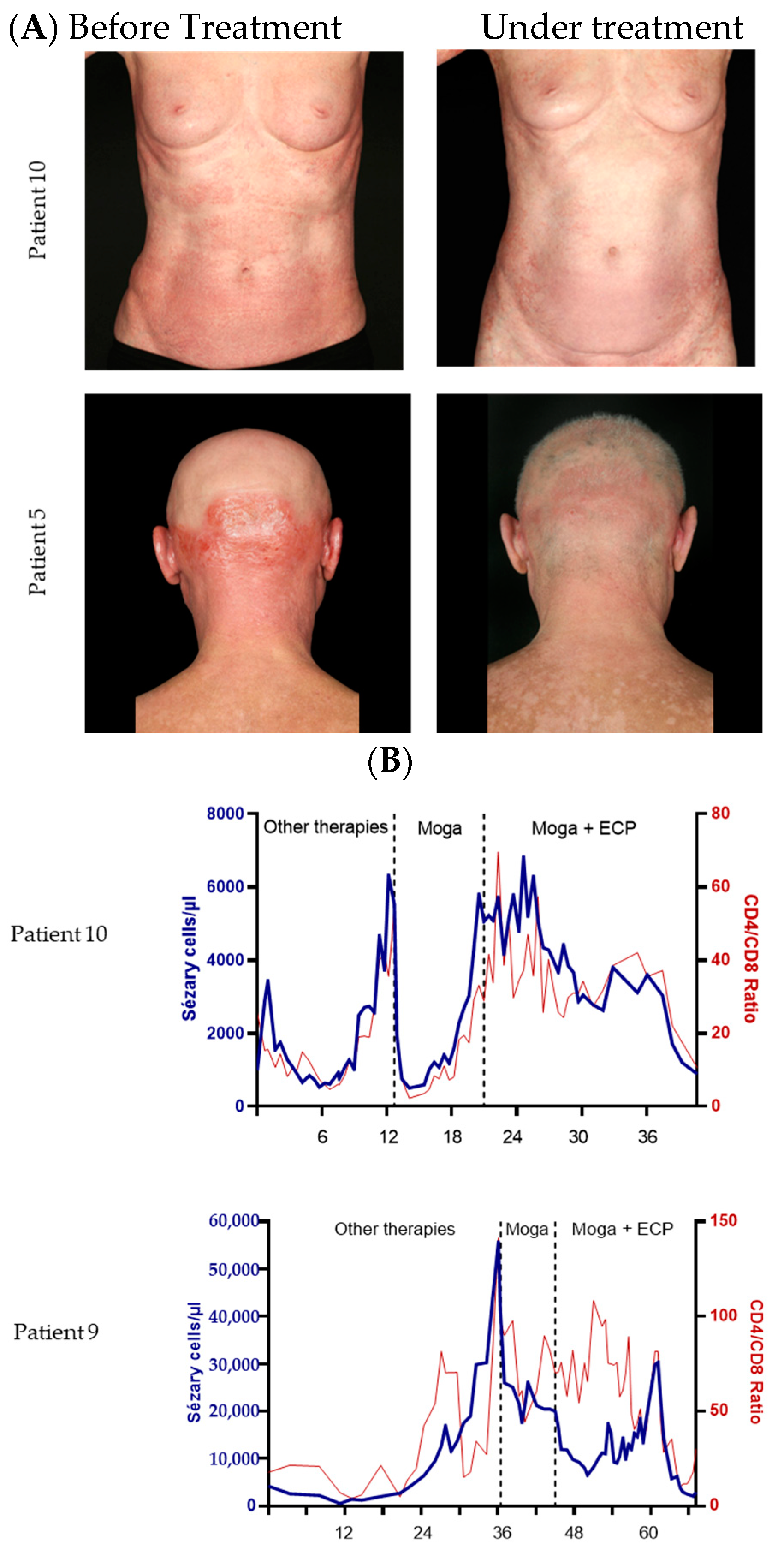
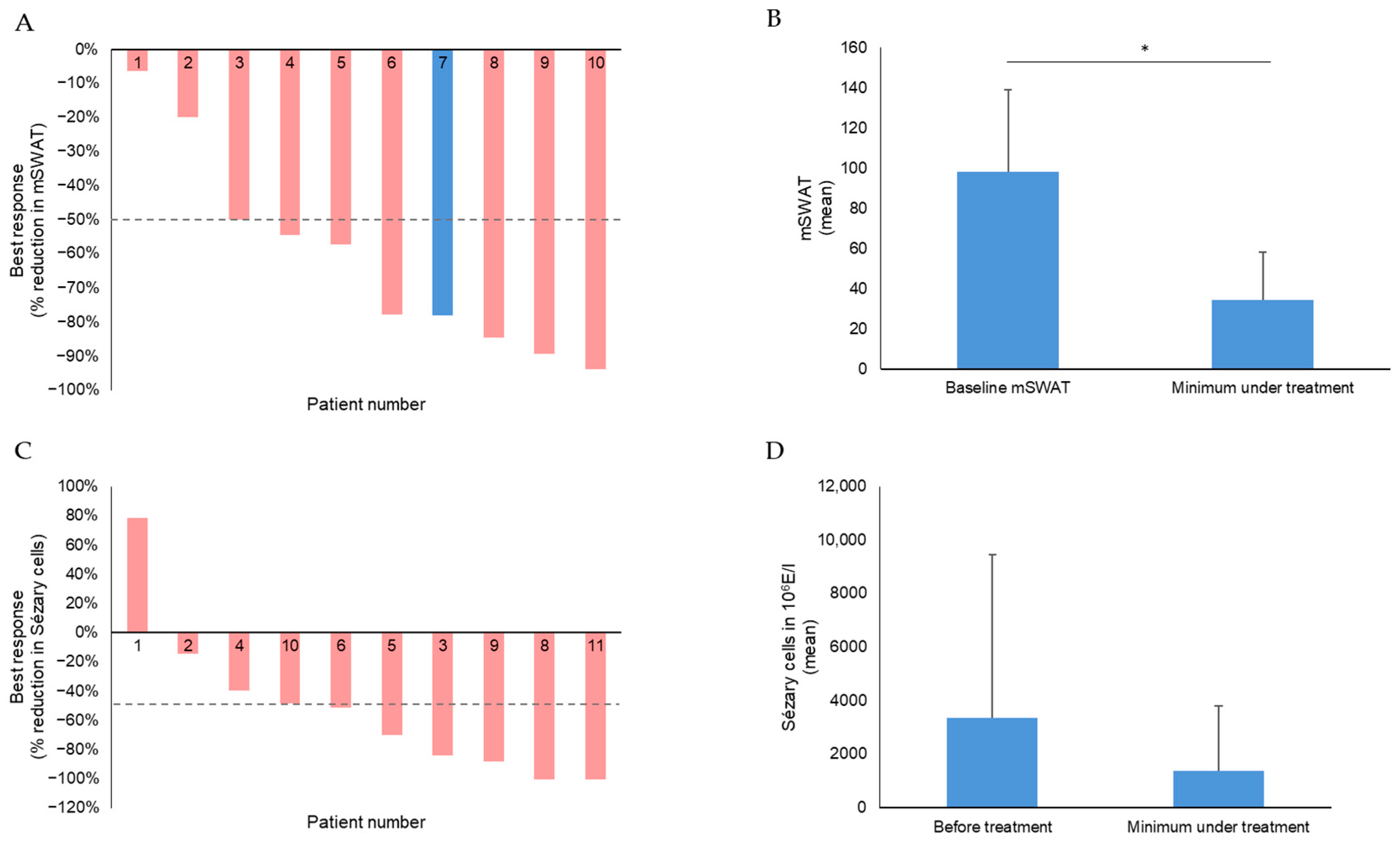
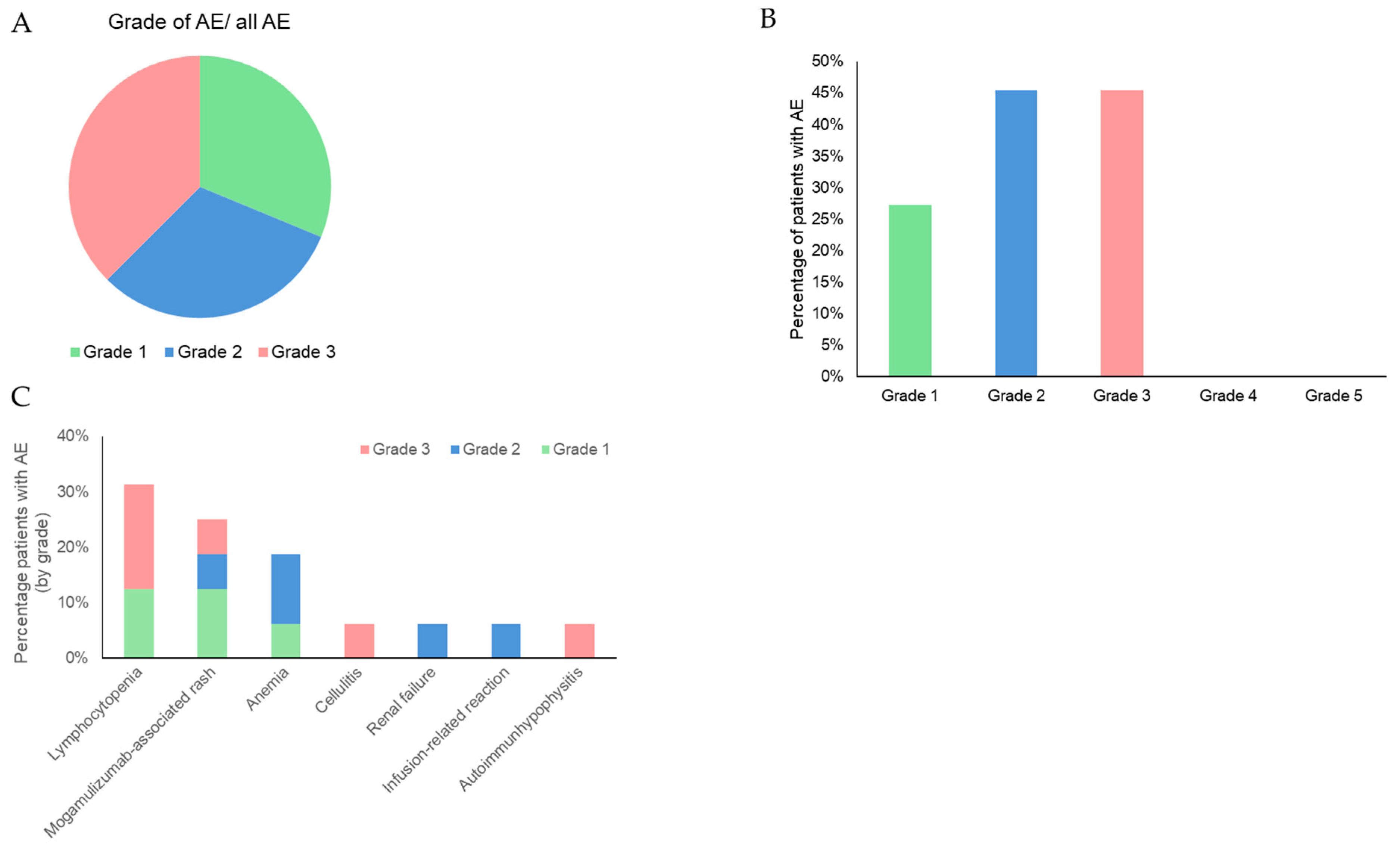
| Attribute | Attribute Level |
|---|---|
| Sex | n (%) |
| Female | 4 (36%) |
| Male | 7 (64%) |
| Age (mean; in years ± SD) | 72.0 ± 9.2 |
| Ethnicity | n (%) |
| Caucasian | 11 (100%) |
| ECOG | n (%) |
|
0 1 2 | 3 (27%) 6 (55%) 2 (18%) |
| Time since initial diagnosis (mean; years ± SD) | 1.80 (±1.44) |
| Disease type | n (%) |
| Mycosis fungoides | 1 (9%) |
| Sézary syndrome | 10 (91%) |
| TNMB stage before combination treatment | n (%) |
| T T3 T4 | 1 (9%) 10 (91%) |
| N Nx N0 N3 | 7 (64%) 2 (18%) 2 (18%) |
| M M0 | 11 (100%) |
| B B0 B2 | 1 (9%) 10 (91%) |
| CTCL stage before combination treatment IIIA IVA1 IVA2 | 1 (9%) 8 (73%) 2 (18%) |
| Sézary cells before combination treatment Mean (in 106 E/L ± SD) | 3365.3 (± 6103.2) (n = 10) |
| Lymyphocytes before combination treatment Mean (in 106 E/L ± SD) | 3728.7 (± 6032.3) (n = 11) |
| CD4/CD8 ratio before combination treatment Mean (± SD) | 41.2 (± 31.9) (n = 10) |
| LDH before combination treatment | |
| Mean (in U/L ± SD) | 290.0 (± 62.1) (n = 7) |
| mSWAT before combination treatment | |
| Mean (± SD) | 98.2 (± 40.8) (n = 10) |
| Number of combination sessions Mean (± SD) | 12.5 (± 11.3) (n = 11) |
| Observation period (months) Mean (± SD) Minimum Maximum | 7.8 (±6.2) 1.8 19.1 |
| Therapy | First Line (n) | Second Line (n) | Third Line (n) | Previous Treatments (n) |
|---|---|---|---|---|
| Antibiotics | 0 | 1 | 0 | 0 |
| Syst. PUVA | 2 | 0 | 0 | 1 |
| Toctino | 0 | 1 | 0 | 0 |
| BXT | 2 | 1 | 1 | 0 |
| Brentuximab | 0 | 1 | 2 | 2 |
| Mogamulizumab | 0 | 1 | 0 | 1 |
| MTX | 2 | 1 | 2 | 2 |
| PDN | 0 | 0 | 0 | 0 |
| Chlorambucil | 0 | 0 | 0 | 0 |
| ECP | 7 | 4 | 5 | 5 |
| Dimethyl fumarate | 0 | 0 | 0 | 2 |
| TSEBT | 0 | 0 | 0 | 1 |
| Interferon | 2 | 4 | 3 | 4 |
| Attribute | Share (%) | |
|---|---|---|
| Best Response (n) | ||
| CR | 0 | 0% |
| PR | 8 | 73% |
| SD | 1 | 9% |
| PD | 2 | 18% |
| PD (n) In total | 4 | 36% |
| PD after initial response | 2 | 27% |
| Mean time (months) to… | ||
| CR | n/a | |
| PR | 3.0 | |
| PD | 4.8 | |
| Mean minimum known period witho ut progress (months) | 7.2 | |
| Minimum mSWAT after combination treatment | ||
| Mean (± SD) | 34.6 (±23.8) | |
| Decrease in mSWAT | ||
| Mean (± SD) | 63.6 (±40.9) |
| Attribute | Share (%) | |
|---|---|---|
| Best Response (n) | ||
| CR | 2 | 18% |
| PR | 5 | 45% |
| SD | 3 | 27% |
| PD | 1 | 9% |
| PD (n) In total | 1 | 9% |
| PD after initial response | 0 | 0% |
| Mean time (months) to… | ||
| CR | 7.1 | |
| PR | 4.0 | |
| Overall response | 4.9 | |
| PD | 1.0 | |
| Mean minimum period without known progress (months) | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninosu, N.; Melchers, S.; Kappenstein, M.; Booken, N.; Hansen, I.; Blanchard, M.; Guenova, E.; Assaf, C.; Goerdt, S.; Nicolay, J.P. Mogamulizumab Combined with Extracorporeal Photopheresis as a Novel Therapy in Erythrodermic Cutaneous T-cell Lymphoma. Cancers 2024, 16, 141. https://doi.org/10.3390/cancers16010141
Ninosu N, Melchers S, Kappenstein M, Booken N, Hansen I, Blanchard M, Guenova E, Assaf C, Goerdt S, Nicolay JP. Mogamulizumab Combined with Extracorporeal Photopheresis as a Novel Therapy in Erythrodermic Cutaneous T-cell Lymphoma. Cancers. 2024; 16(1):141. https://doi.org/10.3390/cancers16010141
Chicago/Turabian StyleNinosu, Nadia, Susanne Melchers, Max Kappenstein, Nina Booken, Inga Hansen, Maël Blanchard, Emmanuella Guenova, Chalid Assaf, Sergij Goerdt, and Jan P. Nicolay. 2024. "Mogamulizumab Combined with Extracorporeal Photopheresis as a Novel Therapy in Erythrodermic Cutaneous T-cell Lymphoma" Cancers 16, no. 1: 141. https://doi.org/10.3390/cancers16010141
APA StyleNinosu, N., Melchers, S., Kappenstein, M., Booken, N., Hansen, I., Blanchard, M., Guenova, E., Assaf, C., Goerdt, S., & Nicolay, J. P. (2024). Mogamulizumab Combined with Extracorporeal Photopheresis as a Novel Therapy in Erythrodermic Cutaneous T-cell Lymphoma. Cancers, 16(1), 141. https://doi.org/10.3390/cancers16010141








