Simple Summary
Takotsubo syndrome (TTS), also known as “broken heart” syndrome, is a rare but serious heart event associated with physical or emotional stress. Patients usually complain about intense chest pain and difficulty in breathing, resembling acute myocardial infarction. Cancer patients are more likely to suffer from TTS and the condition has been linked with the use of several anticancer remedies, such as chemotherapy and targeted therapy. Immunotherapy is a new, viable option for many patients and is currently used in the management of several cancers. There are emerging reports of TTS in cancer patients treated with immunotherapy; however, its causality on TTS development remains uncertain. As TTS may be life-threatening and impact anticancer management, it is crucial to identify any possible association with immunotherapy. In this literature review, we tried to explore the extent of the condition in immunotherapy-treated patients, and to provide information regarding accurate diagnosis, management, and outcomes.
Abstract
Background: There are emerging reports of Takotsubo syndrome (TTS) in cancer patients treated with immune checkpoint inhibitors (ICIs); however, the association of the two remains uncertain. Methods: A systematic literature review was performed in the PubMed database and web sources (Google Scholar) according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines. Case reports/series or studies including cancer patients treated with ICIs and presenting with TTS were considered. Results: Seventeen cases were included in the systematic review. Most patients were males (59%) with median age of 70 years (30–83). Most common tumor types were lung cancer (35%) and melanoma (29%). Most patients were on first-line immunotherapy (35%) and after the first cycle (54%) of treatment. The median time on immunotherapy at the time of TTS presentation was 77 days (1–450). The most used agents were pembrolizumab and the combination of nivolumab–ipilimumab (35%, respectively). Potential stressors were recognized in 12 cases (80%). Six patients (35%) presented with concurrent cardiac complications. Corticosteroids were used in the management of eight patients (50%). Fifteen patients (88%) recovered from TTS, two patients (12%) relapsed, and one patient died. Immunotherapy was reintroduced in five cases (50%). Conclusion: TTS may be associated with immunotherapy for cancer. Physicians should be alert for TTS diagnosis in any patient with myocardial infarction-like presentation under treatment with ICIs.
1. Introduction
Since the approval of the cytotoxic T-lymphocyte associated protein-4 (CTLA-4) inhibitor ipilimumab for the treatment of patients with metastatic melanoma in 2011 [1,2], immune checkpoint inhibitors (ICIs) have revolutionized the management of cancer patients. ICIs, including programmed death protein receptor-1 (PD-1), programmed death ligand-1 (PD-L1), and CTLA-4 inhibitors, are monoclonal antibodies used for the treatment of various malignancies across different clinical settings and have significantly improved the cancer-related outcomes [3]. However, immune activation may lead to a novel spectrum of toxicities, collectively known as immune-related adverse events (irAEs) [4]. These adverse events may involve any organ system and have variable clinical presentations [4].
Immune-related cardiovascular toxicity (irCVT) is rare but constitutes a serious concern as it can be life threatening [5]. In a recent systematic review and meta-analysis, the incidence of irCVT among different studies was 1.3% [6]. Myocarditis represents the most frequent irCVT, accounting for 45% of observed events in case reports/series [5]. Other irCVTs, including arrhythmias (atrial fibrillation, supraventricular tachycardia, and bradyarrhythmias), pericarditis, and pericardial effusion, are less frequent [5]. IrCVT has been reported to occur after single-agent immunotherapy (pembrolizumab, nivolumab), but the incidence is higher with the combined use of different ICIs (nivolumab and ipilimumab compared to nivolumab alone) [7]. As ICIs are considered drugs with potency to induce myocarditis, which may be life-threatening, treatment with high-dose corticosteroids is indicated upon clinical suspicion [7].
Takotsubo cardiomyopathy (TTS), also known as “broken heart” syndrome, is characterized by acute chest pain, dyspnea, or syncope, usually following emotional or physical stress that may resemble acute myocardial infarction (MI) [8]. Distinct echocardiography findings include apical ballooning and midventricular, basal, or focal wall motion abnormalities; a normal coronary angiography (CAG) usually distinguishes TTS from MI scenarios by excluding true type-1 atherosclerotic MI [8]. There is no consensus for TTS diagnostic criteria, since many research groups have proposed different diagnostic approaches based on the respective ethnic phenotypes [8]. The Mayo Clinic Diagnostic Criteria are the most widely known [9], while, in 2018 the InterTAK Diagnostic Criteria were proposed in an effort to incorporate the discrepancies and provide a worldwide consensus [8]. Management is initially directed to acute myocardial ischemia, since the syndrome mimics an acute coronary syndrome, and then supportive measures are indicated with the intention to relieve heart failure symptoms and treat any underline triggers [10]. After the acute phase, the syndrome tends to be spontaneously resolved, but long-term complications may invariably persist [10].
TTS has been associated with cancer and the use of several anticancer agents; however, its association with immunotherapy remains vastly unknown [8]. To date, TTS has only been linked with the use of ICIs in scarce case reports [11]. As a result, although the European Society of Medical Oncology (ESMO) has identified a possible correlation of TTS with immunotherapy, TTS is not currently included in the list of irCVTs [7]. Other Oncology societies (American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN)) have also not incorporated TTS management in irCVTs management guidelines. Indeed, the association of TTS with immunotherapy is difficult not only due to the difficulties in diagnosis and the rarity of the condition but also because other cardiac events (e.g., myocarditis) tend to simultaneously present in many cases [11].
Increasing use of ICIs would be expected to raise the incidence of a potentially immune-related TTS. Since the condition may be life-threatening and it would impact the anticancer management and outcomes, investigation of its correlation with immunotherapy is crucial, with subsequent implications on accurate diagnosis and management.
As the association of TTS with immunotherapy for cancer remains uncertain, we were prompted to investigate the reported extent of the condition. We herein conducted a systematic literature review aiming to identify relevant reports of TTS in patients treated with ICIs. We then sought to summarize the clinicopathologic characteristics and outcomes of included cases. Moreover, we add to current knowledge with the inclusion of one relevant and previously unpublished case, managed in our hospital.
2. Materials and Methods
2.1. Design
The study design was discussed and agreed upon in advance by all authors. A PICO-S (Population, Intervention, Comparison, Outcomes, Study selection) [12] approach for the facilitation of our search strategy was used. The population included cancer patients, while intervention was treatment with ICIs. A comparison group was not included. The outcome of interest was TTS, and we performed a search to identify any type of relevant study. The PICO-S approach is demonstrated in Supplementary File, Table S1.
2.2. Reporting and Protocol Registration
The systematic review was performed in line with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [13]. The PRISMA 2020 checklist for the present systematic review is presented in Supplementary File, Table S2. The protocol was registered in the PROSPERO international prospective register of systematic reviews (ID: 348753).
2.3. Search Strategy
A systematic search of PubMed was performed until 13 March 2023 using the following search algorithm: (“takotsubo cardiomyopathy” OR “takotsubo syndrome” OR “TTS” OR “stress-induced cardiomyopathy” OR “broken-heart syndrome” OR “non-ischemic cardiomyopathy” OR “transient cardiomyopathy”) AND (“immunotherapy” OR “cancer immunotherapy” OR “immune checkpoint inhibitors” OR “checkpoint blockade” OR “anti-PD-1” OR “PD-1” OR “programmed death ligand-1” OR “PD-L1” OR “anti-PD-L1” OR “programmed death ligand-1” OR “CTLA-4” OR “anti-CTLA-4” OR “cytotoxic T-lymphocyte antigen-4” OR “pembrolizumab” OR “nivolumab” OR “ipilimumab” OR “durvalumab” OR “avelumab” OR “cemiplimab” OR “atezolizumab”). An additional search with the same algorithm was performed in Google Scholar. Reference lists of eligible articles were also systematically searched for any additional reports (‘snowball procedure’).
2.4. Cases and Studies Selection
Included cases were retrieved by four reviewers (I.P.T., K.G.K, A.S., and Z.S.) who worked independently. Disagreements were resolved after consensus with two senior authors (E.A.K and K.N.S.) was reached. Eligible items were full-text articles written in the English language. Isolated case reports, case series, and retrospective studies of TTS events in patients treated with ICIs were assessed for inclusion. Non-English articles, publications not providing full manuscript, and not-peer-reviewed reports were excluded.
2.5. Data Extraction and Tabulation
Five reviewers (I.P.T., I.A.V., K.G.K., A.S. and Z.S.) worked independently for extraction and tabulation of data regarding the main characteristics of patients described in the included articles (age, gender, tumor type, potential stressors, type of immunotherapeutic agent, line of therapy, cycles of therapy, concurrent/previous systemic therapies, time on ICI treatment at TTS presentation, time from last ICI treatment, symptoms at TTS presentation, echocardiography findings, CAG findings, electrocardiography (ECG) findings, cardiac enzymes elevation, concurrent cardiac complications, management interventions, outcome, and reintroduction of immunotherapy). History of cardiovascular (CVS) disease, active smoking, and any physical or emotional stress were regarded as potential stressors, in line with the available literature [14].
Communication with the authors of eligible reports was sought with the intention of individual patients’ demographic/outcome data analysis. Two author teams provided raw data after correspondence [15,16]. As a result, details regarding the outcomes of TTS on overall anticancer management, on survival of affected patients, and several baseline clinical characteristics (e.g., ECOG performance status, non-CVS comorbidities) were not included in descriptive analysis. The rest of the clinicopathological characteristics were synthesized based on the available evidence of published sources.
3. Results
3.1. Literature Search and Selection of Reports
Of the seventy-seven articles initially retrieved through the literature search, fifteen met all eligibility criteria and were included in the systematic review [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. One additional unpublished case from the Third Department of Internal Medicine, Sotiria General Hospital for Chest Diseases, National and Kapodistrian University of Athens, Athens, Greece, was also included. Two retrospective studies, reporting a further seventeen cases, were excluded due to possible overlap with other cases included in our study, after unsuccessful communication with corresponding authors [30,31]. Overall, seventeen cases were included in the systematic review and descriptive analysis. The PRISMA 2020 flow diagram for systematic reviews and meta-analyses of studies selection is illustrated in Figure 1.
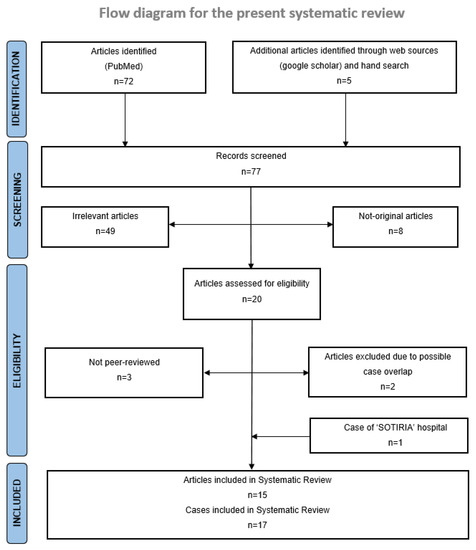
Figure 1.
PRISMA 2020 flow diagram for systematic reviews and meta-analyses of studies selection [13]. For more information, visit: http://www.prisma-statement.org/.
3.2. Baseline Clinicopathologic Characteristics
Baseline characteristics of all included cases are presented in Table 1. Descriptive analysis of each characteristic was based on the total number of cases (N) providing the respective data. Out of 17 patients, 10 (59%) were males with median age of 70 years (30–83). Tumor type included lung cancer (35% (n = 6, N = 17)), melanoma (29% (n = 5)), hepatocellular carcinoma (12% (n = 2)), renal cell carcinoma (12% (n = 2)), head and neck squamous cell carcinoma (6% (n = 1)), and breast cancer (6% (n = 1)). Evidence regarding line of treatment and cycle of therapy at TTS presentation was provided in 13 cases. Among them, 10 (77%) were on first-line treatment, 2 (15%) were on second-line treatment, and 1 (8%) was on adjuvant treatment. Seven patients (54%) presented with TTS after the first immunotherapy cycle, one (8%) after the third cycle, two (15%) after the fourth cycle, and three (23%) in later cycles (seventh and beyond). The median time on ICI treatment at the time of TTS was 77 days (1–450 (N = 15)), and median time from last ICI administration was seven days (1–136 (N = 10)). Ten patients (59% (N = 17)) were treated with monotherapy, while the rest received combination immunotherapy. With respect to ICI type, seven patients (41% (N = 17)) were treated with anti-PD-1 agents (pembrolizumab, 35% (n = 6); nivolumab, 6% (n = 1)), six (35%) with anti-PD-1/anti-CTLA-4 combination (nivolumab/ipilimumab), two (12%) with anti-PD-L1 monotherapy (atezolizumab), and two with anti-CTLA-4 monotherapy (ipilimumab) or anti-PD-L1/anti-CTLA-4 combination (durvalumab/tremelimumab) (6%, respectively). Five patients (31% (N = 16)) were receiving concurrent systematic treatment and six (38%) had received other systemic treatment prior to ICIs, including chemotherapy (cisplatin, carboplatin, pemetrexed, gemcitabine, paclitaxel, anthracycline-based regimens, 5-FU, and vinorelbine) and targeted therapy (trastuzumab, bevacizumab, cetuximab, axitinib, and sorafenib). Potential stressors were identified in 12 patients (80% (N = 15)), and those included history of active smoking, CVS disease, and physical (concurrent cardiac complications, other irAEs, infection, and infusion reactions) or emotional stress (intense stress on the ground of major depression).

Table 1.
Baseline characteristics and immunotherapy setting.
3.3. Clinical Characteristics and Outcomes of Takotsubo Syndrome
Clinical characteristics at TTS presentation, management, and outcomes are presented in Table 2. Main symptoms at presentation included dyspnea (67% (n = 10, N = 15)) with or without respiratory distress, chest pain (47% (n = 7)), and weakness/fatigue (13% (n = 2)). Other symptoms were nausea/vomiting, gastrointestinal symptoms (diarrhea, abdominal cramping), confusion, diaphoresis, palpitations, wheezing, fever, and generalized pain. One patient presented with signs of cardiogenic shock. All included reports provided echocardiographic findings at admission, with the most prominent features being apical ballooning (76% (n = 13)) and hyperkinesis of not affected cardiac segments (29% (n = 5)), mainly of basal and septal walls. Notably, two patients (12%) presented with atypical TTS with akinesia of middle and basal segments. Moreover, 12 patients (92% (n = 13)) had diminished left ventricular ejection fraction (LVEF). All patients with available data demonstrated ECG changes suggestive of MI (ST elevation, T-wave inversion (N = 16)), had abnormalities of cardiac enzymes (elevation of troponin and/or brain natriuretic peptide (N = 16)), and CAG without evidence of acute coronary obstruction (N = 15). In addition, six patients (35% (N = 17)) demonstrated concurrent cardiac complications (myocarditis, pericarditis/pericardial effusion, myopericardial carcinomatous infiltration, malignant embolization of coronary arteries, and ventricular tachycardia) and ten (62% (N = 16)) had concurrent irAEs (pneumonitis, diabetic ketoacidosis, colitis, hepatitis, nausea/vomiting, skin toxicity, nephritis, and infusion reaction). Data regarding TTS management were provided in 16 cases. Noteworthy, only eight patients (50%) were managed with high-dose corticosteroids. Other remedies included MI and/or heart failure management, including use of β-blockers, angiotensin-converting enzyme inhibitors, and diuretics, other supportive measures, such as oxygen therapy, IV fluids, and administration of anxiolytics, and treatment of the underlying trigger (e.g., insulin for diabetic ketoacidosis). Regarding outcomes, TTS resolved completely in 15 cases (88% (N = 17)). In three patients, resolution of apical ballooning followed several weeks after clinical improvement or did not resolve at all (sustained apical ballooning on echocardiography one year later), and one patient died due to ir-CVT (including TTS) and cancer complications. The median time to resolution was 8 days (3–35 (N = 9)). ICIs were reintroduced in five patients (50% (N = 10)) after the index event. It should be noted that two patients experienced TTS relapse: the first event occurred right after the reintroduction of the ICI (four days), while the second occurred seven months after the last ICI dose.

Table 2.
Characteristics and outcomes of Takotsubo syndrome.
4. Discussion
TTS as a potential complication of anticancer treatment is an uncommon but potentially life-threatening condition. The widespread use of ICIs has brought up a wide spectrum of irAEs. However, a direct link between ICIs’ use and TTS remains uncertain.
Indeed, an association between TTS and ICIs is difficult to establish as the diagnosis of the former is challenging and may overlap with other forms of cardiotoxicity (e.g., myocarditis, pericarditis) [17,22,27]. Moreover, history of malignancy appears to be an independent risk factor for TTS, possibly due to intense physical and emotional stress in patients with cancer [32]. Thus, diagnosis of an immune-related TTS is complex, and case-by-case examination is necessary. Notably, cancer patients experience worse TTS outcomes compared with those without malignancy [33], which highlights the need for thorough reporting and careful monitoring of cancer-associated TTS.
To the best of our knowledge, this is the first systematic review specifically designed to retrieve published cases of cancer patients treated with ICIs that developed TTS. Other reviews on the topic have investigated either the impact of anticancer agents to the development of TTS [11,32,34] or the overall cardiotoxic effect of ICIs [5,35,36,37,38,39]. Our systematic search and study design allowed us to retrieve 17 cases, the most reports recruited up to date in the literature, and to meticulously describe several TTS characteristics. Despite that, the number of recruited cases remains small, which was an anticipated limitation of our study. Another limitation lies in the poor evidence for a direct association of TTS with ICIs in the included cases. We investigated several validated algorithms (e.g., Naranjo, Yale, Jone’s, Karch, Begaud, ADRAC, WHO-UMC, Bradford-Hill) [40] for the assessment of probability of adverse drug reaction; however, their application was not feasible either due to lack of sufficient evidence, lack of control group, or due to structural issues of the algorithms. As a result, we acknowledge that the association of ICIs and TTS in the included cases does not necessarily imply causation.
Noteworthy, one of the patients included in our study presented with TTS soon after the first introduction of ICIs (nivolumab–ipilimumab for melanoma) and relapsed soon after the second infusion, which led to immunotherapy discontinuation. This case may imply direct causality of ICIs to the TTS event. Another patient treated with pembrolizumab for lung cancer relapsed with TTS; however, in that case, the relapse occurred seven months after last ICI infusion; thus, other underlying triggers may be implicated as well.
We believe that the challenges in TTS diagnosis may mask the true impact of the condition in immunotherapy-treated patients. In respect to that, seventeen more cases have been described in two retrospective studies [30,31], which were excluded from our systematic review as described earlier. In the first study, Ederhy et al. [30] performed an analysis using data from the World Health Organization (WHO) global database of individual case safety reports. The authors compared the proportion of TTS in patients receiving ICI vs. those receiving paracetamol, adrenaline, and venlafaxine (controls). ICIs were associated with a higher proportion of TTS; this analysis using control groups may enhance the hypothesis of causal relation between ICIs and TTS. In the second study, Escudier et al. [31] demonstrated that TTS may not be extremely rare among patients suffering from ir-CVT after examining the cardiotoxicity cases identified in the databases of two cardio-oncology clinics and in the published literature until 2017 (TTS was present in four out of 29 ir-CVT cases). This emerging evidence alerts us to the possible association of the condition with ICIs’ use, so we encourage our peers in the clinical setting to rigorously report similar cases.
Furthermore, we tried to investigate possible stressors triggering TTS in those patients. We observed that other cardiac complications coexisted in several cases [15,17,20,22,27], mainly myocarditis. As has been previously discussed, ICIs are recognized as therapeutic agents with strong potential to induce myocarditis [5,6]. Since presentation of myocarditis may resemble, or overlap with, TTS [5,17,38,39], a cardiac magnetic resonance imaging (MRI) with gadolinium enhancement or even a myocardial biopsy are needed for the exclusion of immune-related myocarditis. Differentiation of the two clinical entities is crucial, as the presence of myocarditis alerts us to urgent ICIs’ discontinuation and corticosteroids administration [7]. In our review, we identified five cases (29%) with concurrent myocarditis (possible or diagnosed) [13,15,20,22,25] and one with myopericardial effusion due to malignant infiltration [18]. However, out of the remaining eleven cases not reporting concurrent cardiac complications, the necessary diagnostic workup for myocarditis exclusion was performed only in two patients. One more case reported exclusion of myocarditis based on MRI-findings; however, relevant data to interpret the results were not provided. Based on the cases included in our review, there is insufficient evidence to support whether TTS is a phenotype of immunotherapy-related myocarditis or if it is a distinct cardiotoxic effect. A larger sample size following the necessary diagnostic approaches for the precise exclusion of other concurrent irCVTs (mainly myocarditis) would provide better insight.
Other possible stressors included the presence of non-cardiac irAEs [15,17,18,19,23,26,28,29], infections [21], and infusion reactions [16]. Other risk factors, such as active smoking and cardiovascular comorbidities, have been previously described [14] and were also identified in our study [17,18,19]. In addition, several anticancer drugs (e.g., trastuzumab, anthracyclines, axitinib, 5-FU, etc.), of which the association with TTS has been previously described [11,32], may also be also implicated in triggering TTS in some of the cases [20,25,26,29]. The abundance of potential stressors suggests that TTS may not be a direct outcome of immune-related toxicity, and that therapy-induced immune stimulation may aggravate other underlying mechanisms.
Regarding time of TTS presentation, there are lines of evidence in our review (median time on immunotherapy at the time of TTS presentation was found to be 77 days) and in other studies [11,29] denoting that immune-related TTS represents a rather delayed form of cardiotoxicity. However, we believe that these observations may be biased from the small sample size. This is highlighted in cases where TTS occurred after hours or several days [15,16,17,18,24,27,29] from the last ICI administration.
In addition, a detailed description of other critical parameters, such as the impact of corticosteroids in the management of TTS or that of TTS in anticancer outcomes, was not provided, as respective data were not mentioned in most cases and correspondence with authors was unsuccessful.
Better understanding of the underlying mechanisms would provide an insight into TTS and its potential association with ICIs. Although pathophysiology of TTS remains vastly unknown, various mechanisms have been proposed in the literature, including catecholamine excess, coronary vasospasm, microvascular dysfunction, and upregulation of certain cardiac genes [32,41]. The hormonal influence on TTS is supported by the higher frequency of the condition in women (90% of the cases) and in older patients (80% of the cases are older than 50 years old) [8]. Interestingly, a lower incidence of TTS in females with cancer has been reported in several studies [11,42], possibly attributed to the different underlying mechanisms of the condition compared to TTS in non-cancer patients. Lower incidence of TTS on females is consistent with our findings (59% male gender). The different underlying mechanisms of TTS in cancer patients may be at least partially attributed to anticancer drugs effects. Several mechanisms of immunotherapy-related TTS have been proposed in the literature. It has been suggested that autoreactive T-cells may cross-react with myocardium [35], that ICIs may directly act on coronary arteries, leading to coronary vasospam in multiple areas, or that an increased release of catecholamines is triggered from the adrenal glands and postganglionic sympathetic nerves upon immune blockade, thus inducing myocardial response [43,44]. A more thorough assessment of the reported cases is needed for more solid conclusions. A comprehensive illustration summarizing the proposed series of events leading to the development of TTS in ICI-treated cancer patients is provided in Figure 2.
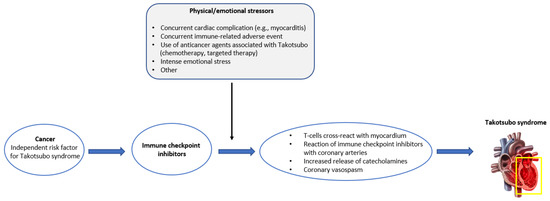
Figure 2.
Graphical illustration of the proposed series of events leading to Takotsubo syndrome in cancer patients treated with immune checkpoint inhibitors.
With the expanding use of ICIs, physicians are more likely to encounter TTS cases with an immune component in the future. Some critical questions include the following: (i) Is there a role of immunosuppressant therapy in the management of an immune-related TTS? (ii) Is it safe to reintroduce ICIs after a life-threatening but reversible event? (iii) What is the impact of TTS in the cancer-related outcomes. As a result, management of such cases should be individualized upon physicians’ and tumor boards’ discretion.
5. Conclusions
Malignancy represents an independent risk factor for TTS. Although accumulating reports have linked TTS with ICIs treatment, a causal association between the two has not yet been established. Physicians should be alert for TTS diagnosis in any patient with MI-like presentation under treatment with ICIs. Moreover, there is an unmet need regarding the management of immune-related TTS. As such, therapeutic decisions should currently be individualized; thorough reporting of similar cases will provide an insight into the underlying mechanisms with implications for accurate diagnosis and effective treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15092637/s1, Table S1: PICO-S model for structuring search approach; Table S2: PRISMA 2020 checklist for the present systematic review.
Author Contributions
Conceptualization: I.P.T., A.C., E.A.K. and K.N.S.; data curation: I.P.T., I.A.V., K.G.K., A.S. and Z.S.; investigation: I.P.T., K.G.K., A.S. and Z.S.; methodology: I.P.T., I.A.V., K.G.K. and A.C.; project administration: I.P.T., A.C., E.A.K. and K.N.S.; resources: I.P.T., A.S., Z.S. and ImmunoTTS Collaborative Group; software: I.P.T., I.A.V. and K.G.K.; supervision: A.C., E.A.K. and K.N.S.; visualization: I.P.T. and K.G.K.; writing—original draft: I.P.T., I.A.V. and K.G.K.; writing—review and editing: A.C., E.A.K., K.N.S. and ImmunoTTS Collaborative Group. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was granted from the patient described as the ‘Sotiria’ hospital case for the anonymous description of data from her medical records. The rest of the consent statements are provided in their respective original sources.
Data Availability Statement
All data are available in the manuscript, tables, and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Drake, C.G. Ipilimumab: An Anti-CTLA-4 Antibody for Metastatic Melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Ghosh, R.K.; Wongsaengsak, S.; Bandyopadhyay, D.; Ghosh, G.C.; Aronow, W.S.; Fonarow, G.C.; Lenihan, D.J.; Bhatt, D.L. Cardiovascular Toxicities of Immune Checkpoint Inhibitors: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Malaty, M.M.; Amarasekera, A.T.; Li, C.; Scherrer-Crosbie, M.; Tan, T.C. Incidence of immune checkpoint inhibitor mediated cardiovascular toxicity: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13831. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; Committee, E.G. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Bybee, K.A.; Kara, T.; Prasad, A.; Lerman, A.; Barsness, G.W.; Wright, R.S.; Rihal, C.S. Systematic Review: Transient Left Ventricular Apical Ballooning: A Syndrome That Mimics ST-Segment Elevation Myocardial Infarction. Ann. Intern. Med. 2004, 141, 858–865. [Google Scholar] [CrossRef]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef]
- Carbone, A.; Bottino, R.; Russo, V.; D’andrea, A.; Liccardo, B.; Maurea, N.; Quagliariello, V.; Cimmino, G.; Golino, P. Takotsubo Cardiomyopathy as Epiphenomenon of Cardiotoxicity in Patients with Cancer: A Meta-summary of Case Reports. J. Cardiovasc. Pharmacol. 2021, 78, e20–e29. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kumar, G.; Pant, S.; Rihal, C.; Murugiah, K.; Mehta, J.L. Prevalence of Takotsubo cardiomyopathy in the United States. Am. Heart J. 2012, 164, 66–71.e1. [Google Scholar] [CrossRef] [PubMed]
- Camastra, G.; Arcari, L.; Ciolina, F.; Danti, M.; Cacciotti, L. Cardiac magnetic resonance imaging of transient myocardial dysfunction in a patient treated with checkpoint-targeted immunotherapy. Eur. J. Cancer 2021, 144, 389–391. [Google Scholar] [CrossRef]
- Okamatsu, Y.; Tsubouchi, K.; Ibusuki, R.; Maehara-Keshino, E.; Shimauchi, A.; Kayukawa, T.; Eto, D.; Inoue, K.; Harada, T. Rapid Onset of Takotsubo Cardiomyopathy Induced by an Infusion Reaction to Pembrolizumab in a Patient with NSCLC. JTO Clin. Res. Rep. 2020, 1, 100055. [Google Scholar] [CrossRef]
- Tan, N.Y.L.; Anavekar, N.S.; Wiley, B.M. Concomitant myopericarditis and takotsubo syndrome following immune checkpoint inhibitor therapy. BMJ Case Rep. 2020, 13, e235265. [Google Scholar] [CrossRef]
- Oldfield, K.; Jayasinghe, R.; Niranjan, S.; Chadha, S. Immune checkpoint inhibitor-induced takotsubo syndrome and diabetic ketoacidosis: Rare reactions. BMJ Case Rep. 2021, 14, e237217. [Google Scholar] [CrossRef] [PubMed]
- Geisler, B.P.; Raad, R.A.; Esaian, D.; Sharon, E.; Schwartz, D.R. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: A case of takotsubo-like syndrome. J. Immunother. Cancer 2015, 3, 4. [Google Scholar] [CrossRef]
- Elikowski, W.; Małek-Elikowska, M.; Łazowski, S.; Zawodna, M.; Fertała, N.; Bryl, M. Takotsubo cardiomyopathy in a young male with lung cancer and neoplastic embolization of the coronary microcirculation. Polski Merkur. Lek. Organ Polskiego Towar. Lek. 2018, 44, 54–59. [Google Scholar]
- Khan, N.A.J.; Pacioles, T.; Alsharedi, M. Atypical Takotsubo Cardiomyopathy Secondary to Combination of Chemo-Immunotherapy in a Patient with Non-Small Cell Lung Cancer. Cureus 2020, 12, e9429. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, T.; Yoshikawa, N.; Kai, M.; Yamaguchi, M.; Toida, R.; Kodama, T.; Kajihara, K.; Kawabata, T.; Nakamura, T.; Sakata, K.; et al. The Cytokine Expression in Patients with Cardiac Complication after Immune Checkpoint Inhibitor Therapy. Intern. Med. 2021, 60, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Serzan, M.; Rapisuwon, S.; Krishnan, J.; Chang, I.C.; Barac, A. Takotsubo Cardiomyopathy Associated with Checkpoint Inhibitor Therapy: Endomyocardial Biopsy Provides Pathological Insights to Dual Diseases. JACC Cardio Oncol. 2021, 3, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Cautela, J.; Ancedy, Y.; Escudier, M.; Thuny, F.; Cohen, A. Takotsubo-Like Syndrome in Cancer Patients Treated with Immune Checkpoint Inhibitors. JACC Cardiovasc. Imaging 2018, 11, 1187–1190. [Google Scholar] [CrossRef]
- Anderson, R.D.; Brooks, M. Apical takotsubo syndrome in a patient with metastatic breast carcinoma on novel immunotherapy. Int. J. Cardiol. 2016, 222, 760–761. [Google Scholar] [CrossRef]
- Schwab, K.S.; Kristiansen, G.; Isaak, A.; Held, S.E.A.; Heine, A.; Brossart, P. Long Term Remission and Cardiac Toxicity of a Combination of Ipilimumab and Nivolumab in a Patient with Metastatic Head and Neck Carcinoma After Progression Following Nivolumab Monotherapy. Front. Oncol. 2019, 9, 403. [Google Scholar] [CrossRef]
- Norikane, T.; Mitamura, K.; Yamamoto, Y.; Takami, Y.; Fujimoto, K.; Noma, T.; Nishiyama, Y. Immune checkpoint inhibitor myocarditis mimicking Takotsubo cardiomyopathy on MPI. J. Nucl. Cardiol. 2020, 29, 2694–2698. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Patel, G.; Singh, R.B.; Goyal, A.; Avgush, K.; Koka, J. Atezolizumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis and Takotsubo cardiomyopathy. BMJ Case Rep. 2022, 15, e250662. [Google Scholar] [CrossRef]
- Airò, G.; Maffezzoli, M.; Lazzarin, A.; Bianconcini, M.; Greco, A.; Buti, S.; Leonetti, A. Takotsubo syndrome in a patient with metastatic renal cell carcinoma treated with pembrolizumab plus axitinib. Immunotherapy 2022, 14, 1297–1305. [Google Scholar] [CrossRef]
- Ederhy, S.; Dolladille, C.; Thuny, F.; Alexandre, J.; Cohen, A. Takotsubo syndrome in patients with cancer treated with immune checkpoint inhibitors: A new adverse cardiac complication. Eur. J. Heart Fail. 2019, 21, 945–947. [Google Scholar] [CrossRef]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.-J.; Scemama, U.; Jacquier, A.; et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor–Related Cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Noor, A.; Joshi, S.; Kim, A.S. Takotsubo cardiomyopathy in cancer patients. Cardio-Oncology 2019, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Joy, P.S.; Guddati, A.K.; Shapira, I. Outcomes of Takotsubo cardiomyopathy in hospitalized cancer patients. J. Cancer Res. Clin. Oncol. 2018, 144, 1539–1545. [Google Scholar] [CrossRef]
- Keramida, K.; Farmakis, D.; Filippatos, G. Cancer and Takotsubo syndrome: From rarity to clinical practice. ESC Heart Fail. 2021, 8, 4365–4369. [Google Scholar] [CrossRef]
- Tajiri, K.; Ieda, M. Cardiac Complications in Immune Checkpoint Inhibition Therapy. Front. Cardiovasc. Med. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Abu-Salman, A.; Steckbeck, R.; Mathew Jacob, B.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 5218. [Google Scholar] [CrossRef]
- Mocan-Hognogi, D.L.; Trancǎ, S.; Farcaş, A.D.; Mocan-Hognogi, R.F.; Pârvu, A.V.; Bojan, A.S. Immune Checkpoint Inhibitors and the Heart. Front. Cardiovasc. Med. 2021, 8, 726426. [Google Scholar] [CrossRef]
- Khunger, A.; Battel, L.; Wadhawan, A.; More, A.; Kapoor, A.; Agrawal, N. New Insights into Mechanisms of Immune Checkpoint Inhibitor-Induced Cardiovascular Toxicity. Curr. Oncol. Rep. 2020, 22, 65. [Google Scholar] [CrossRef]
- Frayberg, M.; Yung, A.; Zubiri, L.; Zlotoff, D.A.; Reynolds, K.L. What the Cardiologist Needs to Know About Cancer Immunotherapies and Complications. Curr. Treat. Options Oncol. 2021, 22, 53. [Google Scholar] [CrossRef]
- Doherty, M. Algorithms for assessing the probability of an Adverse Drug Reaction. Respir. Med. CME 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Akashi, Y.J.; Nef, H.M.; Lyon, A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, N.D.; Tarantino, N.; Guastafierro, F.; De Gennaro, L.; Correale, M.; Stiermaier, T.; Möller, C.; Di Biase, M.; Eitel, I.; Santoro, F. Malignancies and outcome in Takotsubo syndrome: A meta-analysis study on cancer and stress cardiomyopathy. Heart Fail. Rev. 2019, 24, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, C.; Rottmann, D.; Nguyen, V.Q.; Baldassarre, L.A. Myocarditis with checkpoint inhibitor immunotherapy: Case report of late gadolinium enhancement on cardiac magnetic resonance with pathology correlate. Eur. Heart J.—Case Rep. 2019, 3, yty149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, W. Cardiovascular immune-related adverse events: Evaluation, diagnosis and management. Asia-Pacific J. Clin. Oncol. 2020, 16, 232–240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).