Autophagic-Related Proteins in Brain Gliomas: Role, Mechanisms, and Targeting Agents
Abstract
Simple Summary
Abstract
1. Introduction
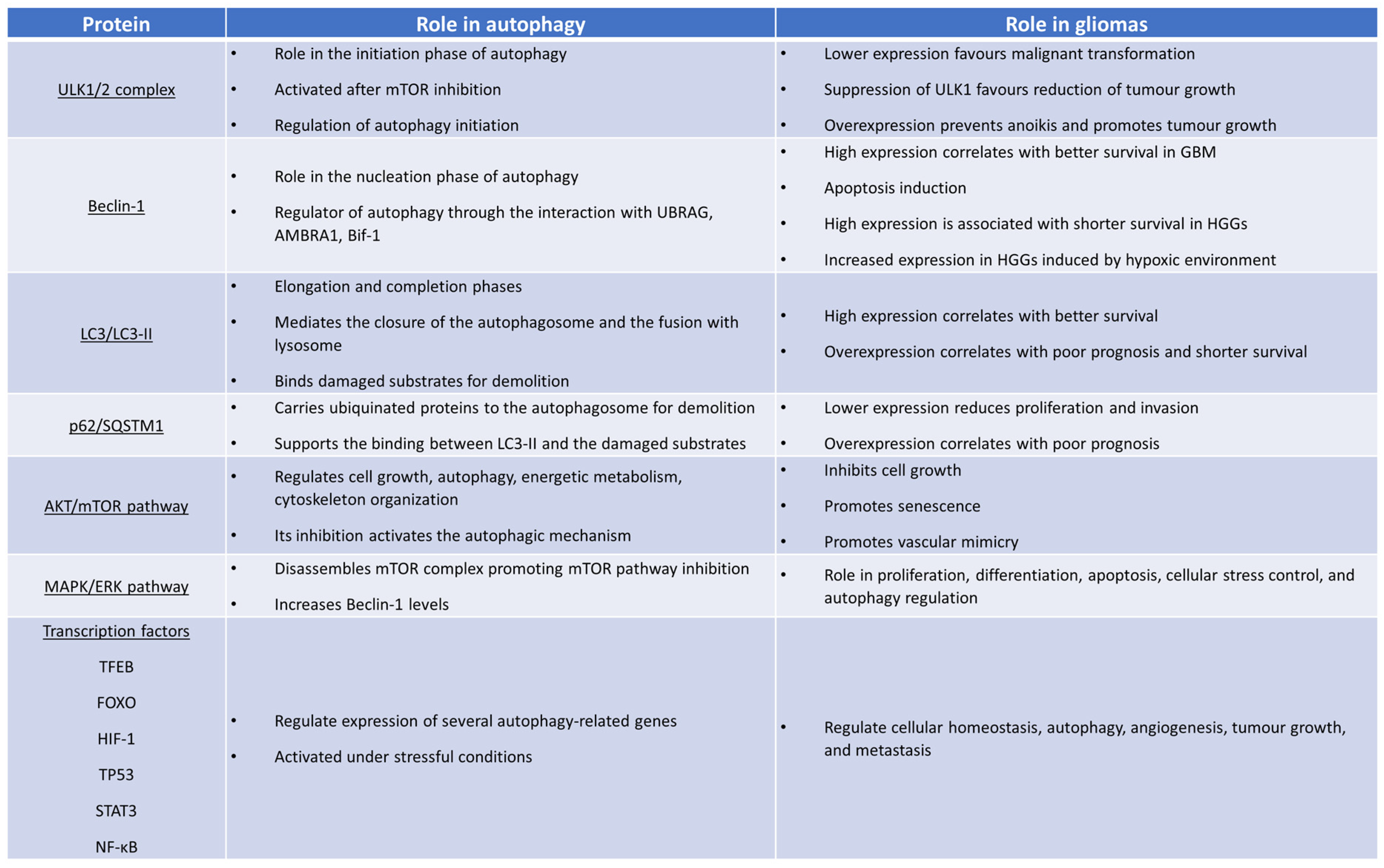
2. Molecular Mechanisms of Autophagy
3. Pathways of Autophagy Regulation
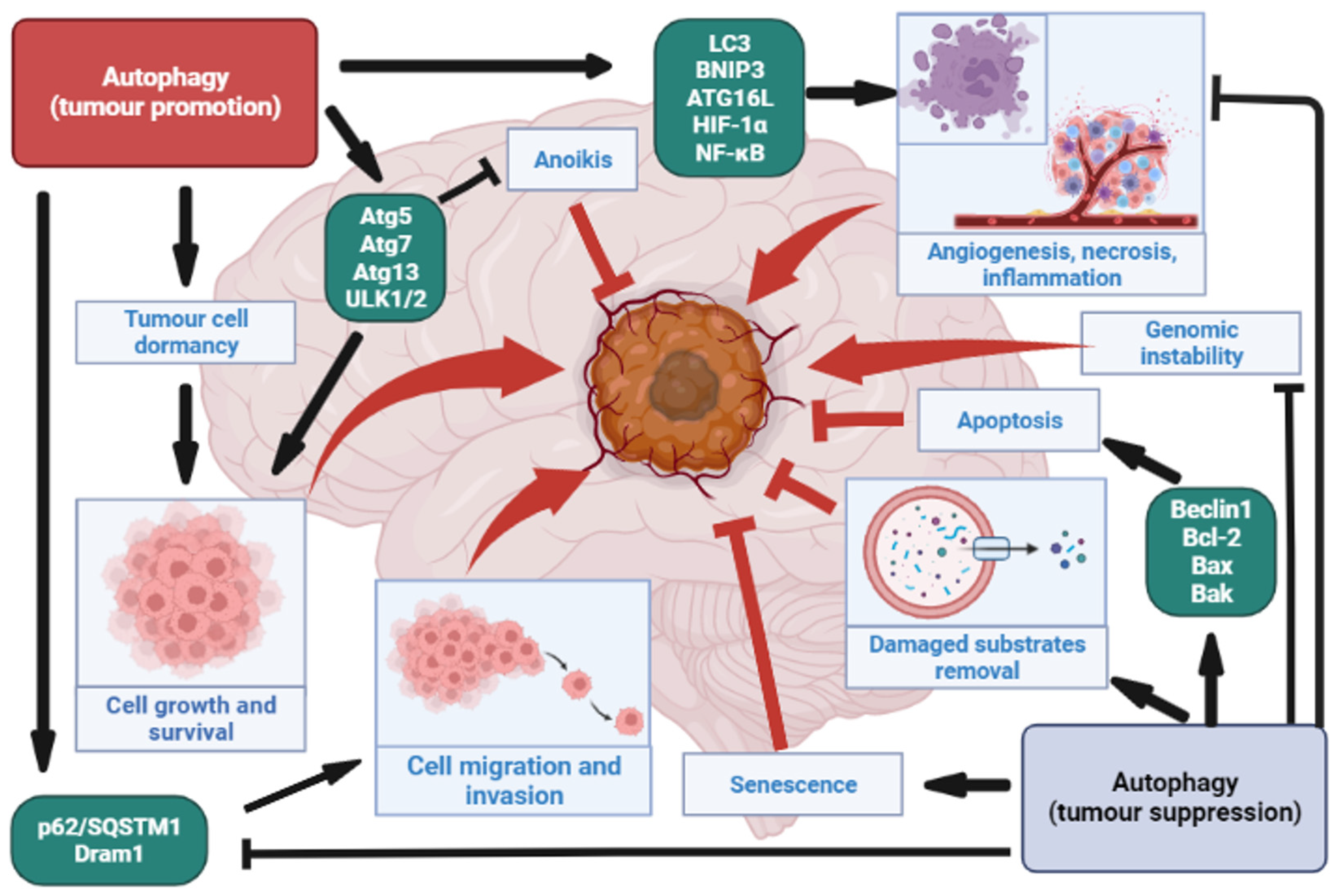
4. The Role of Autophagy in Gliomas
4.1. Autophagy as a Tumour Suppressor in Gliomas
4.2. Autophagy as a Tumour Promoter in Gliomas
5. Autophagy and the Tumour Immune Microenvironment
6. Autophagy and Glioma Stem Cells
7. Resistance to Treatment and Autophagy-Targeting Agents
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Santana-Codina, N.; Mancias, J.D.; Kimmelman, A.C. The Role of Autophagy in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy Fights Disease through Cellular Self-Digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Liang, C.; Feng, P.; Ku, B.; Dotan, I.; Canaani, D.; Oh, B.-H.; Jung, J.U. Autophagic and Tumour Suppressor Activity of a Novel Beclin1-Binding Protein UVRAG. Nat. Cell Biol. 2006, 8, 688–698. [Google Scholar] [CrossRef]
- Liang, C.; Lee, J.; Inn, K.-S.; Gack, M.U.; Li, Q.; Roberts, E.A.; Vergne, I.; Deretic, V.; Feng, P.; Akazawa, C.; et al. Beclin1-Binding UVRAG Targets the Class C Vps Complex to Coordinate Autophagosome Maturation and Endocytic Trafficking. Nat. Cell Biol. 2008, 10, 776–787. [Google Scholar] [CrossRef]
- Takahashi, Y.; Coppola, D.; Matsushita, N.; Cualing, H.D.; Sun, M.; Sato, Y.; Liang, C.; Jung, J.U.; Cheng, J.Q.; Mul, J.J.; et al. Bif-1 Interacts with Beclin 1 through UVRAG and Regulates Autophagy and Tumorigenesis. Nat. Cell Biol. 2007, 9, 1142–1151. [Google Scholar] [CrossRef]
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome Formation from Membrane Compartments Enriched in Phosphatidylinositol 3-Phosphate and Dynamically Connected to the Endoplasmic Reticulum. J. Cell Biol. 2008, 182, 685–701. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 44, 77–88. [Google Scholar] [CrossRef]
- Xu, H.-D.; Qin, Z.-H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 109–126. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- SUN, W.-L. Ambra1 in Autophagy and Apoptosis: Implications for Cell Survival and Chemotherapy Resistance. Oncol. Lett. 2016, 12, 367–374. [Google Scholar] [CrossRef]
- Cianfanelli, V.; Nazio, F.; Cecconi, F. Connecting Autophagy: AMBRA1 and Its Network of Regulation. Mol. Cell. Oncol. 2015, 2, e970059. [Google Scholar] [CrossRef] [PubMed]
- Zachari, M.; Ganley, I.G. The Mammalian ULK1 Complex and Autophagy Initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and Autophagy-Related Proteins in Cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 Links the Autophagy Pathway and the Ubiqutin-Proteasome System Upon Ubiquitinated Protein Degradation. Cell. Mol. Biol. Lett. 2016, 21, 51. [Google Scholar] [CrossRef]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. Int. J. Mol. Sci. 2020, 22, 179. [Google Scholar] [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.-Q.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual Role of Autophagy in Hallmarks of Cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy Promotes Tumor Cell Survival and Restricts Necrosis, Inflammation, and Tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Li, Y.; Yang, L.; Ma, Y.; Peng, X.; Yang, S.; Liu, J.; Li, H. Autophagy: A Novel Mechanism of Chemoresistance in Cancers. Biomed. Pharmacother. 2019, 119, 109415. [Google Scholar] [CrossRef]
- Kung, C.P.; Budina, A.; Balaburski, G.; Bergenstock, M.K.; Murphy, M.E. Autophagy in Tumor Suppression and Cancer Therapy. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 71–100. [Google Scholar] [CrossRef]
- Mathew, R.; Kongara, S.; Beaudoin, B.; Karp, C.M.; Bray, K.; Degenhardt, K.; Chen, G.; Jin, S.; White, E. Autophagy Suppresses Tumor Progression by Limiting Chromosomal Instability. Genes Dev. 2007, 21, 1367–1381. [Google Scholar] [CrossRef]
- Wang, L.; Klionsky, D.J.; Shen, H.-M. The Emerging Mechanisms and Functions of Microautophagy. Nat. Rev. Mol. Cell Biol. 2022, 24, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.; Sequeida, A.; Albornoz, A.; Budini, M. Chaperone Mediated Autophagy Substrates and Components in Cancer. Front. Oncol. 2021, 10, 614677. [Google Scholar] [CrossRef] [PubMed]
- Auzmendi-Iriarte, J.; Otaegi-Ugartemendia, M.; Carrasco-Garcia, E.; Azkargorta, M.; Diaz, A.; Saenz-Antoñanzas, A.; Andermatten, J.A.; Garcia-Puga, M.; Garcia, I.; Elua-Pinin, A.; et al. Chaperone-Mediated Autophagy Controls Proteomic and Transcriptomic Pathways to Maintain Glioma Stem Cell Activity. Cancer Res. 2022, 82, 1283–1297. [Google Scholar] [CrossRef]
- Auzmendi-Iriarte, J.; Matheu, A. Intrinsic role of chaperone-mediated autophagy in cancer stem cell maintenance. Autophagy 2022, 18, 3035–3036. [Google Scholar] [CrossRef]
- Batara, D.C.R.; Choi, M.-C.; Shin, H.-U.; Kim, H.; Kim, S.-H. Friend or Foe: Paradoxical Roles of Autophagy in Gliomagenesis. Cells 2021, 10, 1411. [Google Scholar] [CrossRef]
- Khan, I.; Baig, M.H.; Mahfooz, S.; Rahim, M.; Karacam, B.; Elbasan, E.B.; Ulasov, I.; Dong, J.-J.; Hatiboglu, M.A. Deciphering the Role of Autophagy in Treatment of Resistance Mechanisms in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 1318. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and MTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Hara, T.; Mizushima, N. Role of ULK-FIP200 Complex in Mammalian Autophagy: FIP200, a Counterpart of Yeast Atg17? Autophagy 2009, 5, 85–87. [Google Scholar] [CrossRef]
- Ganley, I.G.; Lam, D.H.; Wang, J.; Ding, X.; Chen, S.; Jiang, X. ULK1·ATG13·FIP200 Complex Mediates MTOR Signaling and Is Essential for Autophagy. J. Biol. Chem. 2009, 284, 12297–12305. [Google Scholar] [CrossRef]
- Kihara, A.; Noda, T.; Ishihara, N.; Ohsumi, Y. Two Distinct Vps34 Phosphatidylinositol 3–Kinase Complexes Function in Autophagy and Carboxypeptidase Y Sorting InSaccharomyces Cerevisiae. J. Cell Biol. 2001, 152, 519–530. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Criollo, A.; Kroemer, G. Crosstalk between Apoptosis and Autophagy within the Beclin 1 Interactome. EMBO J. 2010, 29, 515–516. [Google Scholar] [CrossRef]
- Vega-Rubín-de-Celis, S. The Role of Beclin 1-Dependent Autophagy in Cancer. Biology 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Schulman, B.A. Dynamic Regulation of Macroautophagy by Distinctive Ubiquitin-like Proteins. Nat. Struct. Mol. Biol. 2014, 21, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Mammalian Autophagy: Core Molecular Machinery and Signaling Regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhou, X.-J.; Zhang, H. Exploring the Role of Autophagy-Related Gene 5 (ATG5) Yields Important Insights Into Autophagy in Autoimmune/Autoinflammatory Diseases. Front. Immunol. 2018, 9, 2334. [Google Scholar] [CrossRef]
- Harada, K.; Kotani, T.; Kirisako, H.; Sakoh-Nakatogawa, M.; Oikawa, Y.; Kimura, Y.; Hirano, H.; Yamamoto, H.; Ohsumi, Y.; Nakatogawa, H. Two Distinct Mechanisms Target the Autophagy-Related E3 Complex to the Pre-Autophagosomal Structure. Elife 2019, 8, 43088. [Google Scholar] [CrossRef]
- Romanov, J.; Walczak, M.; Ibiricu, I.; Schüchner, S.; Ogris, E.; Kraft, C.; Martens, S. Mechanism and Functions of Membrane Binding by the Atg5-Atg12/Atg16 Complex during Autophagosome Formation. EMBO J. 2012, 31, 4304–4317. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Lee, J.-A. Role of the Mammalian ATG8/LC3 Family in Autophagy: Differential and Compensatory Roles in the Spatiotemporal Regulation of Autophagy. BMB Rep. 2016, 49, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Lystad; Simonsen Mechanisms and Pathophysiological Roles of the ATG8 Conjugation Machinery. Cells 2019, 8, 973. [CrossRef] [PubMed]
- Hikita, H.; Sakane, S.; Takehara, T. Mechanisms of the Autophagosome-Lysosome Fusion Step and Its Relation to Non-Alcoholic Fatty Liver Disease. Liver Res. 2018, 2, 120–124. [Google Scholar] [CrossRef]
- Jiang, P.; Nishimura, T.; Sakamaki, Y.; Itakura, E.; Hatta, T.; Natsume, T.; Mizushima, N. The HOPS Complex Mediates Autophagosome–Lysosome Fusion through Interaction with Syntaxin 17. Mol. Biol. Cell 2014, 25, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Teng, J.; Chen, J. New Insights Regarding SNARE Proteins in Autophagosome-Lysosome Fusion. Autophagy 2021, 17, 2680–2688. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Farzam, F.; Jia, J.; Gu, Y.; Choi, S.W.; Mudd, M.H.; Claude-Taupin, A.; Wester, M.J.; Lidke, K.A.; et al. Mechanism of Stx17 Recruitment to Autophagosomes via IRGM and Mammalian Atg8 Proteins. J. Cell Biol. 2018, 217, 997–1013. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, S.; Li, K.; Liang, D.; Zhu, X.; Liu, L.; Lu, Z.; Sun, W.; Xu, E. Autophagosome Maturation Mediated by Rab7 Contributes to Neuroprotection of Hypoxic Preconditioning against Global Cerebral Ischemia in Rats. Cell Death Dis. 2017, 8, e2949. [Google Scholar] [CrossRef]
- Kirkin, V.; Lamark, T.; Sou, Y.S.; Bjørkøy, G.; Nunn, J.L.; Bruun, J.A.; Shvets, E.; McEwan, D.G.; Clausen, T.H.; Wild, P.; et al. A Role for NBR1 in Autophagosomal Degradation of Ubiquitinated Substrates. Mol. Cell 2009, 33, 505–516. [Google Scholar] [CrossRef]
- Lamark, T.; Kirkin, V.; Dikic, I.; Johansen, T. NBR1 and P62 as Cargo Receptors for Selective Autophagy of Ubiquitinated Targets. Cell Cycle 2009, 8, 1986–1990. [Google Scholar] [CrossRef]
- Cohen-Kaplan, V.; Ciechanover, A.; Livneh, I. Stress-induced polyubiquitination of proteasomal ubiquitin receptors targets the proteolytic complex for autophagic degradation. Autophagy 2017, 13, 759–760. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Pause, A. MTOR Pathways in Cancer and Autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef]
- Liu, H.-Q.; An, Y.-W.; Hu, A.-Z.; Li, M.-H.; Wu, J.-L.; Liu, L.; Shi, Y.; Cui, G.-H.; Chen, Y. Critical Roles of the PI3K-Akt-MTOR Signaling Pathway in Apoptosis and Autophagy of Astrocytes Induced by Methamphetamine. Open Chem. 2019, 17, 96–104. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The MTOR–Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Regulation of Autophagy by MTOR Signaling Pathway. Adv. Exp. Med. Biol. 2019, 1206, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; You, Z.; Zhou, L.; Xu, Y.; Peng, C.; Zhou, T.; Yi, C.; Shi, Y.; Liu, W. MTORC1-Regulated and HUWE1-Mediated WIPI2 Degradation Controls Autophagy Flux. Mol. Cell 2018, 72, 303–315. [Google Scholar] [CrossRef]
- Matsunaga, K.; Saitoh, T.; Tabata, K.; Omori, H.; Satoh, T.; Kurotori, N.; Maejima, I.; Shirahama-Noda, K.; Ichimura, T.; Isobe, T.; et al. Two Beclin 1-Binding Proteins, Atg14L and Rubicon, Reciprocally Regulate Autophagy at Different Stages. Nat. Cell Biol. 2009, 11, 385–396. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pérez-Rojas, J.M.; Pedraza-Chaverri, J. Novel Compounds for the Modulation of MTOR and Autophagy to Treat Neurodegenerative Diseases. Cell. Signal. 2020, 65, 109442. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.; Lee, H.-K. Mammalian/Mechanistic Target of Rapamycin (MTOR) Complexes in Neurodegeneration. Mol. Neurodegener. 2021, 16, 44. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. MTOR Signaling in Aging and Neurodegeneration: At the Crossroad between Metabolism Dysfunction and Impairment of Autophagy. Neurobiol. Dis. 2015, 84, 39–49. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/MTOR-Mediated Autophagy for Tumor Therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/MTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Qin, Q.-F.; Li, X.-J.; Li, Y.-S.; Zhang, W.K.; Tian, G.-H.; Shang, H.-C.; Tang, H.-B. AMPK-ERK/CARM1 Signaling Pathways Affect Autophagy of Hepatic Cells in Samples of Liver Cancer Patients. Front. Oncol. 2019, 9, 01247. [Google Scholar] [CrossRef]
- Wang, J.; Whiteman, M.W.; Lian, H.; Wang, G.; Singh, A.; Huang, D.; Denmark, T. A Non-Canonical MEK/ERK Signaling Pathway Regulates Autophagy via Regulating Beclin 1. J. Biol. Chem. 2009, 284, 21412–21424. [Google Scholar] [CrossRef]
- Song, T.-T.; Cai, R.-S.; Hu, R.; Xu, Y.-S.; Qi, B.-N.; Xiong, Y.-A. The Important Role of TFEB in Autophagy-Lysosomal Pathway and Autophagy-Related Diseases: A Systematic Review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1641–1649. [Google Scholar] [CrossRef]
- Cortes, C.J.; La Spada, A.R. TFEB Dysregulation as a Driver of Autophagy Dysfunction in Neurodegenerative Disease: Molecular Mechanisms, Cellular Processes, and Emerging Therapeutic Opportunities. Neurobiol. Dis. 2019, 122, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Silva, M.; Li, S.; Yan, F.; Fang, J.; Peng, T.; Hu, J.; Tsao, M.; Little, P.; Zheng, W. The Role of FOXOs and Autophagy in Cancer and Metastasis—Implications in Therapeutic Development. Med. Res. Rev. 2020, 40, 2089–2113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z. The FoxO–Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef]
- Audesse, A.J.; Dhakal, S.; Hassell, L.-A.; Gardell, Z.; Nemtsova, Y.; Webb, A.E. FOXO3 Directly Regulates an Autophagy Network to Functionally Regulate Proteostasis in Adult Neural Stem Cells. PLoS Genet. 2019, 15, e1008097. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Jeon, S.-M.; Bhaskar, P.T.; Nogueira, V.; Sundararajan, D.; Tonic, I.; Park, Y.; Hay, N. FoxOs Inhibit MTORC1 and Activate Akt by Inducing the Expression of Sestrin3 and Rictor. Dev. Cell 2010, 18, 592–604. [Google Scholar] [CrossRef]
- Sánchez-Álvarez, M.; Strippoli, R.; Donadelli, M.; Bazhin, A.V.; Cordani, M. Sestrins as a Therapeutic Bridge between ROS and Autophagy in Cancer. Cancers 2019, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, J.; Fu, Q.; Zhu, L.; Zhang, Z.; Zhang, F.; Lu, N.; Chen, A. Role of Hypoxia-Inducible Factor-1α in Autophagic Cell Death in Microglial Cells Induced by Hypoxia. Mol. Med. Rep. 2017, 15, 2097–2105. [Google Scholar] [CrossRef]
- Daskalaki, I.; Gkikas, I.; Tavernarakis, N. Hypoxia and Selective Autophagy in Cancer Development and Therapy. Front. Cell Dev. Biol. 2018, 6, 104. [Google Scholar] [CrossRef]
- Wu, C.-A.; Huang, D.-Y.; Lin, W.-W. Beclin-1-Independent Autophagy Positively Regulates Internal Ribosomal Entry Site-Dependent Translation of Hypoxia-Inducible Factor 1α under Nutrient Deprivation. Oncotarget 2014, 5, 7525–7539. [Google Scholar] [CrossRef]
- Lu, N.; Li, X.; Tan, R.; An, J.; Cai, Z.; Hu, X.; Wang, F.; Wang, H.; Lu, C.; Lu, H. HIF-1α/Beclin1-Mediated Autophagy Is Involved in Neuroprotection Induced by Hypoxic Preconditioning. J. Mol. Neurosci. 2018, 66, 238–250. [Google Scholar] [CrossRef]
- Shi, Y.; Norberg, E.; Vakifahmetoglu-Norberg, H. Mutant P53 as a Regulator and Target of Autophagy. Front. Oncol. 2021, 10, 607149. [Google Scholar] [CrossRef] [PubMed]
- Errafiy, R.; Aguado, C.; Ghislat, G.; Esteve, J.M.; Gil, A.; Loutfi, M.; Knecht, E. PTEN Increases Autophagy and Inhibits the Ubiquitin-Proteasome Pathway in Glioma Cells Independently of Its Lipid Phosphatase Activity. PLoS ONE 2013, 8, e83318. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Wang, Z.; Li, H.; Shou, J.; Jing, Z.; Xie, J.; Sui, X.; Pan, H.; Han, W. The Role of STAT3 in Autophagy. Autophagy 2015, 11, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Nandy, A.; Lin, L.; Velentzas, P.D.; Wu, L.P.; Baehrecke, E.H.; Silverman, N. The NF-ΚB Factor Relish Regulates Atg1 Expression and Controls Autophagy. Cell Rep. 2018, 25, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Perry, A.; Wesseling, P. Histologic Classification of Gliomas. Handb. Clin. Neurol. 2016, 134, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Aldape, K.; Colman, H.; Figrarella-Branger, D.; Fuller, G.N.; Giannini, C.; Holland, E.C.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Komori, T.; et al. CIMPACT-NOW Update 5: Recommended Grading Criteria and Terminologies for IDH-Mutant Astrocytomas. Acta Neuropathol. 2020, 139, 603–608. [Google Scholar] [CrossRef]
- Velázquez Vega, J.E.; Brat, D.J. Incorporating Advances in Molecular Pathology Into Brain Tumor Diagnostics. Adv. Anat. Pathol. 2018, 25, 143–171. [Google Scholar] [CrossRef]
- Shirahata, M.; Ono, T.; Stichel, D.; Schrimpf, D.; Reuss, D.E.; Sahm, F.; Koelsche, C.; Wefers, A.; Reinhardt, A.; Huang, K.; et al. Novel, Improved Grading System(s) for IDH-Mutant Astrocytic Gliomas. Acta Neuropathol. 2018, 136, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.R.; Shi, Z.; Zhang, Z.; Chan, A.K.; Aibaidula, A.; Wang, W.; Kwan, J.S.H.; Poon, W.S.; Chen, H.; Li, W.; et al. IDH Mutant Lower Grade (WHO Grades II/III) Astrocytomas Can Be Stratified for Risk by CDKN2A, CDK4 and PDGFRA Copy Number Alterations. Brain Pathol. 2020, 30, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Appay, R.; Dehais, C.; Maurage, C.-A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. CDKN2A Homozygous Deletion Is a Strong Adverse Prognosis Factor in Diffuse Malignant IDH-Mutant Gliomas. Neuro-Oncology 2019, 21, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Stichel, D.; Ebrahimi, A.; Reuss, D.; Schrimpf, D.; Ono, T.; Shirahata, M.; Reifenberger, G.; Weller, M.; Hänggi, D.; Wick, W.; et al. Distribution of EGFR Amplification, Combined Chromosome 7 Gain and Chromosome 10 Loss, and TERT Promoter Mutation in Brain Tumors and Their Potential for the Reclassification of IDHwt Astrocytoma to Glioblastoma. Acta Neuropathol. 2018, 136, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dube, C.; Gibert, M.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The P53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef]
- Yang, H.; Han, F.; Hu, R.; Liu, J.; Sui, J.; Xiang, X.; Wang, F.; Chu, L.; Song, S. PTEN Gene Mutations Correlate to Poor Prognosis in Glioma Patients: A Meta-Analysis. OncoTargets Ther. 2016, 9, 3485–3492. [Google Scholar] [CrossRef]
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef]
- Fabro, F.; Lamfers, M.L.M.; Leenstra, S. Advancements, Challenges, and Future Directions in Tackling Glioblastoma Resistance to Small Kinase Inhibitors. Cancers 2022, 14, 600. [Google Scholar] [CrossRef] [PubMed]
- Puputti, M.; Tynninen, O.; Sihto, H.; Blom, T.; Mäenpää, H.; Isola, J.; Paetau, A.; Joensuu, H.; Nupponen, N.N. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in Gliomas. Mol. Cancer Res. 2006, 4, 927–934. [Google Scholar] [CrossRef]
- Meyer, N.; Zielke, S.; Michaelis, J.B.; Linder, B.; Warnsmann, V.; Rakel, S.; Osiewacz, H.D.; Fulda, S.; Mittelbronn, M.; Münch, C.; et al. AT 101 Induces Early Mitochondrial Dysfunction and HMOX1 (Heme Oxygenase 1) to Trigger Mitophagic Cell Death in Glioma Cells. Autophagy 2018, 14, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Zielke, S.; Kardo, S.; Zein, L.; Mari, M.; Covarrubias-Pinto, A.; Kinzler, M.N.; Meyer, N.; Stolz, A.; Fulda, S.; Reggiori, F.; et al. ATF4 Links ER Stress with Reticulophagy in Glioblastoma Cells. Autophagy 2021, 17, 2432–2448. [Google Scholar] [CrossRef] [PubMed]
- Colardo, M.; Segatto, M.; Di Bartolomeo, S. Targeting RTK-PI3K-MTOR Axis in Gliomas: An Update. Int. J. Mol. Sci. 2021, 22, 4899. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Patric, I.R.P.; Patil, V.; Shwetha, S.D.; Hegde, A.S.; Chandramouli, B.A.; Arivazhagan, A.; Santosh, V.; Somasundaram, K. Methylation Silencing of ULK2, an Autophagy Gene, Is Essential for Astrocyte Transformation and Tumor Growth. J. Biol. Chem. 2014, 289, 22306–22318. [Google Scholar] [CrossRef] [PubMed]
- Pirtoli, L.; Cevenini, G.; Tini, P.; Vannini, M.; Oliveri, G.; Marsili, S.; Mourmouras, V.; Rubino, G.; Miracco, C. The Prognostic Role of Beclin 1 Protein Expression in High-Grade Gliomas. Autophagy 2009, 5, 930–936. [Google Scholar] [CrossRef]
- Huang, X.; Bai, H.M.; Chen, L.; Li, B.; Lu, Y.C. Reduced Expression of LC3B-II and Beclin 1 in Glioblastoma Multiforme Indicates a down-Regulated Autophagic Capacity That Relates to the Progression of Astrocytic Tumors. J. Clin. Neurosci. 2010, 17, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor P62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Kumar, A.; Zhang, Y.; Wang, A.S.; Chen, K.; Lim, Y.; Shai, A.; Taylor, J.W.; Clarke, J.; Hilz, S.; et al. PI3K/AKT/MTOR Signaling Pathway Activity in IDH-Mutant Diffuse Glioma and Clinical Implications. Neuro-Oncology 2022, 24, 1471–1481. [Google Scholar] [CrossRef]
- Mecca, C.; Giambanco, I.; Donato, R.; Arcuri, C. Targeting MTOR in Glioblastoma: Rationale and Preclinical/Clinical Evidence. Dis. Markers 2018, 2018, 9230479. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, Q.; Hua, X.; Li, X.; Zhang, W.; Sun, H.; Li, S.; Wang, X.; Li, B. Beclin 1, an Autophagy-Related Gene, Augments Apoptosis in U87 Glioblastoma Cells. Oncol. Rep. 2014, 31, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Q.; Bi, Y. Autophagy and Apoptosis Are Regulated by Stress on Bcl2 by AMBRA1 in the Endoplasmic Reticulum and Mitochondria. Theor. Biol. Med. Model. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Wang, S.; Wang, B.; Hong, X.; Liu, X.; Li, M.; Shen, R.; Dong, Q. The Role of Interaction between Autophagy and Apoptosis in Tumorigenesis (Review). Oncol. Rep. 2022, 48, 208. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, V.; Marino, A.; Caruso, M.; Capodifoglio, S.; Flati, V.; Marynuk, A.; Marricareda, V.; Ursi, S.; Lanuti, P.; Talora, C.; et al. Autophagy Processes Are Dependent on EGF Receptor Signaling. Oncotarget 2018, 9, 30289–30303. [Google Scholar] [CrossRef]
- You, Z.; Jiang, W.-X.; Qin, L.-Y.; Gong, Z.; Wan, W.; Li, J.; Wang, Y.; Zhang, H.; Peng, C.; Zhou, T.; et al. Requirement for P62 Acetylation in the Aggregation of Ubiquitylated Proteins under Nutrient Stress. Nat. Commun. 2019, 10, 5792. [Google Scholar] [CrossRef]
- Xu, H.; Sun, L.; Zheng, Y.; Yu, S.; Ou-yang, J.; Han, H.; Dai, X.; Yu, X.; Li, M.; Lan, Q. GBP3 Promotes Glioma Cell Proliferation via SQSTM1/P62-ERK1/2 Axis. Biochem. Biophys. Res. Commun. 2018, 495, 446–453. [Google Scholar] [CrossRef]
- Wang, H.; Sun, T.; Hu, J.; Zhang, R.; Rao, Y.; Wang, S.; Chen, R.; McLendon, R.E.; Friedman, A.H.; Keir, S.T.; et al. MiR-33a Promotes Glioma-Initiating Cell Self-Renewal via PKA and NOTCH Pathways. J. Clin. Investig. 2014, 124, 4489–4502. [Google Scholar] [CrossRef]
- Guo, X.; Xue, H.; Guo, X.; Gao, X.; Xu, S.; Yan, S.; Han, X.; Li, T.; Shen, J.; Li, G. MiR224-3p Inhibits Hypoxia-Induced Autophagy by Targeting Autophagy-Related Genes in Human Glioblastoma Cells. Oncotarget 2015, 6, 41620–41637. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, H.; Miao, Y.; Feng, X.; Li, Y.; Wang, H.; Song, X. Upregulation of P72 Enhances Malignant Migration and Invasion of Glioma Cells by Repressing Beclin1 Expression. Biochemistry 2016, 81, 574–582. [Google Scholar] [CrossRef]
- Rajendran, P.; Alzahrani, A.M.; Hanieh, H.N.; Kumar, S.A.; Ben Ammar, R.; Rengarajan, T.; Alhoot, M.A. Autophagy and Senescence: A New Insight in Selected Human Diseases. J. Cell. Physiol. 2019, 234, 21485–21492. [Google Scholar] [CrossRef]
- Cayo, A.; Segovia, R.; Venturini, W.; Moore-Carrasco, R.; Valenzuela, C.; Brown, N. MTOR Activity and Autophagy in Senescent Cells, a Complex Partnership. Int. J. Mol. Sci. 2021, 22, 8149. [Google Scholar] [CrossRef]
- Aasland, D.; Götzinger, L.; Hauck, L.; Berte, N.; Meyer, J.; Effenberger, M.; Schneider, S.; Reuber, E.E.; Roos, W.P.; Tomicic, M.T.; et al. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR–CHK1, P21, and NF-ΚB. Cancer Res. 2019, 79, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Beltzig, L.; Schwarzenbach, C.; Leukel, P.; Frauenknecht, K.B.M.; Sommer, C.; Tancredi, A.; Hegi, M.E.; Christmann, M.; Kaina, B. Senescence Is the Main Trait Induced by Temozolomide in Glioblastoma Cells. Cancers 2022, 14, 2233. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Wu, M.; Wu, J.; Liu, J. Synergistic Effects of Resveratrol and Temozolomide Against Glioblastoma Cells: Underlying Mechanism and Therapeutic Implications. Cancer Manag. Res. 2020, 12, 8341–8354. [Google Scholar] [CrossRef] [PubMed]
- Filippi-Chiela, E.C.; Thomé, M.P.; Bueno e Silva, M.M.; Pelegrini, A.L.; Ledur, P.F.; Garicochea, B.; Zamin, L.L.; Lenz, G. Resveratrol Abrogates the Temozolomide-Induced G2 Arrest Leading to Mitotic Catastrophe and Reinforces the Temozolomide-Induced Senescence in Glioma Cells. BMC Cancer 2013, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, Q.; Zhou, W.; Feng, Z.; Huang, B.; Chen, A.; Zhang, D.; Li, W.; Zhang, Q.; Jiang, Z.; et al. Inhibition of Glioma Growth by Flavokawain B Is Mediated through Endoplasmic Reticulum Stress Induced Autophagy. Autophagy 2018, 14, 2007–2022. [Google Scholar] [CrossRef]
- Gammoh, N.; Fraser, J.; Puente, C.; Syred, H.M.; Kang, H.; Ozawa, T.; Lam, D.; Acosta, J.C.; Finch, A.J.; Holland, E.; et al. Suppression of Autophagy Impedes Glioblastoma Development and Induces Senescence. Autophagy 2016, 12, 1431–1439. [Google Scholar] [CrossRef]
- Tamrakar, S.; Yashiro, M.; Kawashima, T.; Uda, T.; Terakawa, Y.; Kuwae, Y.; Ohsawa, M.; Ohata, K. Clinicopathological Significance of Autophagy-Related Proteins and Its Association with Genetic Alterations in Gliomas. Anticancer Res. 2019, 39, 1233–1242. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Mitrakas, A.; Kalamida, D.; Zois, C.E.; Haider, S.; Piperidou, C.; Pappa, A.; Gatter, K.C.; Harris, A.L.; et al. Autophagy and Lysosomal Related Protein Expression Patterns in Human Glioblastoma. Cancer Biol. Ther. 2014, 15, 1468–1478. [Google Scholar] [CrossRef]
- Galavotti, S.; Bartesaghi, S.; Faccenda, D.; Shaked-Rabi, M.; Sanzone, S.; McEvoy, A.; Dinsdale, D.; Condorelli, F.; Brandner, S.; Campanella, M.; et al. The Autophagy-Associated Factors DRAM1 and P62 Regulate Cell Migration and Invasion in Glioblastoma Stem Cells. Oncogene 2013, 32, 699–712. [Google Scholar] [CrossRef]
- Cj, P.; Hv, E.; Vijayakurup, V.; R Menon, G.; Nair, S.; Gopala, S. High LC3/Beclin Expression Correlates with Poor Survival in Glioma: A Definitive Role for Autophagy as Evidenced by In Vitro Autophagic Flux. Pathol. Oncol. Res. 2019, 25, 137–148. [Google Scholar] [CrossRef]
- Wen, Z.; Zeng, W.; Chen, Y.; Li, H.; Wang, J.; Cheng, Q.; Yu, J.; Zhou, H.; Liu, Z.; Xiao, J.; et al. Knockdown ATG4C Inhibits Gliomas Progression and Promotes Temozolomide Chemosensitivity by Suppressing Autophagic Flux. J. Exp. Clin. Cancer Res. 2019, 38, 298. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Williams, J.; Nathans, J. Roles of HIFs and VEGF in Angiogenesis in the Retina and Brain. J. Clin. Investig. 2019, 129, 3807–3820. [Google Scholar] [CrossRef]
- Liao, Y.; Luo, Z.; Lin, Y.; Chen, H.; Chen, T.; Xu, L.; Orgurek, S.; Berry, K.; Dzieciatkowska, M.; Reisz, J.A.; et al. PRMT3 Drives Glioblastoma Progression by Enhancing HIF1A and Glycolytic Metabolism. Cell Death Dis. 2022, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Luo, K.; Liu, H.; Nie, X.; Xue, L.; Wang, R.; Xu, Y.; Cui, J.; Shao, N.; Zhi, F. P62 Acts as an Oncogene and Is Targeted by MiR-124-3p in Glioma. Cancer Cell Int. 2019, 19, 280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wu, Z. Immunohistochemical Assessment of Autophagic Protein LC3B and P62 Levels in Glioma Patients. Int. J. Clin. Exp. Pathol. 2018, 11, 862–868. [Google Scholar]
- Tang, J.; Li, Y.; Xia, S.; Li, J.; Yang, Q.; Ding, K.; Zhang, H. Sequestosome 1/P62: A Multitasker in the Regulation of Malignant Tumor Aggression (Review). Int. J. Oncol. 2021, 59, 77. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, Y.-B.; Fan, Y.; Wu, G.-L. P62/SQSTM1 Is Involved in Caspase-8 Associated Cell Death Induced by Proteasome Inhibitor MG132 in U87MG Cells. Cell Biol. Int. 2014, 38, 1221–1226. [Google Scholar] [CrossRef]
- Ieni, A.; Pizzimenti, C.; Broggi, G.; Caltabiano, R.; Germanò, A.; Barbagallo, G.M.V.; Vigneri, P.; Giuffrè, G.; Tuccari, G. Immunoexpression of P62/SQSTM1/Sequestosome-1 in Human Primary and Recurrent IDH1/2 Wild-Type Glioblastoma: A Pilot Study. Oncol. Lett. 2022, 24, 336. [Google Scholar] [CrossRef]
- Jawhari, S.; Ratinaud, M.-H.; Verdier, M. Glioblastoma, Hypoxia and Autophagy: A Survival-Prone ‘Ménage-à-Trois’. Cell Death Dis. 2016, 7, e2434. [Google Scholar] [CrossRef]
- Duan, S. Silencing the Autophagy-Specific Gene Beclin-1 Contributes to Attenuated Hypoxia-Induced Vasculogenic Mimicry Formation in Glioma. Cancer Biomark. 2018, 21, 565–574. [Google Scholar] [CrossRef]
- Wu, H.-B.; Yang, S.; Weng, H.-Y.; Chen, Q.; Zhao, X.-L.; Fu, W.-J.; Niu, Q.; Ping, Y.-F.; Wang, J.M.; Zhang, X.; et al. Autophagy-Induced KDR/VEGFR-2 Activation Promotes the Formation of Vasculogenic Mimicry by Glioma Stem Cells. Autophagy 2017, 13, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Nah, J.; Yoo, S.-M.; Jung, S.; Jeong, E.I.; Park, M.; Kaang, B.-K.; Jung, Y.-K. Phosphorylated CAV1 Activates Autophagy through an Interaction with BECN1 under Oxidative Stress. Cell Death Dis. 2017, 8, e2822. [Google Scholar] [CrossRef]
- Zhou, H.G.; Zhang, J.D.; Zhang, Y.F. The Effect of Downregulation of MCT1 on the Proliferation of Glioma Cells. Zhonghua Zhong Liu Za Zhi 2019, 41, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Gonçalves, V.; Honavar, M.; Pinheiro, C.; Martinho, O.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; Costa, P.; Palmeirim, I.; et al. Monocarboxylate Transporters (MCTs) in Gliomas: Expression and Exploitation as Therapeutic Targets. Neuro-Oncology 2013, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Chen, F.-C.; Pan, Q.; Zhao, X.; Zhao, C.; Cho, W.; Chen, H.-L. The Different Functions and Clinical Significances of Caveolin-1 in Human Adenocarcinoma and Squamous Cell Carcinoma. OncoTargets Ther. 2017, 10, 819–835. [Google Scholar] [CrossRef]
- Hernandez, S.J.; Fote, G.; Reyes-Ortiz, A.M.; Steffan, J.S.; Thompson, L.M. Cooperation of Cell Adhesion and Autophagy in the Brain: Functional Roles in Development and Neurodegenerative Disease. Matrix Biol. Plus 2021, 12, 100089. [Google Scholar] [CrossRef]
- Talukdar, S.; Pradhan, A.K.; Bhoopathi, P.; Shen, X.-N.; August, L.A.; Windle, J.J.; Sarkar, D.; Furnari, F.B.; Cavenee, W.K.; Das, S.K.; et al. MDA-9/Syntenin Regulates Protective Autophagy in Anoikis-Resistant Glioma Stem Cells. Proc. Natl. Acad. Sci. USA 2018, 115, 5768–5773. [Google Scholar] [CrossRef]
- Kim, J.; Chee, W.-Y.; Yabuta, N.; Kajiwara, K.; Nada, S.; Okada, M. Atg5-Mediated Autophagy Controls Apoptosis/Anoikis via P53/Rb Pathway in Naked Mole-Rat Fibroblasts. Biochem. Biophys. Res. Commun. 2020, 528, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sayers, C.M.; Verginadis, I.I.; Lehman, S.L.; Cheng, Y.; Cerniglia, G.J.; Tuttle, S.W.; Feldman, M.D.; Zhang, P.J.L.; Fuchs, S.Y.; et al. ATF4-Dependent Induction of Heme Oxygenase 1 Prevents Anoikis and Promotes Metastasis. J. Clin. Investig. 2015, 125, 2592–2608. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yi, L.; Liu, P.; Abeysekera, I.; Hai, L.; Li, T.; Tao, Z.; Ma, H.; Xie, Y.; Huang, Y.; et al. Tumour Cell Dormancy as a Contributor to the Reduced Survival of GBM Patients Who Received Standard Therapy. Oncol. Rep. 2018, 40, 463–471. [Google Scholar] [CrossRef]
- Ishii, A.; Kimura, T.; Sadahiro, H.; Kawano, H.; Takubo, K.; Suzuki, M.; Ikeda, E. Histological Characterization of the Tumorigenic “Peri-Necrotic Niche” Harboring Quiescent Stem-Like Tumor Cells in Glioblastoma. PLoS ONE 2016, 11, e0147366. [Google Scholar] [CrossRef]
- Fu, Z.; Luo, W.; Wang, J.; Peng, T.; Sun, G.; Shi, J.; Li, Z.; Zhang, B. Malat1 Activates Autophagy and Promotes Cell Proliferation by Sponging MiR-101 and Upregulating STMN1, RAB5A and ATG4D Expression in Glioma. Biochem. Biophys. Res. Commun. 2017, 492, 480–486. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid Action Induces Autophagy-Mediated Cell Death through Stimulation of ER Stress in Human Glioma Cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Shchors, K.; Massaras, A.; Hanahan, D. Dual Targeting of the Autophagic Regulatory Circuitry in Gliomas with Repurposed Drugs Elicits Cell-Lethal Autophagy and Therapeutic Benefit. Cancer Cell 2015, 28, 456–471. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Domingues, P.; González-Tablas, M.; Otero, Á.; Pascual, D.; Miranda, D.; Ruiz, L.; Sousa, P.; Ciudad, J.; Gonçalves, J.M.; Lopes, M.C.; et al. Tumor Infiltrating Immune Cells in Gliomas and Meningiomas. Brain. Behav. Immun. 2016, 53, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gieryng, A.; Pszczolkowska, D.; Walentynowicz, K.A.; Rajan, W.D.; Kaminska, B. Immune Microenvironment of Gliomas. Lab. Investig. 2017, 97, 498–518. [Google Scholar] [CrossRef]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain Immunology and Immunotherapy in Brain Tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef]
- Gargini, R.; Segura-Collar, B.; Sánchez-Gómez, P. Cellular Plasticity and Tumor Microenvironment in Gliomas: The Struggle to Hit a Moving Target. Cancers 2020, 12, 1622. [Google Scholar] [CrossRef]
- Pires-Afonso, Y.; Niclou, S.P.; Michelucci, A. Revealing and Harnessing Tumour-Associated Microglia/Macrophage Heterogeneity in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 689. [Google Scholar] [CrossRef]
- Molina, M.L.; García-Bernal, D.; Martinez, S.; Valdor, R. Autophagy in the Immunosuppressive Perivascular Microenvironment of Glioblastoma. Cancers 2019, 12, 102. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Y.; Zhang, J.; Dong, X.; Gao, P.; Liu, K.; Ma, C.; Zhao, G. Breaking Bad: Autophagy Tweaks the Interplay between Glioma and the Tumor Immune Microenvironment. Front. Immunol. 2021, 12, 746621. [Google Scholar] [CrossRef]
- Cunha, L.D.; Yang, M.; Carter, R.; Guy, C.; Harris, L.; Crawford, J.C.; Quarato, G.; Boada-Romero, E.; Kalkavan, H.; Johnson, M.D.L.; et al. LC3-Associated Phagocytosis in Myeloid Cells Promotes Tumor Immune Tolerance. Cell 2018, 175, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Zhang, Z.; Gao, Z.; Qi, Y.; Qiu, W.; Pan, Z.; Guo, Q.; Li, B.; Zhao, S.; et al. Hypoxic Glioma-Derived Exosomes Promote M2-like Macrophage Polarization by Enhancing Autophagy Induction. Cell Death Dis. 2021, 12, 373. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front. Immunol. 2021, 12, 741305. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Shao, J.; Qin, Y.; Ji, Q.; Zhang, X.; Du, J. Cathepsin S-Mediated Autophagic Flux in Tumor-Associated Macrophages Accelerate Tumor Development by Promoting M2 Polarization. Mol. Cancer 2014, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, Z.; Cheng, Y.; Ma, C.; Zhong, Y.; Xiao, Y.; Gao, X.; Li, Z. M2 Macrophage-Derived Exosomal MicroRNAs Inhibit Cell Migration and Invasion in Gliomas through PI3K/AKT/MTOR Signaling Pathway. J. Transl. Med. 2021, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, T.; Shen, X.; Xia, X.; Xu, G.; Bai, X.; Liang, T. Macrophage-Induced Tumor Angiogenesis Is Regulated by the TSC2–MTOR Pathway. Cancer Res. 2012, 72, 1363–1372. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.-U. Regulation of the Innate Immune System by Autophagy: Monocytes, Macrophages, Dendritic Cells and Antigen Presentation. Cell Death Differ. 2019, 26, 715–727. [Google Scholar] [CrossRef]
- Houtman, J.; Freitag, K.; Gimber, N.; Schmoranzer, J.; Heppner, F.L.; Jendrach, M. Beclin1-driven Autophagy Modulates the Inflammatory Response of Microglia via NLRP3. EMBO J. 2019, 38, e99430. [Google Scholar] [CrossRef]
- Khan, S.; Mittal, S.; McGee, K.; Alfaro-Munoz, K.D.; Majd, N.; Balasubramaniyan, V.; de Groot, J.F. Role of Neutrophils and Myeloid-Derived Suppressor Cells in Glioma Progression and Treatment Resistance. Int. J. Mol. Sci. 2020, 21, 1954. [Google Scholar] [CrossRef] [PubMed]
- Alissafi, T.; Hatzioannou, A.; Mintzas, K.; Barouni, R.M.; Banos, A.; Sormendi, S.; Polyzos, A.; Xilouri, M.; Wielockx, B.; Gogas, H.; et al. Autophagy Orchestrates the Regulatory Program of Tumor-Associated Myeloid-Derived Suppressor Cells. J. Clin. Investig. 2018, 128, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Wei, Q.; Shin, J.N.; Abdel Fattah, E.; Bonilla, D.L.; Xiang, Q.; Eissa, N.T. Autophagy Is Required for Neutrophil-Mediated Inflammation. Cell Rep. 2015, 12, 1731–1739. [Google Scholar] [CrossRef]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps. Front. Cell Dev. Biol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Mocholi, E.; Dowling, S.D.; Botbol, Y.; Gruber, R.C.; Ray, A.K.; Vastert, S.; Shafit-Zagardo, B.; Coffer, P.J.; Macian, F. Autophagy Is a Tolerance-Avoidance Mechanism That Modulates TCR-Mediated Signaling and Cell Metabolism to Prevent Induction of T Cell Anergy. Cell Rep. 2018, 24, 1136–1150. [Google Scholar] [CrossRef]
- DeVorkin, L.; Pavey, N.; Carleton, G.; Comber, A.; Ho, C.; Lim, J.; McNamara, E.; Huang, H.; Kim, P.; Zacharias, L.G.; et al. Autophagy Regulation of Metabolism Is Required for CD8+ T Cell Anti-Tumor Immunity. Cell Rep. 2019, 27, 502–513. [Google Scholar] [CrossRef]
- Dwivedi, A.R.; Thakur, A.; Kumar, V.; Skvortsova, I.; Kumar, V. Targeting Cancer Stem Cells Pathways for the Effective Treatment of Cancer. Curr. Drug Targets 2020, 21, 258–278. [Google Scholar] [CrossRef]
- Nunes, T.; Hamdan, D.; Leboeuf, C.; El Bouchtaoui, M.; Gapihan, G.; Nguyen, T.; Meles, S.; Angeli, E.; Ratajczak, P.; Lu, H.; et al. Targeting Cancer Stem Cells to Overcome Chemoresistance. Int. J. Mol. Sci. 2018, 19, 4036. [Google Scholar] [CrossRef]
- Abbas, S.; Singh, S.K.; Tiwari, S.; Sharma, L.K.; Tiwari, M. Role of Autophagy in Regulation of Glioma Stem Cells Population during Therapeutic Stress. J. Stem Cells Regen. Med. 2020, 16, 80–89. [Google Scholar] [CrossRef]
- Pan, H.; Cai, N.; Li, M.; Liu, G.; Izpisua Belmonte, J.C. Autophagic Control of Cell ‘Stemness. EMBO Mol. Med. 2013, 5, 327–331. [Google Scholar] [CrossRef]
- Hou, J.; Han, Z.; Jing, Y.; Yang, X.; Zhang, S.; Sun, K.; Hao, C.; Meng, Y.; Yu, F.; Liu, X.; et al. Autophagy Prevents Irradiation Injury and Maintains Stemness through Decreasing ROS Generation in Mesenchymal Stem Cells. Cell Death Dis. 2013, 4, e844. [Google Scholar] [CrossRef]
- Ryskalin, L.; Gaglione, A.; Limanaqi, F.; Biagioni, F.; Familiari, P.; Frati, A.; Esposito, V.; Fornai, F. The Autophagy Status of Cancer Stem Cells in Gliobastoma Multiforme: From Cancer Promotion to Therapeutic Strategies. Int. J. Mol. Sci. 2019, 20, 3824. [Google Scholar] [CrossRef]
- Kaverina, N.V.; Kadagidze, Z.G.; Borovjagin, A.V.; Karseladze, A.I.; Kim, C.K.; Lesniak, M.S.; Miska, J.; Zhang, P.; Baryshnikova, M.A.; Xiao, T.; et al. Tamoxifen Overrides Autophagy Inhibition in Beclin-1-Deficient Glioma Cells and Their Resistance to Adenovirus-Mediated Oncolysis via Upregulation of PUMA and BAX. Oncogene 2018, 37, 6069–6082. [Google Scholar] [CrossRef] [PubMed]
- Buccarelli, M.; Marconi, M.; Pacioni, S.; De Pascalis, I.; D’Alessandris, Q.G.; Martini, M.; Ascione, B.; Malorni, W.; Larocca, L.M.; Pallini, R.; et al. Inhibition of Autophagy Increases Susceptibility of Glioblastoma Stem Cells to Temozolomide by Igniting Ferroptosis. Cell Death Dis. 2018, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Ramírez, A.; Castillo-Rodríguez, R.A.; Zavala-Vega, S.; Jimenez-Farfan, D.; Anaya-Rubio, I.; Briseño, E.; Palencia, G.; Guevara, P.; Cruz-Salgado, A.; Sotelo, J.; et al. Autophagy as a Potential Therapy for Malignant Glioma. Pharmaceuticals 2020, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Serrano-Garcia, N.; Escamilla-Ramírez, Á.; Castillo-Rodríguez, R.A.; Jimenez-Farfan, D.; Palencia, G.; Calvillo, M.; Alvarez-Lemus, M.A.; Flores-Nájera, A.; Cruz-Salgado, A.; et al. Autophagic and Apoptotic Pathways as Targets for Chemotherapy in Glioblastoma. Int. J. Mol. Sci. 2018, 19, 3773. [Google Scholar] [CrossRef]
- Compter, I.; Eekers, D.B.P.; Hoeben, A.; Rouschop, K.M.A.; Reymen, B.; Ackermans, L.; Beckervordersantforth, J.; Bauer, N.J.C.; Anten, M.M.; Wesseling, P.; et al. Chloroquine Combined with Concurrent Radiotherapy and Temozolomide for Newly Diagnosed Glioblastoma: A Phase IB Trial. Autophagy 2021, 17, 2604–2612. [Google Scholar] [CrossRef]
- Hori, Y.S.; Hosoda, R.; Akiyama, Y.; Sebori, R.; Wanibuchi, M.; Mikami, T.; Sugino, T.; Suzuki, K.; Maruyama, M.; Tsukamoto, M.; et al. Chloroquine Potentiates Temozolomide Cytotoxicity by Inhibiting Mitochondrial Autophagy in Glioma Cells. J. Neurooncol. 2015, 122, 11–20. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, H.-K.; Lee, N.-H.; Yi, H.-Y.; Kim, H.-S.; Hong, S.H.; Hong, Y.-K.; Joe, Y.A. The Synergistic Effect of Combination Temozolomide and Chloroquine Treatment Is Dependent on Autophagy Formation and P53 Status in Glioma Cells. Cancer Lett. 2015, 360, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Chen, M.; Cao, F.; Huang, H.; Zhan, R.; Zheng, X. Chloroquine, an Autophagy Inhibitor, Potentiates the Radiosensitivity of Glioma Initiating Cells by Inhibiting Autophagy and Activating Apoptosis. BMC Neurol. 2016, 16, 178. [Google Scholar] [CrossRef]
- Sotelo, J.; Briceño, E.; López-González, M.A. Adding Chloroquine to Conventional Treatment for Glioblastoma Multiforme. Ann. Intern. Med. 2006, 144, 337. [Google Scholar] [CrossRef] [PubMed]
- Briceño, E.; Reyes, S.; Sotelo, J. Therapy of Glioblastoma Multiforme Improved by the Antimutagenic Chloroquine. Neurosurg. Focus 2003, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, N.; Kiang, K.Y.; Zhu, Z.; Leung, G.M.; Cheng, S.; Leung, G.K. Quinacrine Enhances Temozolomide Cytotoxicity in Temozolomide-Sensitive and -Resistant Glioblastoma Cells. Glioma 2018, 1, 175. [Google Scholar] [CrossRef]
- Mudassar, F.; Shen, H.; O’Neill, G.; Hau, E. Targeting Tumor Hypoxia and Mitochondrial Metabolism with Anti-Parasitic Drugs to Improve Radiation Response in High-Grade Gliomas. J. Exp. Clin. Cancer Res. 2020, 39, 208. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.R.; Wang, X.; Gillespie, G.Y.; Woltjer, R.L.; Pike, M.M. Combined Efficacy of Cediranib and Quinacrine in Glioma Is Enhanced by Hypoxia and Causally Linked to Autophagic Vacuole Accumulation. PLoS ONE 2014, 9, e114110. [Google Scholar] [CrossRef]
- Lohitesh, K.; Saini, H.; Srivastava, A.; Mukherjee, S.; Roy, A.; Chowdhury, R. Autophagy Inhibition Potentiates SAHA-mediated Apoptosis in Glioblastoma Cells by Accumulation of Damaged Mitochondria. Oncol. Rep. 2018, 39, 2787–2796. [Google Scholar] [CrossRef]
- Benzeroual, K.; Dharmadhikari, R.; Mehta, V. SAHA Anti-neoplastic Effects in Glioblastoma via IGF-1R Signaling Pathway. FASEB J. 2021, 35, 05439. [Google Scholar] [CrossRef]
- Feng, X.-L.; Deng, H.-B.; Wang, Z.-G.; Wu, Y.; Ke, J.-J.; Feng, X.-B. Suberoylanilide Hydroxamic Acid Triggers Autophagy by Influencing the MTOR Pathway in the Spinal Dorsal Horn in a Rat Neuropathic Pain Model. Neurochem. Res. 2019, 44, 450–464. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Chang, W.-C.; Hsu, T.-I.; Liu, J.-J.; Yeh, S.-H.; Wang, J.-Y.; Liou, J.-P.; Ko, C.-Y.; Chang, K.-Y.; Chuang, J.-Y. Suberoylanilide Hydroxamic Acid Represses Glioma Stem-like Cells. J. Biomed. Sci. 2016, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Jaeckle, K.A.; Maurer, M.J.; Reid, J.M.; Ames, M.M.; Hardwick, J.S.; Reilly, J.F.; Loboda, A.; Nebozhyn, M.; Fantin, V.R.; et al. Phase II Trial of Vorinostat in Recurrent Glioblastoma Multiforme: A North Central Cancer Treatment Group Study. J. Clin. Oncol. 2009, 27, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.B.; Lipp, E.S.; Miller, E.; Herndon, J.E.; McSherry, F.; Desjardins, A.; Reardon, D.A.; Friedman, H.S. Phase I/II Trial of Vorinostat, Bevacizumab, and Daily Temozolomide for Recurrent Malignant Gliomas. J. Neurooncol. 2018, 137, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Kilburn, L.B.; Mansur, D.B.; Krailo, M.; Buxton, A.; Adekunle, A.; Gajjar, A.; Adamson, P.C.; Weigel, B.; Fox, E.; et al. Phase I/II Trial of Vorinostat and Radiation and Maintenance Vorinostat in Children with Diffuse Intrinsic Pontine Glioma: A Children’s Oncology Group Report. Neuro-Oncology 2022, 24, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, B.; Li, W.; Wang, L.; Song, X.; Guo, C.; Li, Y.; Liu, F.; Zhu, F.; Wang, Q.; et al. Systemic Application of 3-Methyladenine Markedly Inhibited Atherosclerotic Lesion in ApoE−/− Mice by Modulating Autophagy, Foam Cell Formation and Immune-Negative Molecules. Cell Death Dis. 2016, 7, e2498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, R.; Chen, Q.; Chang, H. Inhibition of Autophagy Using 3-Methyladenine Increases Cisplatin-Induced Apoptosis by Increasing Endoplasmic Reticulum Stress in U251 Human Glioma Cells. Mol. Med. Rep. 2015, 12, 1727–1732. [Google Scholar] [CrossRef]
- Zhou, N.; Wei, Z.X.; Qi, Z.X. Inhibition of Autophagy Triggers Melatonin-Induced Apoptosis in Glioblastoma Cells. BMC Neurosci. 2019, 20, 63. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, H.; Ruan, J.; Fang, W.; Li, A.; Tian, G.; Niu, X.; Luo, S.; Zhao, P. Autophagy Inhibition Induces Enhanced Proapoptotic Effects of ZD6474 in Glioblastoma. Br. J. Cancer 2013, 109, 164–171. [Google Scholar] [CrossRef]
- Kreisl, T.N.; McNeill, K.A.; Sul, J.; Iwamoto, F.M.; Shih, J.; Fine, H.A. A Phase I/II Trial of Vandetanib for Patients with Recurrent Malignant Glioma. Neuro-Oncology 2012, 14, 1519–1526. [Google Scholar] [CrossRef]
- Lee, E.Q.; Kaley, T.J.; Duda, D.G.; Schiff, D.; Lassman, A.B.; Wong, E.T.; Mikkelsen, T.; Purow, B.W.; Muzikansky, A.; Ancukiewicz, M.; et al. A Multicenter, Phase II, Randomized, Noncomparative Clinical Trial of Radiation and Temozolomide with or without Vandetanib in Newly Diagnosed Glioblastoma Patients. Clin. Cancer Res. 2015, 21, 3610–3618. [Google Scholar] [CrossRef]
- Eimer, S.; Belaud-Rotureau, M.-A.; Airiau, K.; Jeanneteau, M.; Laharanne, E.; Véron, N.; Vital, A.; Loiseau, H.; Merlio, J.-P.; Belloc, F. Autophagy Inhibition Cooperates with Erlotinib to Induce Glioblastoma Cell Death. Cancer Biol. Ther. 2011, 11, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.R.; Rath, P.; Oyinlade, O.; Lopez, H.; Mughal, S.; Xia, S.; Li, Y.; Kaur, H.; Zhou, X.; Ahmed, A.K.; et al. Crizotinib and Erlotinib Inhibits Growth of C-Met+/EGFRvIII+ Primary Human Glioblastoma Xenografts. Clin. Neurol. Neurosurg. 2018, 171, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Karpel-Massler, G.; Westhoff, M.-A.; Kast, R.E.; Dwucet, A.; Karpel-Massler, S.; Nonnenmacher, L.; Siegelin, M.D.; Wirtz, C.R.; Debatin, K.-M.; Halatsch, M.-E. Simultaneous Interference with HER1/EGFR and RAC1 Signaling Drives Cytostasis and Suppression of Survivin in Human Glioma Cells in Vitro. Neurochem. Res. 2017, 42, 1543–1554. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Brandes, A.A.; Rampling, R.; Kouwenhoven, M.C.M.; Kros, J.M.; Carpentier, A.F.; Clement, P.M.; Frenay, M.; Campone, M.; Baurain, J.-F.; et al. Randomized Phase II Trial of Erlotinib Versus Temozolomide or Carmustine in Recurrent Glioblastoma: EORTC Brain Tumor Group Study 26034. J. Clin. Oncol. 2009, 27, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Giglio, P.; Hu, J.; Groves, M.; Merrell, R.; Conrad, C.; Phuphanich, S.; Puduvalli, V.K.; Loghin, M.; Paleologos, N.; et al. A Phase II Study of Bevacizumab and Erlotinib after Radiation and Temozolomide in MGMT Unmethylated GBM Patients. J. Neurooncol. 2016, 126, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Peereboom, D.M.; Ahluwalia, M.S.; Ye, X.; Supko, J.G.; Hilderbrand, S.L.; Phuphanich, S.; Nabors, L.B.; Rosenfeld, M.R.; Mikkelsen, T.; Grossman, S.A. NABTT 0502: A Phase II and Pharmacokinetic Study of Erlotinib and Sorafenib for Patients with Progressive or Recurrent Glioblastoma Multiforme. Neuro-Oncology 2013, 15, 490–496. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Kuan, Y.-H.; Ou, Y.-C.; Li, J.-R.; Wu, C.-C.; Pan, P.-H.; Chen, W.-Y.; Huang, H.-Y.; Chen, C.-J. Autophagy Contributes to Gefitinib-Induced Glioma Cell Growth Inhibition. Exp. Cell Res. 2014, 327, 102–112. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Li, J.-R.; Wu, C.-C.; Ou, Y.-C.; Chen, W.-Y.; Kuan, Y.-H.; Wang, W.-Y.; Chen, C.-J. Valproic Acid Sensitizes Human Glioma Cells to Gefitinib-Induced Autophagy. IUBMB Life 2015, 67, 869–879. [Google Scholar] [CrossRef]
- Uhm, J.H.; Ballman, K.V.; Wu, W.; Giannini, C.; Krauss, J.C.; Buckner, J.C.; James, C.D.; Scheithauer, B.W.; Behrens, R.J.; Flynn, P.J.; et al. Phase II Evaluation of Gefitinib in Patients with Newly Diagnosed Grade 4 Astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int. J. Radiat. Oncol. 2011, 80, 347–353. [Google Scholar] [CrossRef]
- Chakravarti, A.; Wang, M.; Robins, H.I.; Lautenschlaeger, T.; Curran, W.J.; Brachman, D.G.; Schultz, C.J.; Choucair, A.; Dolled-Filhart, M.; Christiansen, J.; et al. RTOG 0211: A Phase 1/2 Study of Radiation Therapy with Concurrent Gefitinib for Newly Diagnosed Glioblastoma Patients. Int. J. Radiat. Oncol. 2013, 85, 1206–1211. [Google Scholar] [CrossRef]
- Shingu, T.; Fujiwara, K.; Bögler, O.; Akiyama, Y.; Moritake, K.; Shinojima, N.; Tamada, Y.; Yokoyama, T.; Kondo, S. Inhibition of Autophagy at a Late Stage Enhances Imatinib-Induced Cytotoxicity in Human Malignant Glioma Cells. Int. J. Cancer 2009, 124, 1060–1071. [Google Scholar] [CrossRef]
- Sautter, L.; Hofheinz, R.; Tuettenberg, J.; Grimm, M.; Vajkoczy, P.; Groden, C.; Schmieder, K.; Hochhaus, A.; Wenz, F.; Giordano, F.A. Open-Label Phase II Evaluation of Imatinib in Primary Inoperable or Incompletely Resected and Recurrent Glioblastoma. Oncology 2020, 98, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Brandes, A.A.; Dittrich, C.; Fumoleau, P.; Coudert, B.; Clement, P.M.J.; Frenay, M.; Rampling, R.; Stupp, R.; Kros, J.M.; et al. Phase II Study of Imatinib in Patients with Recurrent Gliomas of Various Histologies: A European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J. Clin. Oncol. 2008, 26, 4659–4665. [Google Scholar] [CrossRef]
- Jakubowicz-Gil, J.; Bądziul, D.; Langner, E.; Wertel, I.; Zając, A.; Rzeski, W. Temozolomide and Sorafenib as Programmed Cell Death Inducers of Human Glioma Cells. Pharmacol. Rep. 2017, 69, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, K.; Wang, H.; Dai, Y. Inhibition of Autophagy by Chloroquine Enhances the Antitumor Efficacy of Sorafenib in Glioblastoma. Cell. Mol. Neurobiol. 2016, 36, 1197–1208. [Google Scholar] [CrossRef]
- Hamed, H.A.; Tavallai, S.; Grant, S.; Poklepovic, A.; Dent, P. Sorafenib/Regorafenib and Lapatinib Interact to Kill CNS Tumor Cells. J. Cell. Physiol. 2015, 230, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zustovich, F.; Landi, L.; Lombardi, G.; Porta, C.; Galli, L.; Fontana, A.; Amoroso, D.; Galli, C.; Andreuccetti, M.; Falcone, A.; et al. Sorafenib plus Daily Low-Dose Temozolomide for Relapsed Glioblastoma: A Phase II Study. Anticancer Res. 2013, 33, 3487–3494. [Google Scholar] [CrossRef]
- Pan, J.; Chen, B.; Su, C.-H.; Zhao, R.; Xu, Z.-X.; Sun, L.; Lee, M.-H.; Yeung, S.-C.J. Autophagy Induced by Farnesyltransferase Inhibitors in Cancer Cells. Cancer Biol. Ther. 2008, 7, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Yust-Katz, S.; Liu, D.; Yuan, Y.; Liu, V.; Kang, S.; Groves, M.; Puduvalli, V.; Levin, V.; Conrad, C.; Colman, H.; et al. Phase 1/1b Study of Lonafarnib and Temozolomide in Patients with Recurrent or Temozolomide Refractory Glioblastoma. Cancer 2013, 119, 2747–2753. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Thompson, J.C.; Griesinger, A.M.; Amani, V.; Donson, A.M.; Birks, D.K.; Morgan, M.J.; Mirsky, D.M.; Handler, M.H.; Foreman, N.K.; et al. Autophagy Inhibition Improves Chemosensitivity in BRAFV600E Brain Tumors. Cancer Discov. 2014, 4, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kaley, T.; Touat, M.; Subbiah, V.; Hollebecque, A.; Rodon, J.; Lockhart, A.C.; Keedy, V.; Bielle, F.; Hofheinz, R.-D.; Joly, F.; et al. BRAF Inhibition in BRAF V600-Mutant Gliomas: Results From the VE-BASKET Study. J. Clin. Oncol. 2018, 36, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dobrikov, M.; Keir, S.T.; Gromeier, M.; Pastan, I.H.; Reisfeld, R.; Bigner, D.D.; Chandramohan, V. Synergistic Antitumor Effects of 9.2.27-PE38KDEL and ABT-737 in Primary and Metastatic Brain Tumors. PLoS ONE 2019, 14, e0210608. [Google Scholar] [CrossRef]
- Rahman, M.A.; Engelsen, A.S.T.; Sarowar, S.; Bindesbøll, C.; Birkeland, E.; Goplen, D.; Lotsberg, M.L.; Knappskog, S.; Simonsen, A.; Chekenya, M. Bortezomib Abrogates Temozolomide-Induced Autophagic Flux through an ATG5 Dependent Pathway. Front. Cell Dev. Biol. 2022, 10, 1022191. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Wang, C.; Leng, X.; Lian, S.; Feng, J.; Li, J.; Wang, H. Inhibition of Autophagy Enhances Apoptosis Induced by Proteasome Inhibitor Bortezomib in Human Glioblastoma U87 and U251 Cells. Mol. Cell. Biochem. 2014, 385, 265–275. [Google Scholar] [CrossRef]
- Tang, J.-H.; Yang, L.; Chen, J.-X.; Li, Q.-R.; Zhu, L.-R.; Xu, Q.-F.; Huang, G.-H.; Zhang, Z.-X.; Xiang, Y.; Du, L.; et al. Bortezomib Inhibits Growth and Sensitizes Glioma to Temozolomide (TMZ) via down-Regulating the FOXM1–Survivin Axis. Cancer Commun. 2019, 39, 81. [Google Scholar] [CrossRef]
- Kong, X.-T.; Nguyen, N.T.; Choi, Y.J.; Zhang, G.; Nguyen, H.N.; Filka, E.; Green, S.; Yong, W.H.; Liau, L.M.; Green, R.M.; et al. Phase 2 Study of Bortezomib Combined with Temozolomide and Regional Radiation Therapy for Upfront Treatment of Patients with Newly Diagnosed Glioblastoma Multiforme: Safety and Efficacy Assessment. Int. J. Radiat. Oncol. 2018, 100, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Petővári, G.; Hujber, Z.; Krencz, I.; Dankó, T.; Nagy, N.; Tóth, F.; Raffay, R.; Mészáros, K.; Rajnai, H.; Vetlényi, E.; et al. Targeting Cellular Metabolism Using Rapamycin and/or Doxycycline Enhances Anti-Tumour Effects in Human Glioma Cells. Cancer Cell Int. 2018, 18, 211. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Cheng, F.; Wen, X.; Feng, S.; Yu, F.; Tang, H.; Liu, Z.; Teng, X. Rapamycin Inhibits Glioma Cells Growth and Promotes Autophagy by MiR-26a-5p/DAPK1 Axis. Cancer Manag. Res. 2021, 13, 2691–2700. [Google Scholar] [CrossRef]
- Zhuang, W.-Z.; Long, L.-M.; Ji, W.-J.; Liang, Z.-Q. Rapamycin Induces Differentiation of Glioma Stem/Progenitor Cells by Activating Autophagy. Chin. J. Cancer 2011, 30, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.P.C.; Kuo, J.S.; Chiang, H.-C.; Wang, H.-E.; Wang, Y.-S.; Huang, C.-C.; Huang, Y.-C.; Chi, M.-S.; Mehta, M.P.; Chi, K.-H. Temozolomide, Sirolimus and Chloroquine Is a New Therapeutic Combination That Synergizes to Disrupt Lysosomal Function and Cholesterol Homeostasis in GBM Cells. Oncotarget 2018, 9, 6883–6896. [Google Scholar] [CrossRef]
- Chandrika, G.; Natesh, K.; Ranade, D.; Chugh, A.; Shastry, P. Mammalian Target of Rapamycin Inhibitors, Temsirolimus and Torin 1, Attenuate Stemness-Associated Properties and Expression of Mesenchymal Markers Promoted by Phorbol-Myristate-Acetate and Oncostatin-M in Glioblastoma Cells. Tumor Biol. 2017, 39, 101042831769592. [Google Scholar] [CrossRef]
- Geoerger, B.; Kieran, M.W.; Grupp, S.; Perek, D.; Clancy, J.; Krygowski, M.; Ananthakrishnan, R.; Boni, J.P.; Berkenblit, A.; Spunt, S.L. Phase II Trial of Temsirolimus in Children with High-Grade Glioma, Neuroblastoma and Rhabdomyosarcoma. Eur. J. Cancer 2012, 48, 253–262. [Google Scholar] [CrossRef]
- Wen, P.Y.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Norden, A.D.; Cloughesy, T.F.; Robins, H.I.; Lieberman, F.S.; Gilbert, M.R.; Mehta, M.P.; et al. Phase I/II Study of Erlotinib and Temsirolimus for Patients with Recurrent Malignant Gliomas: North American Brain Tumor Consortium Trial 04-02. Neuro-Oncology 2014, 16, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Lassen, U.; Sorensen, M.; Gaziel, T.B.; Hasselbalch, B.; Poulsen, H.S. Phase II Study of Bevacizumab and Temsirolimus Combination Therapy for Recurrent Glioblastoma Multiforme. Anticancer Res. 2013, 33, 1657–1660. [Google Scholar] [PubMed]
- Lee, E.Q.; Kuhn, J.; Lamborn, K.R.; Abrey, L.; DeAngelis, L.M.; Lieberman, F.; Robins, H.I.; Chang, S.M.; Yung, W.K.A.; Drappatz, J.; et al. Phase I/II Study of Sorafenib in Combination with Temsirolimus for Recurrent Glioblastoma or Gliosarcoma: North American Brain Tumor Consortium Study 05-02. Neuro-Oncology 2012, 14, 1511–1518. [Google Scholar] [CrossRef]
- Josset, E.; Burckel, H.; Noël, G.; Bischoff, P. The MTOR Inhibitor RAD001 Potentiates Autophagic Cell Death Induced by Temozolomide in a Glioblastoma Cell Line. Anticancer Res. 2013, 33, 1845–1851. [Google Scholar] [PubMed]
- Chinnaiyan, P.; Won, M.; Wen, P.Y.; Rojiani, A.M.; Werner-Wasik, M.; Shih, H.A.; Ashby, L.S.; Michael Yu, H.-H.; Stieber, V.W.; Malone, S.C.; et al. A Randomized Phase II Study of Everolimus in Combination with Chemoradiation in Newly Diagnosed Glioblastoma: Results of NRG Oncology RTOG 0913. Neuro-Oncology 2018, 20, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Shih, K.C.; Shepard, G.C.; Tillinghast, G.W.; Brinker, B.T.; Spigel, D.R. Phase II Study of Concurrent Radiation Therapy, Temozolomide, and Bevacizumab Followed by Bevacizumab/Everolimus as First-Line Treatment for Patients with Glioblastoma. Clin. Adv. Hematol. Oncol. 2012, 10, 240–246. [Google Scholar] [PubMed]
- Kreisl, T.N.; Lassman, A.B.; Mischel, P.S.; Rosen, N.; Scher, H.I.; Teruya-Feldstein, J.; Shaffer, D.; Lis, E.; Abrey, L.E. A Pilot Study of Everolimus and Gefitinib in the Treatment of Recurrent Glioblastoma (GBM). J. Neurooncol. 2009, 92, 99–105. [Google Scholar] [CrossRef]
- Liu, T.; Li, A.; Xu, Y.; Xin, Y. Momelotinib Sensitizes Glioblastoma Cells to Temozolomide by Enhancement of Autophagy via JAK2/STAT3 Inhibition. Oncol. Rep. 2019, 41, 1883–1892. [Google Scholar] [CrossRef]
- De Santi, M.; Baldelli, G.; Diotallevi, A.; Galluzzi, L.; Schiavano, G.F.; Brandi, G. Metformin Prevents Cell Tumorigenesis through Autophagy-Related Cell Death. Sci. Rep. 2019, 9, 66. [Google Scholar] [CrossRef]
- Ohno, M.; Kitanaka, C.; Miyakita, Y.; Tanaka, S.; Sonoda, Y.; Mishima, K.; Ishikawa, E.; Takahashi, M.; Yanagisawa, S.; Ohashi, K.; et al. Metformin with Temozolomide for Newly Diagnosed Glioblastoma: Results of Phase I Study and a Brief Review of Relevant Studies. Cancers 2022, 14, 4222. [Google Scholar] [CrossRef]
- Sesen, J.; Dahan, P.; Scotland, S.J.; Saland, E.; Dang, V.-T.; Lemarié, A.; Tyler, B.M.; Brem, H.; Toulas, C.; Cohen-Jonathan Moyal, E.; et al. Metformin Inhibits Growth of Human Glioblastoma Cells and Enhances Therapeutic Response. PLoS ONE 2015, 10, e0123721. [Google Scholar] [CrossRef]
- Carmignani, M.; Volpe, A.R.; Aldea, M.; Soritau, O.; Irimie, A.; Florian, I.S.; Tomuleasa, C.; Baritchii, A.; Petrushev, B.; Crisan, G.; et al. Glioblastoma Stem Cells: A New Target for Metformin and Arsenic Trioxide. J. Biol. Regul. Homeost. Agents 2014, 28, 1–15. [Google Scholar]
- O’Rawe, M.; Wickremesekera, A.C.; Pandey, R.; Young, D.; Sim, D.; FitzJohn, T.; Burgess, C.; Kaye, A.H.; Tan, S.T. Treatment of Glioblastoma with Re-Purposed Renin-Angiotensin System Modulators: Results of a Phase I Clinical Trial. J. Clin. Neurosci. 2022, 95, 48–54. [Google Scholar] [CrossRef]
- Porper, K.; Shpatz, Y.; Plotkin, L.; Pechthold, R.G.; Talianski, A.; Champ, C.E.; Furman, O.; Shimoni-Sebag, A.; Symon, Z.; Amit, U.; et al. A Phase I Clinical Trial of Dose-Escalated Metabolic Therapy Combined with Concomitant Radiation Therapy in High-Grade Glioma. J. Neurooncol. 2021, 153, 487–496. [Google Scholar] [CrossRef]
- Seliger, C.; Genbrugge, E.; Gorlia, T.; Chinot, O.; Stupp, R.; Nabors, B.; Weller, M.; Hau, P. Use of Metformin and Outcome of Patients with Newly Diagnosed Glioblastoma: Pooled Analysis. Int. J. Cancer 2020, 146, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Koleini, N.; Samiei, E.; Aghaei, M.; Cole, L.K.; Alizadeh, J.; Islam, M.I.; Vosoughi, A.; Albokashy, M.; Butterfield, Y.; et al. Simvastatin Increases Temozolomide-induced Cell Death by Targeting the Fusion of Autophagosomes and Lysosomes. FEBS J. 2020, 287, 1005–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, P.; Li, N.; Kiang, K.M.Y.; Cheng, S.Y.; Wong, V.K.-W.; Leung, G.K.-K. Lovastatin Enhances Cytotoxicity of Temozolomide via Impairing Autophagic Flux in Glioblastoma Cells. BioMed Res. Int. 2019, 2019, 2710693. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Cantó, A.; López-Abellán, M.D.; Castillo-Guardiola, V.; Hurtado, A.M.; Martínez-Penella, M.; Luengo-Gil, G.; Conesa-Zamora, P. Antitumoral Effects of Tricyclic Antidepressants: Beyond Neuropathic Pain Treatment. Cancers 2022, 14, 3248. [Google Scholar] [CrossRef]
- Hartleben, G.; Schorpp, K.; Kwon, Y.; Betz, B.; Tsokanos, F.; Dantes, Z.; Schäfer, A.; Rothenaigner, I.; Monroy Kuhn, J.M.; Morigny, P.; et al. Combination Therapies Induce Cancer Cell Death through the Integrated Stress Response and Disturbed Pyrimidine Metabolism. EMBO Mol. Med. 2021, 13, e12461. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, H.; Peng, R.; Ding, X.; Jiang, B.; Yuan, X.; Xi, J. MicroRNA-30a Increases the Chemosensitivity of U251 Glioblastoma Cells to Temozolomide by Directly Targeting Beclin�1 and Inhibiting Autophagy. Exp. Ther. Med. 2018, 15, 4798–4804. [Google Scholar] [CrossRef]
- Chen, P.-H.; Cheng, C.-H.; Shih, C.-M.; Ho, K.-H.; Lin, C.-W.; Lee, C.-C.; Liu, A.-J.; Chang, C.-K.; Chen, K.-C. The Inhibition of MicroRNA-128 on IGF-1-Activating MTOR Signaling Involves in Temozolomide-Induced Glioma Cell Apoptotic Death. PLoS ONE 2016, 11, e0167096. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Li, J.; Zhou, Q.; Huang, A.; Liu, W.; Wang, K.; Gao, L.; Qi, S.; Lu, Y. MiR-519a Enhances Chemosensitivity and Promotes Autophagy in Glioblastoma by Targeting STAT3/Bcl2 Signaling Pathway. J. Hematol. Oncol. 2018, 11, 70. [Google Scholar] [CrossRef]
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.-C.; Pentheroudakis, G. High-Grade Glioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2014, 25, iii93–iii101. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of Temozolomide Resistance in Glioblastoma—A Comprehensive Review. Cancer Drug Resist. 2020, 4, 17–43. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Chen, H.; Lei, Y.; Zhang, T.; Wang, Y.; Jin, P.; Lan, J.; Zhou, L.; Huang, Z.; et al. Regorafenib Induces Lethal Autophagy Arrest by Stabilizing PSAT1 in Glioblastoma. Autophagy 2020, 16, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, J.; Liu, Z.; Pan, L.; Xu, G. Autophagy Activation Promotes Bevacizumab Resistance in Glioblastoma by Suppressing Akt/MTOR Signaling Pathway. Oncol. Lett. 2017, 15, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.V.; Bergers, G. Mechanisms of Evasive Resistance to Anti-VEGF Therapy in Glioblastoma. CNS Oncol. 2013, 2, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-C.; Loh, J.-K.; Li, Y.-Y.; Huang, W.-S.; Chou, C.-H.; Cheng, J.-T.; Wang, Y.-T.; Lieu, A.-S.; Howng, S.-L.; Hong, Y.-R.; et al. Bcl2L12 with a BH3-like Domain in Regulating Apoptosis and TMZ-Induced Autophagy: A Prospective Combination of ABT-737 and TMZ for Treating Glioma. Int. J. Oncol. 2015, 46, 1304–1316. [Google Scholar] [CrossRef]
- Nam, H.Y.; Han, M.W.; Chang, H.W.; Lee, Y.S.; Lee, M.; Lee, H.J.; Lee, B.W.; Lee, H.J.; Lee, K.E.; Jung, M.K.; et al. Radioresistant Cancer Cells Can Be Conditioned to Enter Senescence by MTOR Inhibition. Cancer Res. 2013, 73, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Gorlia, T.; Bady, P.; Platten, M.; van den Bent, M.J.; Taphoorn, M.J.B.; Steuve, J.; Brandes, A.A.; Hamou, M.-F.; Wick, A.; et al. Phase II Study of Radiotherapy and Temsirolimus versus Radiochemotherapy with Temozolomide in Patients with Newly Diagnosed Glioblastoma without MGMT Promoter Hypermethylation (EORTC 26082). Clin. Cancer Res. 2016, 22, 4797–4806. [Google Scholar] [CrossRef]
- Graham-Gurysh, E.G.; Murthy, A.B.; Moore, K.M.; Hingtgen, S.D.; Bachelder, E.M.; Ainslie, K.M. Synergistic Drug Combinations for a Precision Medicine Approach to Interstitial Glioblastoma Therapy. J. Control. Release 2020, 323, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, J.; Liu, H.; Sun, S.; Wang, Y. Effects of MTOR Inhibitor, Everolimus, on Proliferation, Autophagy and Temozolomide Sensitivity of Glioma Cells. Trop. J. Pharm. Res. 2020, 19, 77–82. [Google Scholar] [CrossRef]
- Momota, H.; Nerio, E.; Holland, E.C. Perifosine Inhibits Multiple Signaling Pathways in Glial Progenitors and Cooperates with Temozolomide to Arrest Cell Proliferation in Gliomas In Vivo. Cancer Res. 2005, 65, 7429–7435. [Google Scholar] [CrossRef]
- Holohan, B.; Hagiopian, M.M.; Lai, T.-P.; Huang, E.; Friedman, D.R.; Wright, W.E.; Shay, J.W. Perifosine as a Potential Novel Anti-Telomerase Therapy. Oncotarget 2015, 6, 21816–21826. [Google Scholar] [CrossRef]
- Kaley, T.J.; Panageas, K.S.; Mellinghoff, I.K.; Nolan, C.; Gavrilovic, I.T.; DeAngelis, L.M.; Abrey, L.E.; Holland, E.C.; Lassman, A.B. Phase II Trial of an AKT Inhibitor (Perifosine) for Recurrent Glioblastoma. J. Neurooncol. 2019, 144, 403–407. [Google Scholar] [CrossRef]
- Kaley, T.J.; Panageas, K.S.; Pentsova, E.I.; Mellinghoff, I.K.; Nolan, C.; Gavrilovic, I.; DeAngelis, L.M.; Abrey, L.E.; Holland, E.C.; Omuro, A.; et al. Phase I Clinical Trial of Temsirolimus and Perifosine for Recurrent Glioblastoma. Ann. Clin. Transl. Neurol. 2020, 7, 429–436. [Google Scholar] [CrossRef]
- Ramezani, S.; Vousooghi, N.; Ramezani Kapourchali, F.; Joghataei, M.T. Perifosine Enhances Bevacizumab-Induced Apoptosis and Therapeutic Efficacy by Targeting PI3K/AKT Pathway in a Glioblastoma Heterotopic Model. Apoptosis 2017, 22, 1025–1034. [Google Scholar] [CrossRef]
- Aili, Y.; Maimaitiming, N.; Mahemuti, Y.; Qin, H.; Wang, Y.; Wang, Z. The Role of Exosomal MiRNAs in Glioma: Biological Function and Clinical Application. Front. Oncol. 2021, 11, 686369. [Google Scholar] [CrossRef]
- Huang, T.; Wan, X.; Alvarez, A.A.; James, C.D.; Song, X.; Yang, Y.; Sastry, N.; Nakano, I.; Sulman, E.P.; Hu, B.; et al. MIR93 (MicroRNA -93) Regulates Tumorigenicity and Therapy Response of Glioblastoma by Targeting Autophagy. Autophagy 2019, 15, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Yokoda, R.; Nagalo, B.; Vernon, B.; Oklu, R.; Albadawi, H.; DeLeon, T.; Zhou, Y.; Egan, J.; Duda, D.; Borad, M. Oncolytic Virus Delivery: From Nano-Pharmacodynamics to Enhanced Oncolytic Effect. Oncolytic Virother. 2017, 6, 39–49. [Google Scholar] [CrossRef] [PubMed]
| Direct Effects on Autophagy Pathway | ||
|---|---|---|
| Agent | Mechanism of action | Clinical trials targeting autophagy in glioma |
| Choloquine (CQ) | ||
| Quinacrine (QC) |
| |
| Suberoylanilide Hydroxamic Acid (SAHA) | ||
| 3-Methyladenine (3-MA) |
| |
| Agent | Indirect effects on autophagy | Clinical trials targeting autophagy in glioma |
| Vandetanib Tyrosine-kinase inhibitors (TKI) | ||
| Erlotinib EGFR kinase inhibitor | ||
| Gefitinib EGFR kinase inhibitor | ||
| Imatinib Tyrosine-kinase inhibitors (TKI) |
| |
| Sorafenib Tyrosine-kinase inhibitors (TKI) |
|
|
| Lonarfanib |
|
|
| Vemurafenib |
| |
| ABT-737 |
|
|
| Bortezomib |
|
|
| Sirolimus (rapamycin) | ||
| Temsirolimus | ||
| Everolimus | ||
| Momelotinib (MTB) |
| |
| Metformin | ||
| Simvastatin |
|
|
| Lovastatin |
| |
| Imipramine |
| |
| Micro-RNAs (miRNAs) |
| |
| CRISPR-Cas9 Genome Editing Application in GBM | miRNA | Target (s) |
|---|---|---|
| ATM | miR-93 | Beclin 1, ATG5, ATG4B, SQSTM1/p62 |
| ATG5 | miR-30a | Beclin 1 |
| ATG7 | miR-224-3p | ATG5 |
| TSC2 | miR-17 | ATG7 |
| miR-224-3p | ATG5, | |
| miR-7-1-3p | mTOR, SQSTM1, p62 | |
| miR-138 | LC3-II, BIM | |
| miR-30e | Beclin-1 | |
| miR-590-3p | LC3-II, Beclin-1, | |
| miR-155-3p | LC3B-II, SQSTM1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzimenti, C.; Fiorentino, V.; Franchina, M.; Martini, M.; Giuffrè, G.; Lentini, M.; Silvestris, N.; Di Pietro, M.; Fadda, G.; Tuccari, G.; et al. Autophagic-Related Proteins in Brain Gliomas: Role, Mechanisms, and Targeting Agents. Cancers 2023, 15, 2622. https://doi.org/10.3390/cancers15092622
Pizzimenti C, Fiorentino V, Franchina M, Martini M, Giuffrè G, Lentini M, Silvestris N, Di Pietro M, Fadda G, Tuccari G, et al. Autophagic-Related Proteins in Brain Gliomas: Role, Mechanisms, and Targeting Agents. Cancers. 2023; 15(9):2622. https://doi.org/10.3390/cancers15092622
Chicago/Turabian StylePizzimenti, Cristina, Vincenzo Fiorentino, Mariausilia Franchina, Maurizio Martini, Giuseppe Giuffrè, Maria Lentini, Nicola Silvestris, Martina Di Pietro, Guido Fadda, Giovanni Tuccari, and et al. 2023. "Autophagic-Related Proteins in Brain Gliomas: Role, Mechanisms, and Targeting Agents" Cancers 15, no. 9: 2622. https://doi.org/10.3390/cancers15092622
APA StylePizzimenti, C., Fiorentino, V., Franchina, M., Martini, M., Giuffrè, G., Lentini, M., Silvestris, N., Di Pietro, M., Fadda, G., Tuccari, G., & Ieni, A. (2023). Autophagic-Related Proteins in Brain Gliomas: Role, Mechanisms, and Targeting Agents. Cancers, 15(9), 2622. https://doi.org/10.3390/cancers15092622











