The Application of an Extracellular Vesicle-Based Biosensor in Early Diagnosis and Prediction of Chemoresponsiveness in Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Samples
2.2. Interrogation of OVCA Public Datasets
2.3. Reagents
2.4. Ovarian Cancer Cell Lines
2.5. pGSN Gene Interference
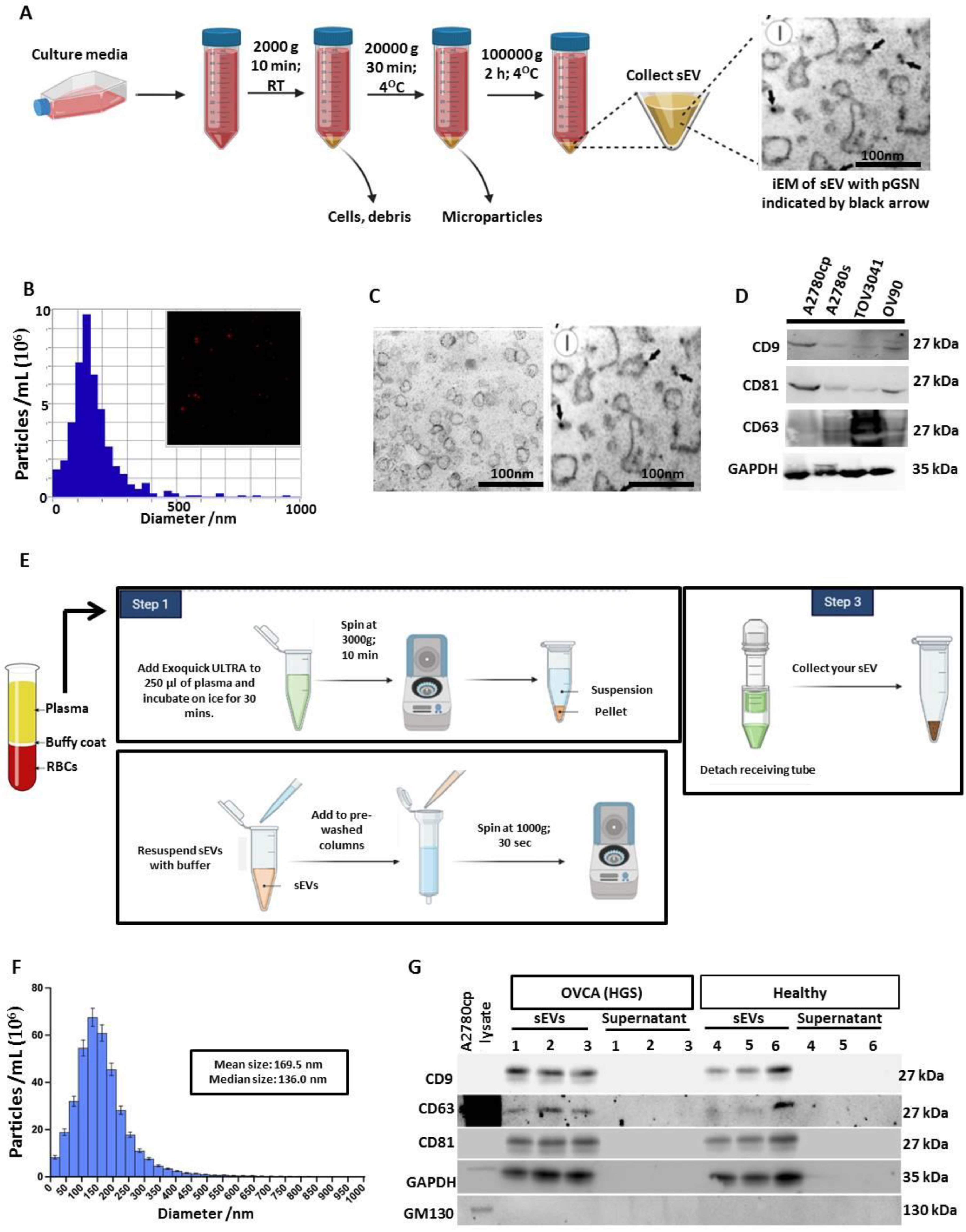
2.6. Extracellular Vesicle (EVs) Isolation and Characterization
2.7. Nanoparticle Tracking Analysis (NTA)
2.8. Protein Extraction and Western Blot Analysis
2.9. Assessment of Cell Proliferation and Apoptosis
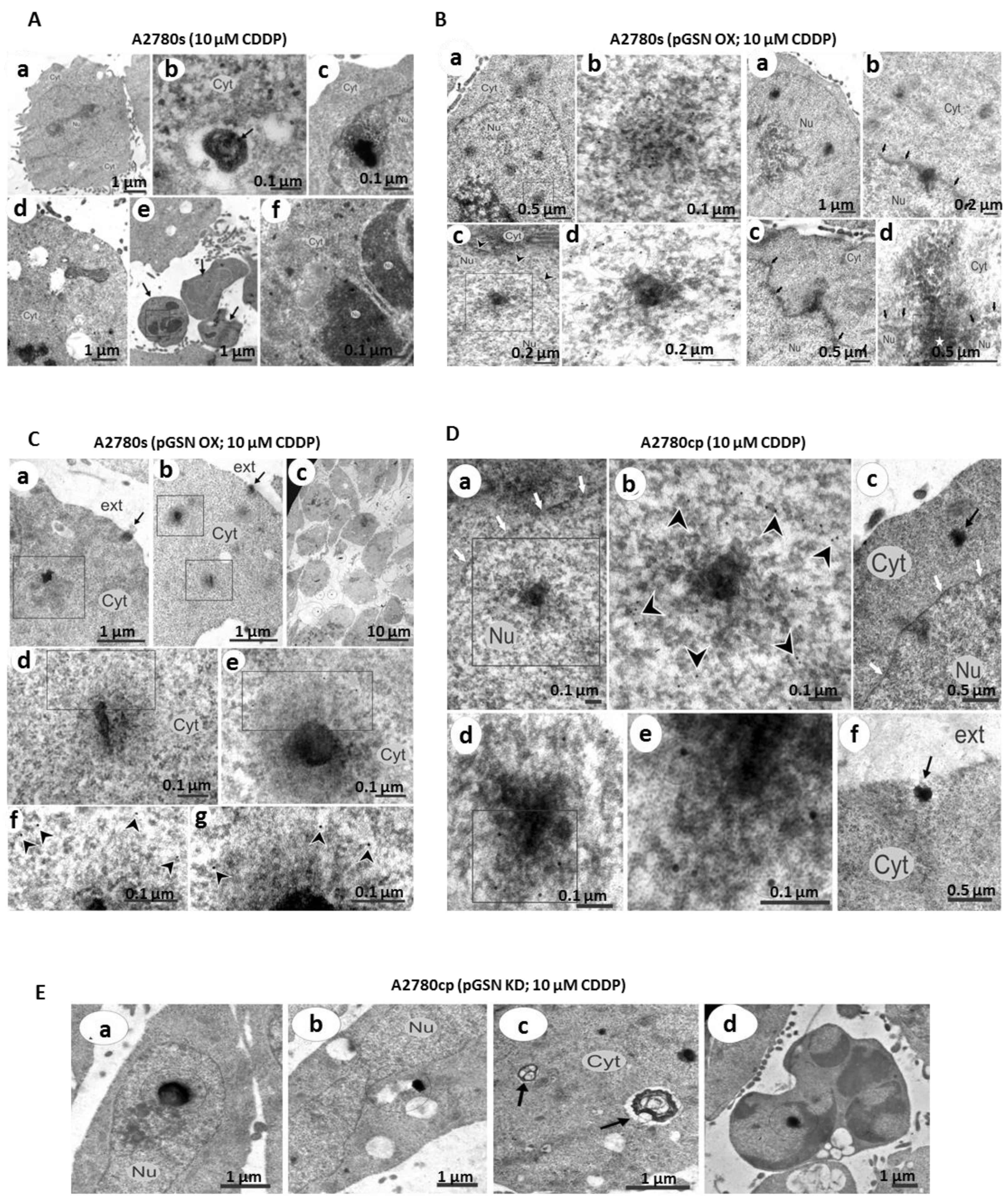
2.10. Transmission Electron Microscopy (TEM)
2.11. Immunoelectron Microscopy (iEM)
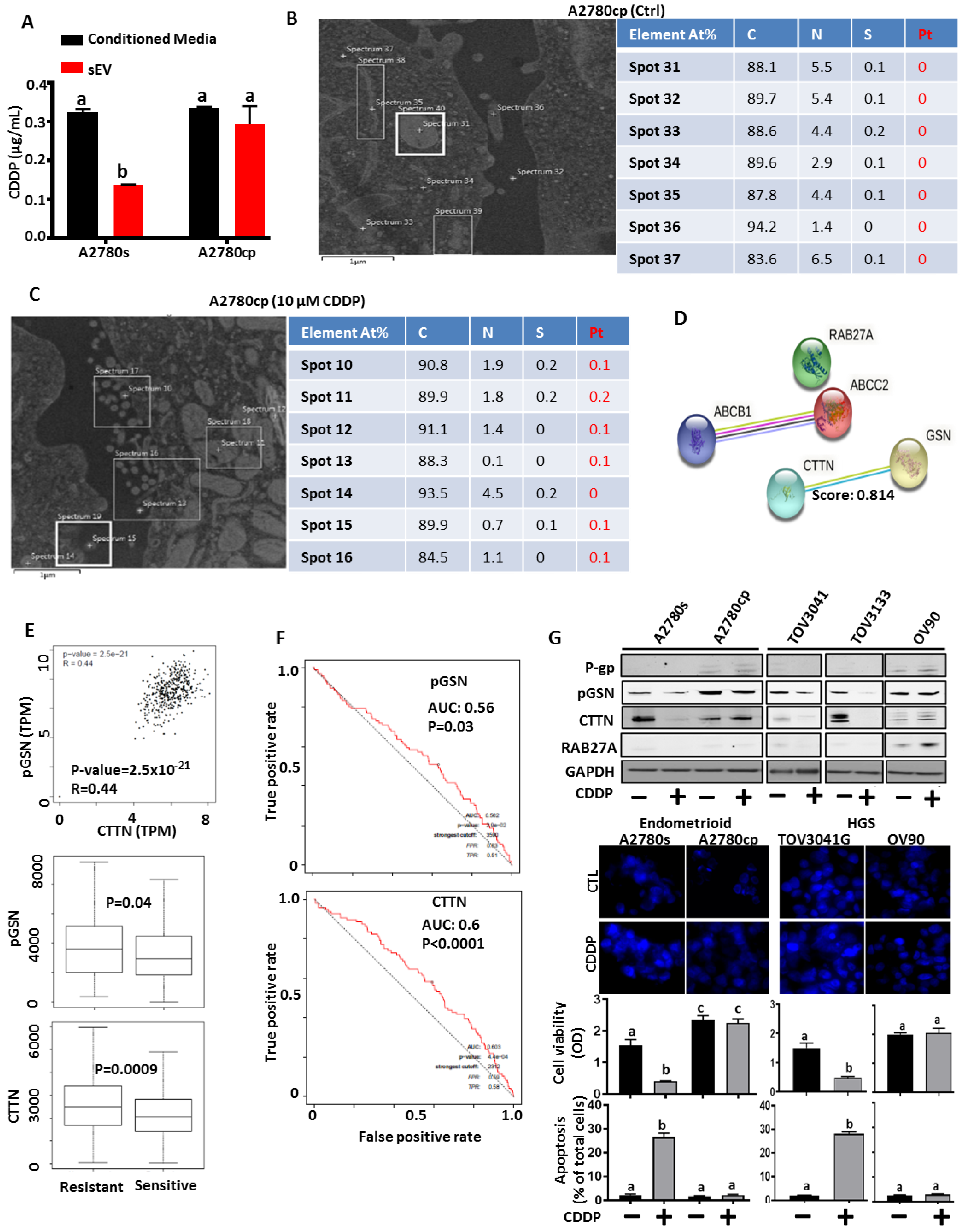
2.12. EDS and ICP-MS
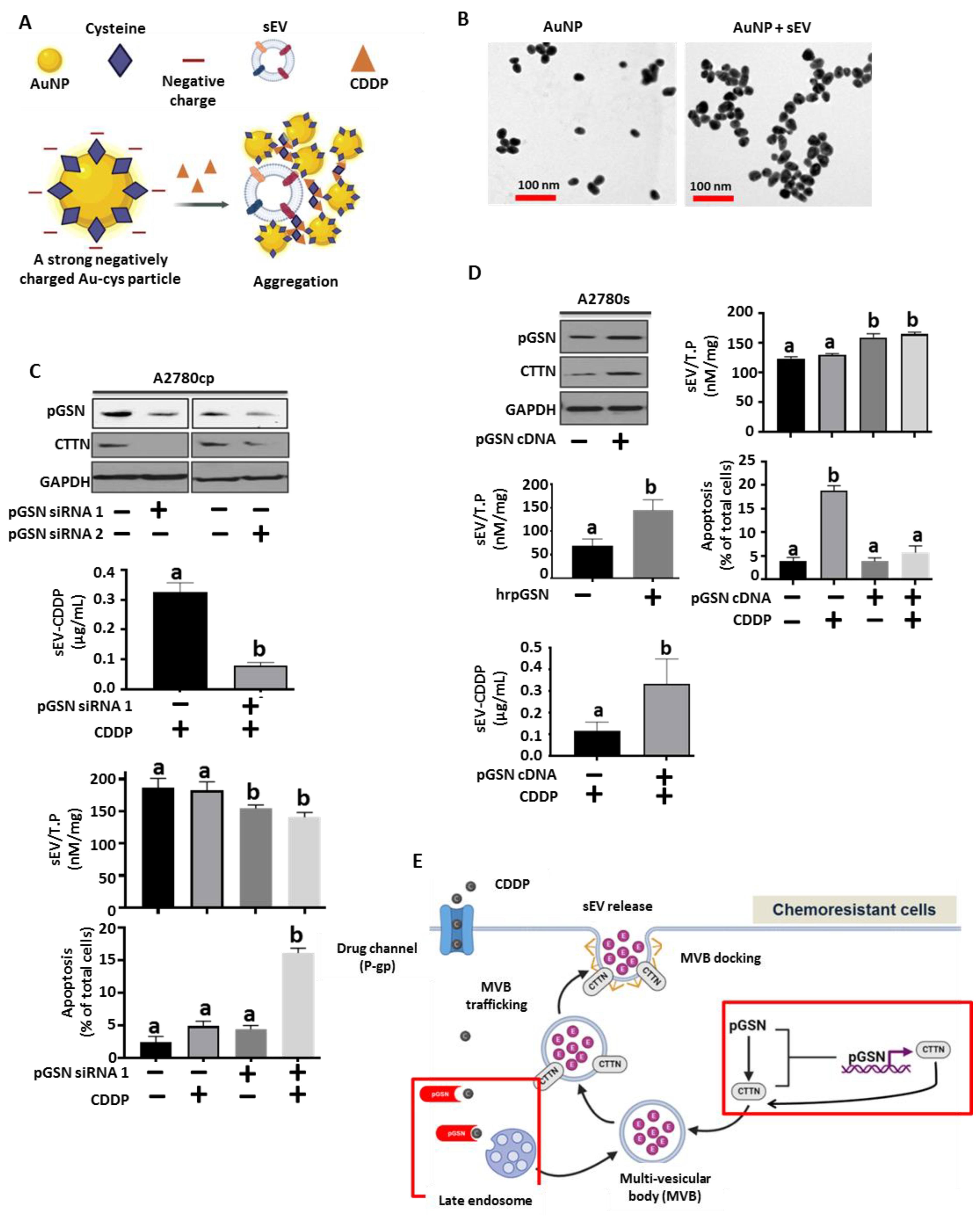
2.13. SERS Quantification of sEV and CDDP
2.14. Statistical Analyses
3. Results
3.1. Extracellular Vesicle Isolation and Characterizations from OVCA Cell Lines
3.2. Chemoresistant OVCA Cells Express High pGSN and CTTN and Are Associated with Increased sEV-CDDP
3.3. pGSN Regulates sEV Release of CDDP and Chemoresponsiveness in OVCA Cells
3.4. pGSN-Mediated Release of sEV-CDDP Is Associated with the Formation of Extranuclear and Extracellular Electron-Dense Granules
3.5. pGSN Positively Correlates with Dense-Granule-Related Proteins and Are Associated with Chemoresistance
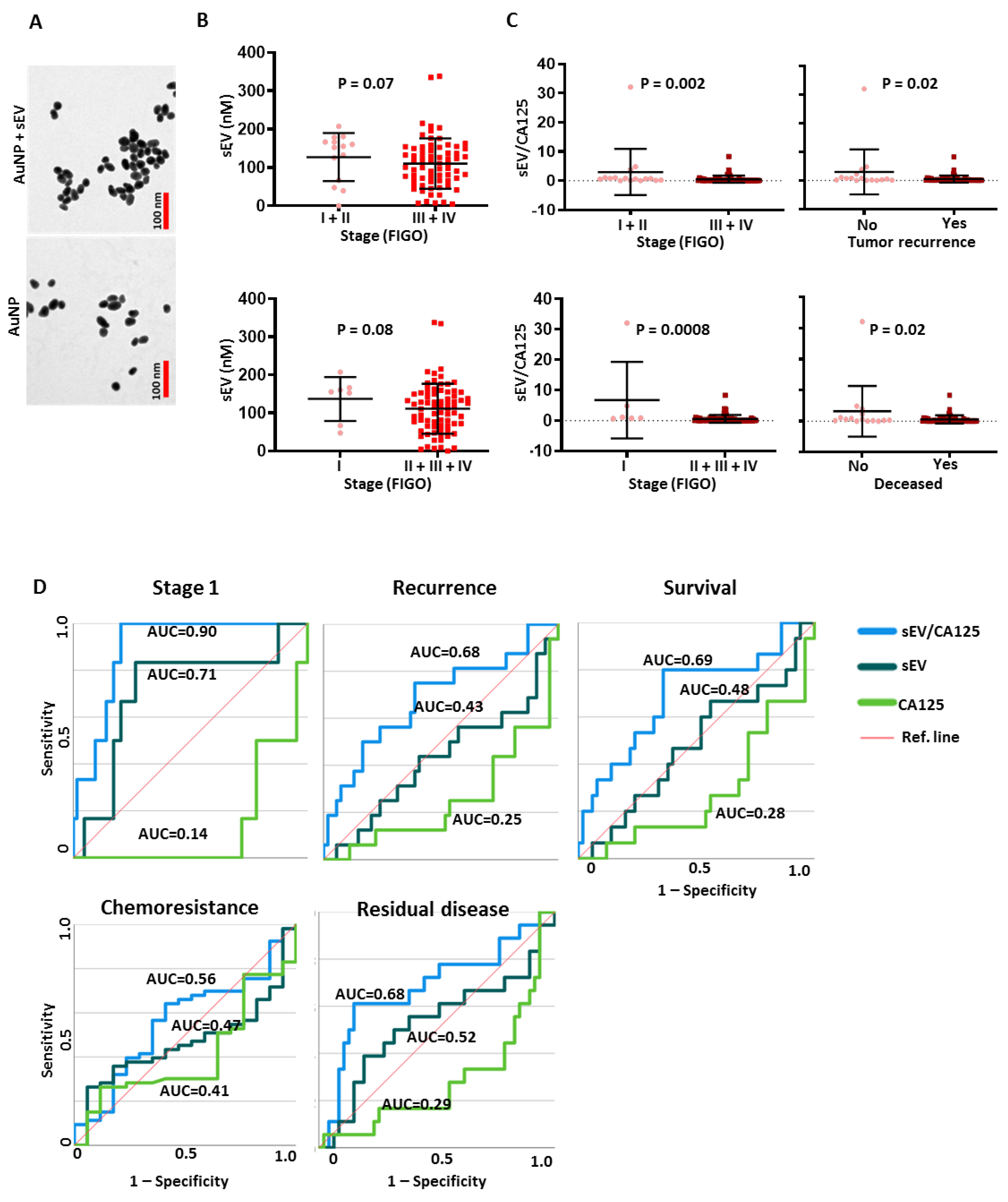
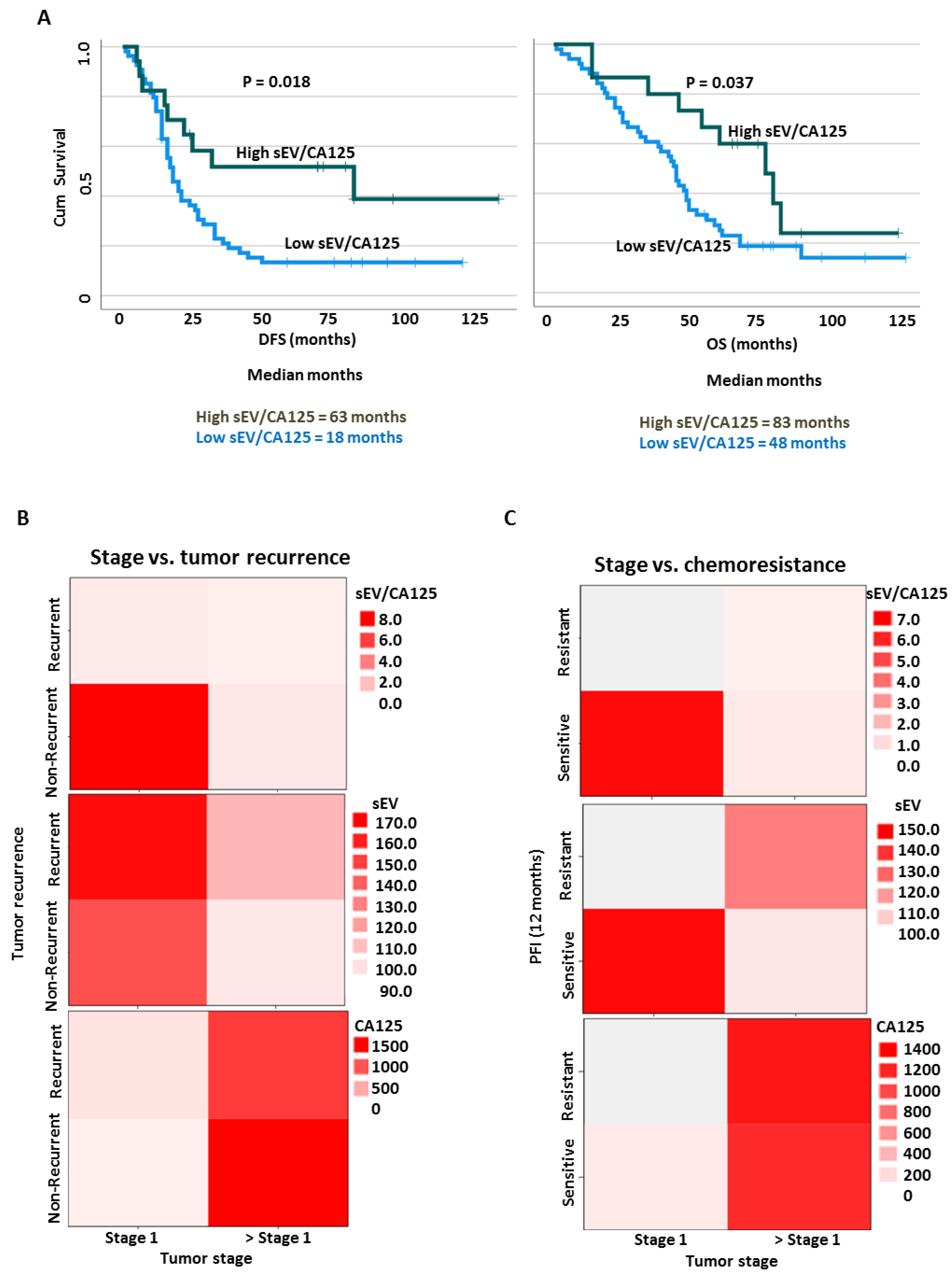
3.6. SEV to CA125 Ratio (sEV/CA125) Is a Strong Predictor of Stage 1 Disease, Chemoresponsiveness and Favorable Survival Outcomes in OVCA Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canada Statistics; Canadian Cancer Society. Release notice—Canadian cancer statistics 2019. Health Promot. Chronic Dis. Prev. Can. 2019, 39, 255. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Pan, L.L.; Wu, S.Q.; Sun, L.; Huang, G. Ca 125, pet alone, pet-ct, ct and mri in diagnosing recurrent ovarian carcinoma: A systematic review and meta-analysis. Eur. J. Radiol. 2009, 71, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, C.; Leon, A.E.; Fabricio, A.S.C.; Taborelli, M.; Polesel, J.; Del Pup, L.; Steffan, A.; Cervo, S.; Ravaggi, A.; Zanotti, L.; et al. He4, ca125 and risk of ovarian malignancy algorithm (roma) as diagnostic tools for ovarian cancer in patients with a pelvic mass: An italian multicenter study. Gynecol. Oncol. 2016, 141, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Janas, L.; Stachowiak, G.; Stetkiewicz, T.; Wilczynski, J.R. Current clinical application of serum biomarkers to detect ovarian cancer. Prz. Menopauzalny Menopause Rev. 2015, 14, 254–259. [Google Scholar] [CrossRef]
- Kwiatkowski, D.J.; Stossel, T.P.; Orkin, S.H.; Mole, J.E.; Colten, H.R.; Yin, H.L. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature 1986, 323, 455–458. [Google Scholar] [CrossRef]
- Yin, H.L.; Kwiatkowski, D.J.; Mole, J.E.; Cole, F.S. Structure and biosynthesis of cytoplasmic and secreted variants of gelsolin. J. Biol. Chem. 1984, 259, 5271–5276. [Google Scholar] [CrossRef]
- Feldt, J.; Schicht, M.; Garreis, F.; Welss, J.; Schneider, U.W.; Paulsen, F. Structure, regulation and related diseases of the actin-binding protein gelsolin. Expert Rev. Mol. Med. 2019, 20, e7. [Google Scholar] [CrossRef]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.T.; Lee, W.T.; Abedini, M.R.; Wang, P.W.; Shieh, D.B.; Dilworth, F.J.; Carmona, E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2020, 39, 1600–1616. [Google Scholar] [CrossRef]
- Ma, X.; Sun, W.; Shen, J.; Hua, Y.; Yin, F.; Sun, M.; Cai, Z. Gelsolin promotes cell growth and invasion through the upregulation of p-akt and p-p38 pathway in osteosarcoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 7165–7174. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.W.; Abedini, M.R.; Yang, L.X.; Ding, A.A.; Figeys, D.; Chang, J.Y.; Tsang, B.K.; Shieh, D.B. Gelsolin regulates cisplatin sensitivity in human head-and-neck cancer. Int. J. Cancer. J. Int. Du Cancer 2014, 135, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Wu, C.C.; Peng, P.H.; Liang, Y.; Hsiao, Y.C.; Chien, K.Y.; Chen, J.T.; Lin, S.J.; Tang, R.P.; Hsieh, L.L.; et al. Identification of secretory gelsolin as a plasma biomarker associated with distant organ metastasis of colorectal cancer. J. Mol. Med. 2012, 90, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Wang, L.; Zhao, R.; Qiao, Y.; Han, D.; Sun, Q.; Dong, N.; Liu, Y.; Wu, D.; et al. Mir-200a targets gelsolin: A novel mechanism regulating secretion of microvesicles in hepatocellular carcinoma cells. Oncol. Rep. 2017, 37, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Asare-Werehene, M.; Tsuyoshi, H.; Zhang, H.; Salehi, R.; Chang, C.Y.; Carmona, E.; Librach, C.L.; Mes-Masson, A.M.; Chang, C.C.; Burger, D.; et al. Plasma gelsolin confers chemoresistance in ovarian cancer by resetting the relative abundance and function of macrophage subtypes. Cancers 2022, 14, 1039. [Google Scholar] [CrossRef]
- Asare-Werehene, M.; Communal, L.; Carmona, E.; Han, Y.; Song, Y.S.; Burger, D.; Mes-Masson, A.M.; Tsang, B.K. Plasma gelsolin inhibits cd8(+) t-cell function and regulates glutathione production to confer chemoresistance in ovarian cancer. Cancer Res. 2020, 80, 3959–3971. [Google Scholar] [CrossRef]
- Chen, C.C.; Chiou, S.H.; Yang, C.L.; Chow, K.C.; Lin, T.Y.; Chang, H.W.; You, W.C.; Huang, H.W.; Chen, C.M.; Chen, N.C.; et al. Secreted gelsolin desensitizes and induces apoptosis of infiltrated lymphocytes in prostate cancer. Oncotarget 2017, 8, 77152–77167. [Google Scholar] [CrossRef]
- Giampazolias, E.; Schulz, O.; Lim, K.H.J.; Rogers, N.C.; Chakravarty, P.; Srinivasan, N.; Gordon, O.; Cardoso, A.; Buck, M.D.; Poirier, E.Z.; et al. Secreted gelsolin inhibits dngr-1-dependent cross-presentation and cancer immunity. Cell 2021, 184, 4016–4031.e4022. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018, 27, 237–251.e234. [Google Scholar] [CrossRef]
- Budnik, V.; Ruiz-Canada, C.; Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 2016, 17, 160–172. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal pd-l1 contributes to immunosuppression and is associated with anti-pd-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular notch signaling. Nat. Commun. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Marimpietri, D.; Airoldi, I.; Faini, A.C.; Malavasi, F.; Morandi, F. The role of extracellular vesicles in the progression of human neuroblastoma. Int. J. Mol. Sci. 2021, 22, 3964. [Google Scholar] [CrossRef] [PubMed]
- Jermyn, M.; Mercier, J.; Aubertin, K.; Desroches, J.; Urmey, K.; Karamchandiani, J.; Marple, E.; Guiot, M.C.; Leblond, F.; Petrecca, K. Highly accurate detection of cancer in situ with intraoperative, label-free, multimodal optical spectroscopy. Cancer Res. 2017, 77, 3942–3950. [Google Scholar] [CrossRef]
- Paidi, S.K.; Rizwan, A.; Zheng, C.; Cheng, M.; Glunde, K.; Barman, I. Label-free raman spectroscopy detects stromal adaptations in premetastatic lungs primed by breast cancer. Cancer Res. 2017, 77, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.A.; Asare-Werehene, M.; Mandour, A.; Tsang, B.K.; Anis, H. Determination of chemoresistance in ovarian cancer by simultaneous quantification of exosomes and exosomal cisplatin with surface enhanced raman scattering. Sens. Actuators B Chem. 2022, 354, 131237. [Google Scholar] [CrossRef]
- Abedini, M.R.; Qiu, Q.; Yan, X.; Tsang, B.K. Possible role of flice-like inhibitory protein (flip) in chemoresistant ovarian cancer cells in vitro. Oncogene 2004, 23, 6997–7004. [Google Scholar] [CrossRef]
- Abedini, M.R.; Muller, E.J.; Bergeron, R.; Gray, D.A.; Tsang, B.K. Akt promotes chemoresistance in human ovarian cancer cells by modulating cisplatin-induced, p53-dependent ubiquitination of flice-like inhibitory protein. Oncogene 2010, 29, 11–25. [Google Scholar] [CrossRef]
- Ali, A.Y.; Abedini, M.R.; Tsang, B.K. The oncogenic phosphatase ppm1d confers cisplatin resistance in ovarian carcinoma cells by attenuating checkpoint kinase 1 and p53 activation. Oncogene 2012, 31, 2175–2186. [Google Scholar] [CrossRef]
- Abedini, M.R.; Wang, P.W.; Huang, Y.F.; Cao, M.; Chou, C.Y.; Shieh, D.B.; Tsang, B.K. Cell fate regulation by gelsolin in human gynecologic cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 14442–14447. [Google Scholar] [CrossRef]
- Burger, D.; Vinas, J.L.; Akbari, S.; Dehak, H.; Knoll, W.; Gutsol, A.; Carter, A.; Touyz, R.M.; Allan, D.S.; Burns, K.D. Human endothelial colony-forming cells protect against acute kidney injury: Role of exosomes. Am. J. Pathol. 2015, 185, 2309–2323. [Google Scholar] [CrossRef] [PubMed]
- Vinas, J.L.; Burger, D.; Zimpelmann, J.; Haneef, R.; Knoll, W.; Campbell, P.; Gutsol, A.; Carter, A.; Allan, D.S.; Burns, K.D. Transfer of microrna-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int. 2016, 90, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Reunov, A.; Pimenova, E.; Reunova, Y.; Menchinskaiya, E.; Lapshina, L.; Aminin, D. The study of the calpain and caspase-1 expression in ultrastructural dynamics of ehrlich ascites carcinoma necrosis. Gene 2018, 658, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Pospichalova, V.; Huang, Z.; Murphy, S.K.; Payne, S.; Wang, F.; Kennedy, M.; Cianciolo, G.J.; Bryja, V.; Pizzo, S.V.; et al. Ascites increases expression/function of multidrug resistance proteins in ovarian cancer cells. PLoS ONE 2015, 10, e0131579. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ali-Osman, F. Glutathione-associated cis-diamminedichloroplatinum(ii) metabolism and atp-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J. Biol. Chem. 1993, 268, 20116–20125. [Google Scholar] [CrossRef]
- Morrow, C.S.; Peklak-Scott, C.; Bishwokarma, B.; Kute, T.E.; Smitherman, P.K.; Townsend, A.J. Multidrug resistance protein 1 (mrp1, abcc1) mediates resistance to mitoxantrone via glutathione-dependent drug efflux. Mol. Pharmacol. 2006, 69, 1499–1505. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Sodani, K.; Dai, C.L.; Ashby, C.R., Jr.; Chen, Z.S. Revisiting the abcs of multidrug resistance in cancer chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 570–594. [Google Scholar] [CrossRef]
- Stock, K.; Borrink, R.; Mikesch, J.H.; Hansmeier, A.; Rehkämper, J.; Trautmann, M.; Wardelmann, E.; Hartmann, W.; Sperveslage, J.; Steinestel, K. Overexpression and tyr421-phosphorylation of cortactin is induced by three-dimensional spheroid culturing and contributes to migration and invasion of pancreatic ductal adenocarcinoma (pdac) cells. Cancer Cell Int. 2019, 19, 77. [Google Scholar] [CrossRef]
- Möltgen, S.; Piumatti, E.; Massafra, G.M.; Metzger, S.; Jaehde, U.; Kalayda, G.V. Cisplatin protein binding partners and their relevance for platinum drug sensitivity. Cells 2020, 9, 1322. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Ferraro, G.; Merlino, A. Cisplatin-protein interactions: Unexpected drug binding to n-terminal amine and lysine side chains. Inorg. Chem. 2016, 55, 7814–7816. [Google Scholar] [CrossRef]
- Ambrosio, A.L.; Di Pietro, S.M. Storage pool diseases illuminate platelet dense granule biogenesis. Platelets 2017, 28, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, Y.; Li, W. Sorting machineries: How platelet-dense granules differ from α-granules. Biosci. Rep. 2018, 38, BSR20180458. [Google Scholar] [CrossRef] [PubMed]
- Provencher, D.M.; Lounis, H.; Champoux, L.; Tétrault, M.; Manderson, E.N.; Wang, J.C.; Eydoux, P.; Savoie, R.; Tonin, P.N.; Mes-Masson, A.M. Characterization of four novel epithelial ovarian cancer cell lines. Vitr. Cell. Dev. Biol.-Anim. 2000, 36, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Zietarska, M.; Portelance, L.; Lafontaine, J.; Madore, J.; Puiffe, M.L.; Arcand, S.L.; Shen, Z.; Hébert, J.; Tonin, P.N.; et al. Characterization of three new serous epithelial ovarian cancer cell lines. BMC Cancer 2008, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Fleury, H.; Communal, L.; Carmona, E.; Portelance, L.; Arcand, S.L.; Rahimi, K.; Tonin, P.N.; Provencher, D.; Mes-Masson, A.M. Novel high-grade serous epithelial ovarian cancer cell lines that reflect the molecular diversity of both the sporadic and hereditary disease. Genes Cancer 2015, 6, 378–398. [Google Scholar] [CrossRef]
- Leroy, B.; Girard, L.; Hollestelle, A.; Minna, J.D.; Gazdar, A.F.; Soussi, T. Analysis of TP53 mutation status in human cancer cell lines: A reassessment. Hum. Mutat. 2014, 35, 756–765. [Google Scholar] [CrossRef]
- Létourneau, I.J.; Quinn, M.C.; Wang, L.L.; Portelance, L.; Caceres, K.Y.; Cyr, L.; Delvoye, N.; Meunier, L.; de Ladurantaye, M.; Shen, Z.; et al. Derivation and characterization of matched cell lines from primary and recurrent serous ovarian cancer. BMC Cancer 2012, 12, 379. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Wiegand, K.C.; Melnyk, N.; Chow, C.; Salamanca, C.; Prentice, L.M.; Senz, J.; Yang, W.; Spillman, M.A.; Cochrane, D.R.; et al. Correction: Type-Specific Cell Line Models for Type-Specific Ovarian Cancer Research. PLoS ONE 2013, 8, e72162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asare-Werehene, M.; Hunter, R.A.; Gerber, E.; Reunov, A.; Brine, I.; Chang, C.-Y.; Chang, C.-C.; Shieh, D.-B.; Burger, D.; Anis, H.; et al. The Application of an Extracellular Vesicle-Based Biosensor in Early Diagnosis and Prediction of Chemoresponsiveness in Ovarian Cancer. Cancers 2023, 15, 2566. https://doi.org/10.3390/cancers15092566
Asare-Werehene M, Hunter RA, Gerber E, Reunov A, Brine I, Chang C-Y, Chang C-C, Shieh D-B, Burger D, Anis H, et al. The Application of an Extracellular Vesicle-Based Biosensor in Early Diagnosis and Prediction of Chemoresponsiveness in Ovarian Cancer. Cancers. 2023; 15(9):2566. https://doi.org/10.3390/cancers15092566
Chicago/Turabian StyleAsare-Werehene, Meshach, Robert A. Hunter, Emma Gerber, Arkadiy Reunov, Isaiah Brine, Chia-Yu Chang, Chia-Ching Chang, Dar-Bin Shieh, Dylan Burger, Hanan Anis, and et al. 2023. "The Application of an Extracellular Vesicle-Based Biosensor in Early Diagnosis and Prediction of Chemoresponsiveness in Ovarian Cancer" Cancers 15, no. 9: 2566. https://doi.org/10.3390/cancers15092566
APA StyleAsare-Werehene, M., Hunter, R. A., Gerber, E., Reunov, A., Brine, I., Chang, C.-Y., Chang, C.-C., Shieh, D.-B., Burger, D., Anis, H., & Tsang, B. K. (2023). The Application of an Extracellular Vesicle-Based Biosensor in Early Diagnosis and Prediction of Chemoresponsiveness in Ovarian Cancer. Cancers, 15(9), 2566. https://doi.org/10.3390/cancers15092566





