Expression of Hemangioblast Proteins in von Hippel-Lindau Disease Related Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Selection
2.2. Tissue Preparation and Histological Classification
2.3. Immunohistochemistry
2.4. Evaluation of Immunohistochemical Staining
2.5. Statistical Analysis
3. Results
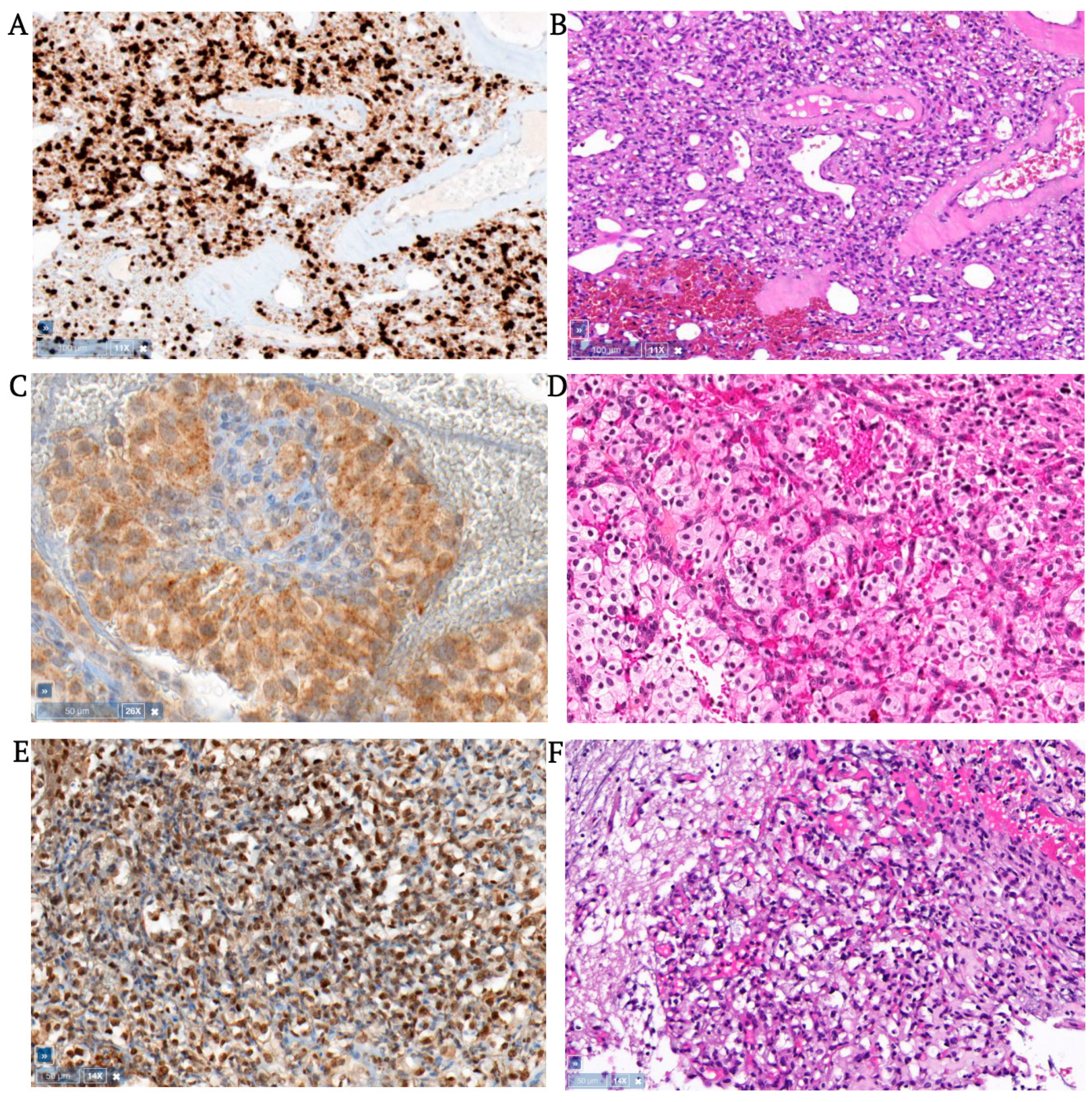
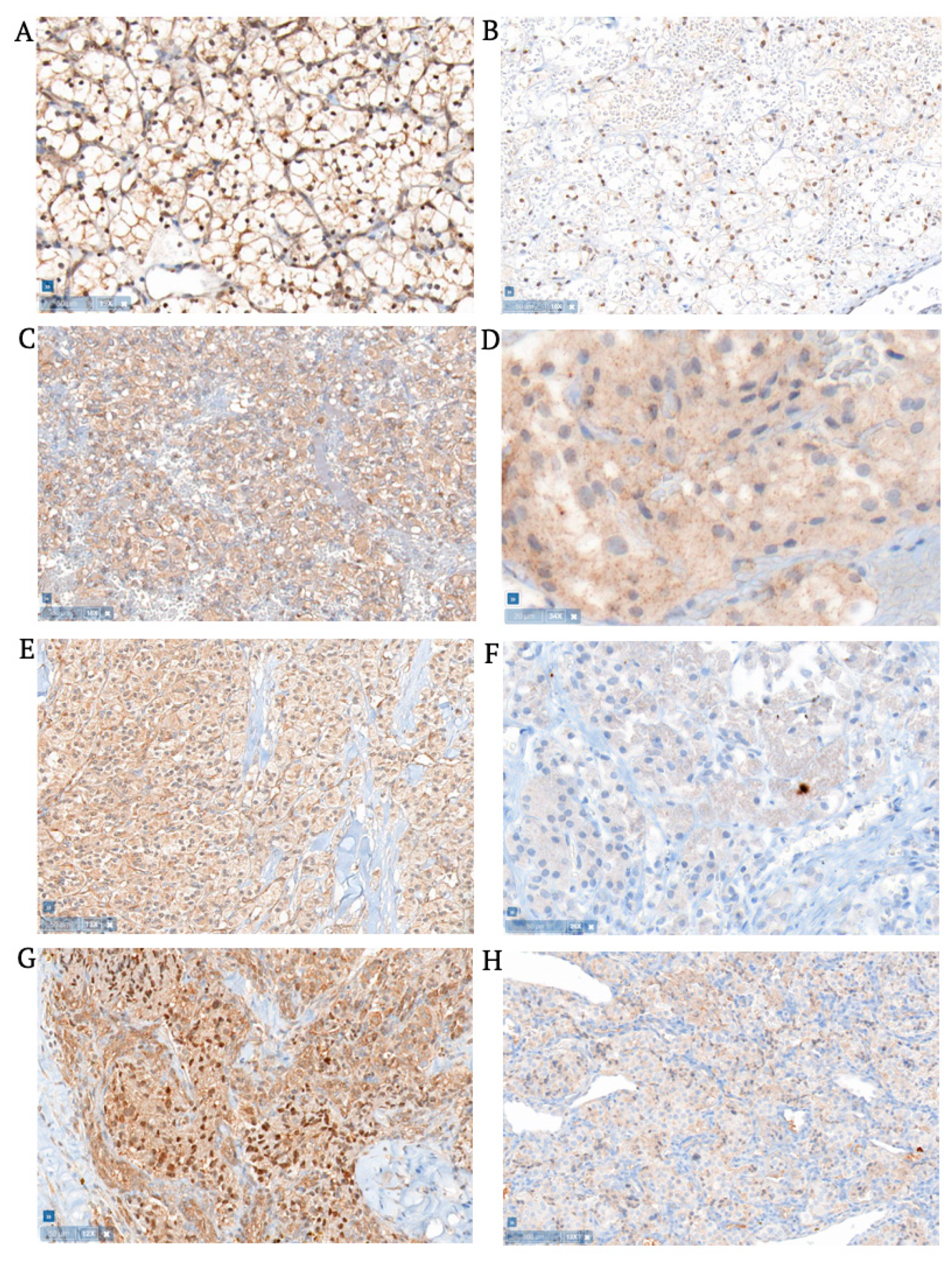
3.1. Hemangioblastomas
3.2. Clear Cell Renal Cell Carcinomas
3.3. Pheochromocytomas
3.4. Pancreatic Neuroendocrine Tumors
3.5. Extra-Adrenal Aragangliomas
3.6. Statistical Analysis
4. Discussion
4.1. The Embryonic Hemangioblast: Fact or Fiction?
4.2. Hypothesis of Embryonic Origin
4.3. New Insights into Brachyury Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seizinger, B.R.; Rouleau, G.A.; Ozelius, L.J.; Lane, A.H.; Farmer, G.E.; Lamiell, J.M.; Haines, J.; Yuen, J.W.; Collins, D.; Majoor-Krakauer, D.; et al. Von Hippel-Lindau Disease Maps to the Region of Chromosome 3 Associated with Renal Cell Carcinoma. Nature 1988, 332, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L.; et al. Identification of the Von Hippel-Lindau Disease Tumor Suppressor Gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Li, F.P.; Berg, S.; Marchetto, D.J.; Tsai, S.; Jacobs, S.C.; Brown, R.S. Hereditary Renal-Cell Carcinoma Associated with a Chromosomal Translocation. N. Engl. J. Med. 1979, 301, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Whaley, J.M.; Naglich, J.; Gelbert, L.; Hsia, Y.E.; Lamiell, J.M.; Green, J.S.; Collins, D.; Neumann, H.P.; Laidlaw, J.; Li, F.P.; et al. Germ-Line Mutations in the Von Hippel-Lindau Tumor-Suppressor Gene Are Similar to Somatic Von Hippel-Lindau Aberrations in Sporadic Renal Cell Carcinoma. Am. J. Hum. Genet. 1994, 55, 1092–1102. [Google Scholar]
- Gaal, J.; Van Nederveen, F.H.; Erlic, Z.; Korpershoek, E.; Oldenburg, R.; Boedeker, C.C.; Kontny, U.; Neumann, H.P.; Dinjens, W.N.; De Krijger, R.R. Parasympathetic Paragangliomas Are Part of the Von Hippel-Lindau Syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 4367–4371. [Google Scholar] [CrossRef]
- Brandt, R. Zur Frage Der Angiomatosis Retinae. Albrecht Von Graæes Arch. Für Ophthalmol. 1921, 106, 126–136. [Google Scholar] [CrossRef]
- Manski, T.J.; Heffner, D.K.; Glenn, G.M.; Patronas, N.J.; Pikus, A.T.; Katz, D.; Lebovics, R.; Sledjeski, K.; Choyke, P.L.; Zbar, B.; et al. Endolymphatic Sac Tumors. A Source of Morbid Hearing Loss in Von Hippel-Lindau Disease. JAMA 1997, 277, 1461–1466. [Google Scholar] [CrossRef]
- Lindau, A. Studien ÜBer Kleinhirncysten: Bau, Pathogenese Und Beziehungen Zur Angiomatosis Retinae. Acta Pathol. Et Microbiol. Scandinavica Suppl. 1926, 1, 1–128. [Google Scholar]
- Cushing, H.; Bailey, P. Tumors Arising from Blood-Vessels of the Brain: Angiomatous Malformations and Hemangioblastomas; Charles C. Thomas: Springfield, IL, USA, 1928. [Google Scholar]
- Plate, K.H.; Aldape, K.D.; Vortmeyer, A.O.; Zagzag, D.; Neumann, H.P. WHO Classification of Tumours of the Central Nervous System: Haemangioblastoma, 4th ed.; Louis, D.N., Ohgaki, H., Wiestler, O.D., Cavenee, W.K., Eds.; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- Vortmeyer, A.O.; Frank, S.; Jeong, S.Y.; Yuan, K.; Ikejiri, B.; Lee, Y.S.; Bhowmick, D.; Lonser, R.R.; Smith, R.; Rodgers, G.; et al. Developmental Arrest of Angioblastic Lineage Initiates Tumorigenesis in Von Hippel-Lindau Disease. Cancer Res. 2003, 63, 7051–7055. [Google Scholar]
- Glasker, S.; Smith, J.; Raffeld, M.; Li, J.; Oldfield, E.H.; Vortmeyer, A.O. Vhl-Deficient Vasculogenesis in Hemangioblastoma. Exp. Mol. Pathol. 2014, 96, 162–167. [Google Scholar] [CrossRef]
- Vortmeyer, A.O.; Yuan, Q.; Lee, Y.S.; Zhuang, Z.; Oldfield, E.H. Developmental Effects of Von Hippel-Lindau Gene Deficiency. Ann. Neurol. 2004, 55, 721–728. [Google Scholar] [CrossRef]
- Vortmeyer, A.O.; Alomari, A.K. Pathology of the Nervous System in Von Hippel-Lindau Disease. J. Kidney Cancer VHL 2015, 2, 114–129. [Google Scholar] [CrossRef]
- Srigley, J.R.; Delahunt, B.; Eble, J.N.; Egevad, L.; Epstein, J.I.; Grignon, D.; Hes, O.; Moch, H.; Montironi, R.; Tickoo, S.K.; et al. The International Society of Urological Pathology (Isup) Vancouver Classification of Renal Neoplasia. Am. J. Surg. Pathol. 2013, 37, 1469–1489. [Google Scholar] [CrossRef]
- Thompson, L.D. Pheochromocytoma of the Adrenal Gland Scaled Score (Pass) to Separate Benign from Malignant Neoplasms: A Clinicopathologic and Immunophenotypic Study of 100 Cases. Am. J. Surg. Pathol. 2002, 26, 551–566. [Google Scholar] [CrossRef]
- Rindi, G.; Arnold, R.; Bosman, F.T. Nomenclature and Classification of Neuroendocrine Neoplasms of the Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; International Agency for Reseach on Cancer: Lyon, France, 2010. [Google Scholar]
- Fedchenko, N.; Reifenrath, J. Different Approaches for Interpretation and Reporting of Immunohistochemistry Analysis Results in the Bone Tissue—A Review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for Uniform Definition of an Immunoreactive Score (Irs) for Immunohistochemical Estrogen Receptor Detection (Er-Ica) in Breast Cancer Tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Herberth, B.; Minko, K.; Csillag, A.; Jaffredo, T.; Madarasz, E. Scl, Gata-2 and Lmo2 Expression in Neurogenesis. Int. J. Dev. Neurosci. 2005, 23, 449–463. [Google Scholar] [CrossRef]
- His, W. Anatomie Menslicher Embyronen: Die Aortenbogen; FCW Vogel: Leipzig, Germany, 1882. [Google Scholar]
- Sabin, F. Preliminary Note on the Differentiation of Angioblasts and the Method by Which They Produce Blood-Vessels, Blood-Plasma and Red Blood-Cells as Seen in the Living Chick. Anat. Rec. 1917, 13, 199–204. [Google Scholar] [CrossRef]
- Murray, P. The Development in Vitro of the Blood of the Early Chick Embryo. Proc. R. Soc. B Biol. Sci. 1932, 111, 497–521. [Google Scholar]
- Gritz, E.; Hirschi, K.K. Specification and Function of Hemogenic Endothelium During Embryogenesis. Cell Mol. Life Sci. 2016, 73, 1547–1567. [Google Scholar] [CrossRef]
- Lacaud, G.; Kouskoff, V. Hemangioblast, Hemogenic Endothelium, and Primitive Versus Definitive Hematopoiesis. Exp. Hematol 2017, 49, 19–24. [Google Scholar] [CrossRef]
- Huber, T.L.; Kouskoff, V.; Fehling, H.J.; Palis, J.; Keller, G. Haemangioblast Commitment Is Initiated in the Primitive Streak of the Mouse Embryo. Nature 2004, 432, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Lancrin, C.; Sroczynska, P.; Stephenson, C.; Allen, T.; Kouskoff, V.; Lacaud, G. The Haemangioblast Generates Haematopoietic Cells through a Haemogenic Endothelium Stage. Nature 2009, 457, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Lindau, A. Zur Frage Der Angiomatosis Retinae Und Ihrer Hirnkomplikationen. Acta Ophthalmol. Scand. 1926, 4, 193–226. [Google Scholar] [CrossRef]
- Karlefors, J. Die Hirnhauträume Des Kleinhirns, Die Verbindungen Des 4. Ventrikels Mit Den Subarachnoidalräumen Und Der Aquaeductus Cochleae Bei Kindern Und Erwachsenen. Acta Oto-Laryngol. 1924, 6, 87–184. [Google Scholar]
- Paton, L. Discussion on Vascular Tumours of the Brain and Spinal Cord. Proc. R. Soc. Med. 1930, 24, 363. [Google Scholar]
- Stein, A.A.; Schilp, A.O.; Whitfield, R.D. The Histogenesis of Hemangioblastoma of the Brain. A Review of Twenty-One Cases. J. Neurosurg. 1960, 17, 751–761. [Google Scholar] [CrossRef]
- Ma, D.; Zhu, W.; Zhang, M.; Ding, X.; Xu, F.; Hua, W.; Tang, X.; Zhu, J.; Mao, Y.; Zhou, L. Identification of Tumorigenic Cells and Implication of Their Aberrant Differentiation in Human Hemangioblastomas. Cancer Biol. Ther. 2011, 12, 727–736. [Google Scholar] [CrossRef]
- Vortmeyer, A.O.; Tran, M.G.; Zeng, W.; Glasker, S.; Riley, C.; Tsokos, M.; Ikejiri, B.; Merrill, M.J.; Raffeld, M.; Zhuang, Z.; et al. Evolution of Vhl Tumourigenesis in Nerve Root Tissue. J. Pathol. 2006, 210, 374–382. [Google Scholar] [CrossRef]
- Shively, S.B.; Beltaifa, S.; Gehrs, B.; Duong, H.; Smith, J.; Edwards, N.A.; Lonser, R.; Raffeld, M.; Vortmeyer, A.O. Protracted Haemangioblastic Proliferation and Differentiation in Von Hippel-Lindau Disease. J. Pathol. 2008, 216, 514–520. [Google Scholar] [CrossRef]
- Glasker, S.; Lonser, R.R.; Tran, M.G.; Ikejiri, B.; Butman, J.A.; Zeng, W.; Maxwell, P.H.; Zhuang, Z.; Oldfield, E.H.; Vortmeyer, A.O. Effects of Vhl Deficiency on Endolymphatic Duct and Sac. Cancer Res. 2005, 65, 10847–10853. [Google Scholar] [CrossRef]
- Shively, S.B.; Falke, E.A.; Li, J.; Tran, M.G.; Thompson, E.R.; Maxwell, P.H.; Roessler, E.; Oldfield, E.H.; Lonser, R.R.; Vortmeyer, A.O. Developmentally Arrested Structures Preceding Cerebellar Tumors in Von Hippel-Lindau Disease. Mod. Pathol. 2011, 24, 1023–1030. [Google Scholar] [CrossRef]
- Glasker, S.; Tran, M.G.; Shively, S.B.; Ikejiri, B.; Lonser, R.R.; Maxwell, P.H.; Zhuang, Z.; Oldfield, E.H.; Vortmeyer, A.O. Epididymal Cystadenomas and Epithelial Tumourlets: Effects of Vhl Deficiency on the Human Epididymis. J. Pathol. 2006, 210, 32–41. [Google Scholar] [CrossRef]
- Mandriota, S.J.; Turner, K.J.; Davies, D.R.; Murray, P.G.; Morgan, N.V.; Sowter, H.M.; Wykoff, C.C.; Maher, E.R.; Harris, A.L.; Ratcliffe, P.J.; et al. Hif Activation Identifies Early Lesions in Vhl Kidneys: Evidence for Site-Specific Tumor Suppressor Function in the Nephron. Cancer Cell 2002, 1, 459–468. [Google Scholar] [CrossRef]
- Glasker, S.; Li, J.; Xia, J.B.; Okamoto, H.; Zeng, W.; Lonser, R.R.; Zhuang, Z.; Oldfield, E.H.; Vortmeyer, A.O. Hemangioblastomas Share Protein Expression with Embryonal Hemangioblast Progenitor Cell. Cancer Res. 2006, 66, 4167–4172. [Google Scholar] [CrossRef]
- Park, D.M.; Zhuang, Z.; Chen, L.; Szerlip, N.; Maric, I.; Li, J.; Sohn, T.; Kim, S.H.; Lubensky, I.A.; Vortmeyer, A.O.; et al. Von Hippel-Lindau Disease-Associated Hemangioblastomas Are Derived from Embryologic Multipotent Cells. PLoS Med. 2007, 4, e60. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Du, G.; Yang, J.; Tang, Q.; Zhou, L. Cd41 and Cd45 Expression Marks the Angioformative Initiation of Neovascularisation in Human Haemangioblastoma. Tumour. Biol. 2016, 37, 3765–3774. [Google Scholar] [CrossRef]
- Barresi, V.; Vitarelli, E.; Branca, G.; Antonelli, M.; Giangaspero, F.; Barresi, G. Expression of Brachyury in Hemangioblastoma: Potential Use in Differential Diagnosis. Am. J. Surg. Pathol. 2012, 36, 1052–1057. [Google Scholar] [CrossRef]
- Barresi, V.; Ieni, A.; Branca, G.; Tuccari, G. Brachyury: A Diagnostic Marker for the Differential Diagnosis of Chordoma and Hemangioblastoma Versus Neoplastic Histological Mimickers. Dis. Markers 2014, 2014, 514753. [Google Scholar] [CrossRef]
- Jambhekar, N.A.; Rekhi, B.; Thorat, K.; Dikshit, R.; Agrawal, M.; Puri, A. Revisiting Chordoma with Brachyury, a “New Age” Marker: Analysis of a Validation Study on 51 Cases. Arch. Pathol. Lab. Med. 2010, 134, 1181–1187. [Google Scholar] [CrossRef]
- Vujovic, S.; Henderson, S.; Presneau, N.; Odell, E.; Jacques, T.S.; Tirabosco, R.; Boshoff, C.; Flanagan, A.M. Brachyury, a Crucial Regulator of Notochordal Development, Is a Novel Biomarker for Chordomas. J. Pathol. 2006, 209, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Wang, Z.; Lasota, J.; Heery, C.; Schlom, J.; Palena, C. Nuclear Brachyury Expression Is Consistent in Chordoma, Common in Germ Cell Tumors and Small Cell Carcinomas, and Rare in Other Carcinomas and Sarcomas: An Immunohistochemical Study of 5229 Cases. Am. J. Surg. Pathol. 2015, 39, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Tirabosco, R.; Mangham, D.C.; Rosenberg, A.E.; Vujovic, S.; Bousdras, K.; Pizzolitto, S.; De Maglio, G.; den Bakker, M.A.; Di Francesco, L.; Kalil, R.K.; et al. Brachyury Expression in Extra-Axial Skeletal and Soft Tissue Chordomas: A Marker That Distinguishes Chordoma from Mixed Tumor/Myoepithelioma/Parachordoma in Soft Tissue. Am. J. Surg. Pathol. 2008, 32, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Fletcher, C.D. Peripheral Hemangioblastoma: Clinicopathologic Characterization in a Series of 22 Cases. Am. J. Surg. Pathol. 2014, 38, 119–127. [Google Scholar] [CrossRef]
- Hamilton, D.H.; Fernando, R.I.; Schlom, J.; Palena, C. Aberrant Expression of the Embryonic Transcription Factor Brachyury in Human Tumors Detected with a Novel Rabbit Monoclonal Antibody. Oncotarget 2015, 6, 4853–4862. [Google Scholar] [CrossRef]

| Case | Sex | Age (Year) | Tumor Site | Grade | Tumor Size (mm³) | Remelle Brachyury | Remelle TAL1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | ||||||
| Cerebellar hemangioblastoma | WHO | ||||||||
| 1 | M (18) | 17 | unknown | 1 | unknown | 3 | 0 | 2 | 3 |
| 2 | M (6) | 17 | right hemisphere | 1 | 2300 | 0 | 0 | 9 | 0 |
| 3 | M (6) | 18 | left hemisphere | 1 | 4200 | 0 | 0 | 6 | 4 |
| 4 | M (6) | 20 | left hemisphere | 1 | 48,000 | 0 | 0 | 9 | 6 |
| 5 | M (18) | 25 | right hemisphere | 1 | 9880 | 0 | 0 | 4 | 6 |
| 6 | F (7) | 26 | unknown | 1 | 3491 | 0 | 0 | 0 | 0 |
| 7 | M (3) | 27 | unknown | 1 | 1280 | 3 | 0 | 4 | 8 |
| 8 | F | 30 | left hemisphere | 1 | 356 | 0 | 1 | 9 | 6 |
| 9 | M (2) | 30 | left hemisphere | 1 | 700 | 0 | 0 | 4 | 6 |
| 10 | F (7) | 30 | unknown | 1 | unknown | 0 | 0 | 6 | 6 |
| 11 | M (1) | 31 | right hemisphere | 1 | 1601 (3 tumors) | 0 | 0 | 2 | 6 |
| 12 | F | 32 | right hemisphere | 1 | 2265 (2 tumors) | 0 | 0 | 6 | 6 |
| 13 | M (1) | 37 | midline | 1 | 1760 (2 tumors) | 0 | 0 | 6 | 6 |
| 14 | M (4) | 41 | left hemisphere | 1 | 4752 | 0 | 0 | 6 | 6 |
| 15 | F (8) | 42 | left hemisphere | 1 | unknown | 0 | 0 | 4 | 6 |
| 16 | M (1) | 43 | left + right hemisphere | 1 | 7560 (2 tumors) | 0 | 8 | 9 | 6 |
| 17 | F (5) | 43 | intraventricular | 1 | 1920 | 0 | 0 | 6 | 6 |
| 18 | F (8) | 47 | right hemisphere | 1 | 288 | 0 | 3 | 6 | 6 |
| 19 | M (4) | 50 | unknown | 1 | 9478 (2 tumors) | 3 | 2 | 4 | 6 |
| 20 | F | 55 | left hemisphere | 1 | 4756 (2 tumors) | 0 | 0 | 6 | 6 |
| 21 | M | 56 | right hemisphere | 1 | 4048 | 0 | 0 | 6 | 6 |
| 22 | F | 65 | right hemisphere | 1 | 9525 | 0 | 3 | 12 | 6 |
| 23 | F | 67 | vermis | 1 | 1728 | 0 | 0 | 0 | 6 |
| 24 | F | 22 | right hemisphere | 1 | 5400 | 0 | 0 | 4 | 6 |
| 25 | M | 44 | left hemisphere | 1 | 532 | 0 | 0 | 4 | 6 |
| 26 | F (19) | 40 | right hemisphere | 1 | 1241 | 0 | 0 | 6 | 6 |
| Spinal hemangioblastoma | WHO | ||||||||
| 27 | M (6) | 17 | C6 | 1 | 1000 | 0 | 0 | 4 | 2 |
| 28 | F | 24 | C1 + C5 | 1 | 92 (2 tumors) | 0 | 0 | 2 | 4 |
| 29 | F | 24 | T7–8 | 1 | 192 | 3 | 0 | 6 | 0 |
| 30 | M (15) * | 25 | medulla oblongata | 1 | unknown | 0 | 0 | 0 | 2 |
| 31 | F (9) | 26 | medulla, obex | 1 | 3588 | 6 | 0 | 6 | 6 |
| 32 | F (10) | 26 | T10–11 | 1 | 250 | 0 | 0 | 12 | 8 |
| 33 | F (9) | 27 | C5–6 | 1 | 224 | 0 | 2 | 9 | 6 |
| 34 | F (10) | 27 | C5–6 | 1 | 6 | 9 | 0 | 2 | 6 |
| 35 | M (3) | 30 | T12-L1 | 1 | 3360 | 12 | 0 | 1 | 3 |
| 36 | M (14) | 31 | nerve root | 1 | 1500 | 6 | 0 | 6 | 6 |
| 37 | F | 34 | T5 | 1 | 1080 (2 tumors) | 0 | 0 | 9 | 4 |
| 38 | F (11) | 34 | T10 | 1 | 1000 | 2 | 0 | 4 | 2 |
| 39 | F | 36 | C3-C5 | 1 | 6 | 3 | 0 | 2 | 1 |
| 40 | M | 38 | C1-C3 | 1 | 3600 | 3 | 6 | 6 | 6 |
| 41 | F | 42 | C4-C6 | 1 | 180 | 3 | 3 | 4 | 6 |
| 42 | F (12) | 42 | lumbar nerve root | 1 | 1728 | 6 | 0 | 4 | 2 |
| 43 | M (13) | 47 | filum terminale | 1 | 1600 | 0 | 0 | 6 | 6 |
| 44 | F | 58 | T12 | 1 | 43 | 0 | 0 | 0 | 0 |
| 45 | F | 33 | borderzone cerebellum | 1 | 225 | 0 | 0 | 6 | 4 |
| 46 | F (19) | 37 | C4–C5 | 1 | 91 | 0 | 0 | 3 | 4 |
| 47 | M | 70 | L1 | 1 | 2856 | 0 | 0 | 4 | 8 |
| Clear cell renal cell carcinoma | WHO/ISUP | ||||||||
| 48 | F (11) | 31 | left kidney | 2 | 23,400 | 0 | 1 | 0 | 0 |
| 49 | F (16) | 37 | right kidney | 1 | 4335 | 0 | 0 | 2 | 0 |
| 50 | M (14) | 37 | right kidney | 2 | 87,500 | 0 | 0 | 9 | 6 |
| 51 | M | 38 | right kidney | 1 | 5832 | 0 | 0 | 2 | 0 |
| 52 | F (16) | 38 | left kidney | 1 | unknown | 2 | 1 | 2 | 0 |
| 53 | F | 46 | left kidney | 1 | 389,017 | 0 | 0 | 12 | 0 |
| 54 | F (12) | 46 | left kidney | 1 | 6000 | 0 | 0 | 6 | 3 |
| 55 | M (13) | 47 | right kidney | 1 | 7820 | 0 | 0 | 12 | 6 |
| 56 | F (5) | 52 | right kidney | 1 | 8000 | 2 | 2 | 4 | 4 |
| 57 | M (17) * | 52 | right kidney | 2 | 2197 | 0 | 0 | 2 | 8 |
| 58 | F | 57 | unknown | 1 | 12,167 | 0 | 0 | 12 | 0 |
| 59 | F | 35 | right kidney | 2 | 10,166 | 0 | 0 | 6 | 8 |
| 60 | M | 44 | left kidney | 2 | 123,786 (11 tumors) | 0 | 0 | 6 | 4 |
| Pheochromocytoma | PASS (Tompson) | ||||||||
| 61 | M (2) | 13 | right adrenal gland | 6 | 192,500 | 0 | 0 | 0 | 1 |
| 62 | M | 20 | right adrenal gland | 0 | 2240 | 0 | 0 | 4 | 4 |
| 63 | F (11) | 21 | right adrenal gland | 3 | 7920 | 0 | 2 | 0 | 0 |
| 64 | M (6) | 24 | right adrenal gland | 3 | 210 | 0 | 0 | 0 | 1 |
| 65 | M (15) * | 46 | right adrenal gland | 0 | 2520 | 0 | 0 | 3 | 4 |
| 66 | M (17) * | 47 | right adrenal gland | 2 | 14,520 | 0 | 0 | 0 | 8 |
| 67 | M | 51 | left adrenal gland | 0 | 21,000 | 0 | 2 | 2 | 4 |
| 68 | F | 54 | unknown | 7 | 61,845 | 0 | 4 | 0 | 8 |
| Pancreatic neuroendocrine tumor | WHO/AJCC | ||||||||
| 69 | F | 23 | pancreatic head | 2 | 10,648 | 0 | 0 | 2 | 6 |
| 70 | F | 29 | pancreatic head | 4 | 8800 | 0 | 0 | 4 | 0 |
| 71 | F | 45 | pancreatic head | 3 | unknown | 3 | 0 | 0 | 4 |
| 72 | F | 46 | pancreatic head + body | 3 | unknown | 0 | 1 | 0 | 2 |
| 73 | M | 48 | pancreatic head | 3 | unknown | 3 | 0 | 2 | 4 |
| Extra-adrenal paraganglioma | |||||||||
| 74 | F | 31 | carotid body | 0 | 20,825 | 0 | 0 | 0 | 8 |
| 75 | M | 60 | carotid body | 1 | 151,740 | 3 | 1 | 6 | 8 |
| Target Protein | Type of Antibody | Concentration, Dilution | Distributor | Cellular Localization | Positive Control |
|---|---|---|---|---|---|
| TAL1 | Rabbit polyclonal IgG to Tal1 C-terminal (ab155195) | 1 mg/mL, 1:2000 | Abcam, Cambridge, UK | Nuclear/cytoplasmic | Human T-ALL (DSMZ) |
| Brachyury | Rabbit monoclonal IgG [EPR18113] to Brachyury (ab209665) | 0.413 mg/mL, 1:50 | Abcam, Cambridge, UK | Nuclear/cytoplasmic | Human chordoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergauwen, E.; Forsyth, R.; Vortmeyer, A.; Gläsker, S. Expression of Hemangioblast Proteins in von Hippel-Lindau Disease Related Tumors. Cancers 2023, 15, 2551. https://doi.org/10.3390/cancers15092551
Vergauwen E, Forsyth R, Vortmeyer A, Gläsker S. Expression of Hemangioblast Proteins in von Hippel-Lindau Disease Related Tumors. Cancers. 2023; 15(9):2551. https://doi.org/10.3390/cancers15092551
Chicago/Turabian StyleVergauwen, Evelynn, Ramses Forsyth, Alexander Vortmeyer, and Sven Gläsker. 2023. "Expression of Hemangioblast Proteins in von Hippel-Lindau Disease Related Tumors" Cancers 15, no. 9: 2551. https://doi.org/10.3390/cancers15092551
APA StyleVergauwen, E., Forsyth, R., Vortmeyer, A., & Gläsker, S. (2023). Expression of Hemangioblast Proteins in von Hippel-Lindau Disease Related Tumors. Cancers, 15(9), 2551. https://doi.org/10.3390/cancers15092551








