Radiotherapy—Dose Escalated for Large Volume Primary Tumors—And Cetuximab with or without Induction Chemotherapy for HPV Associated Squamous Cell Carcinoma of the Head and Neck—A Randomized Phase II Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Objectives
2.3. Patients
2.4. Treatment
2.4.1. Chemotherapy
2.4.2. Radiotherapy
2.5. Procedures during Study/Follow-Up
2.6. Statistical Analysis
2.7. Sample Size Calculation
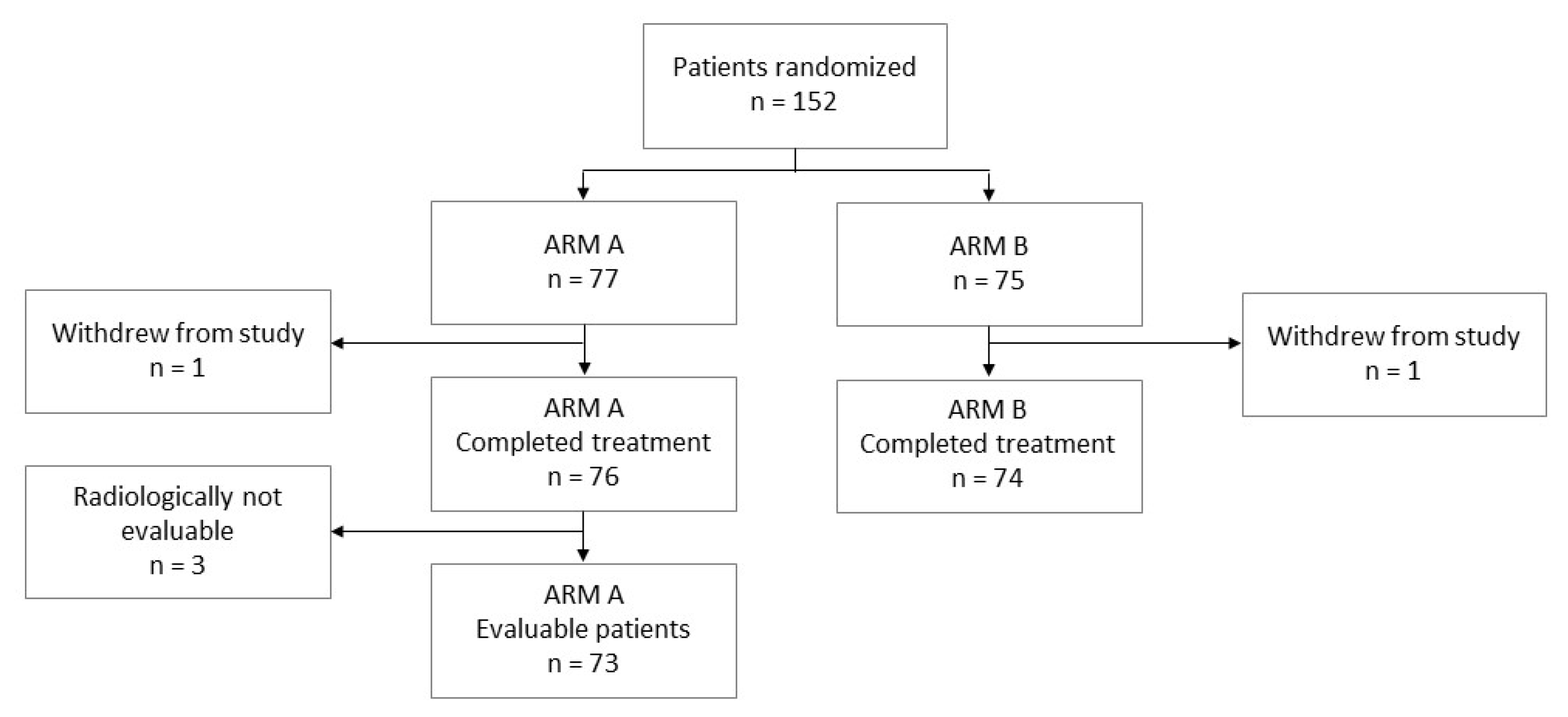
3. Results
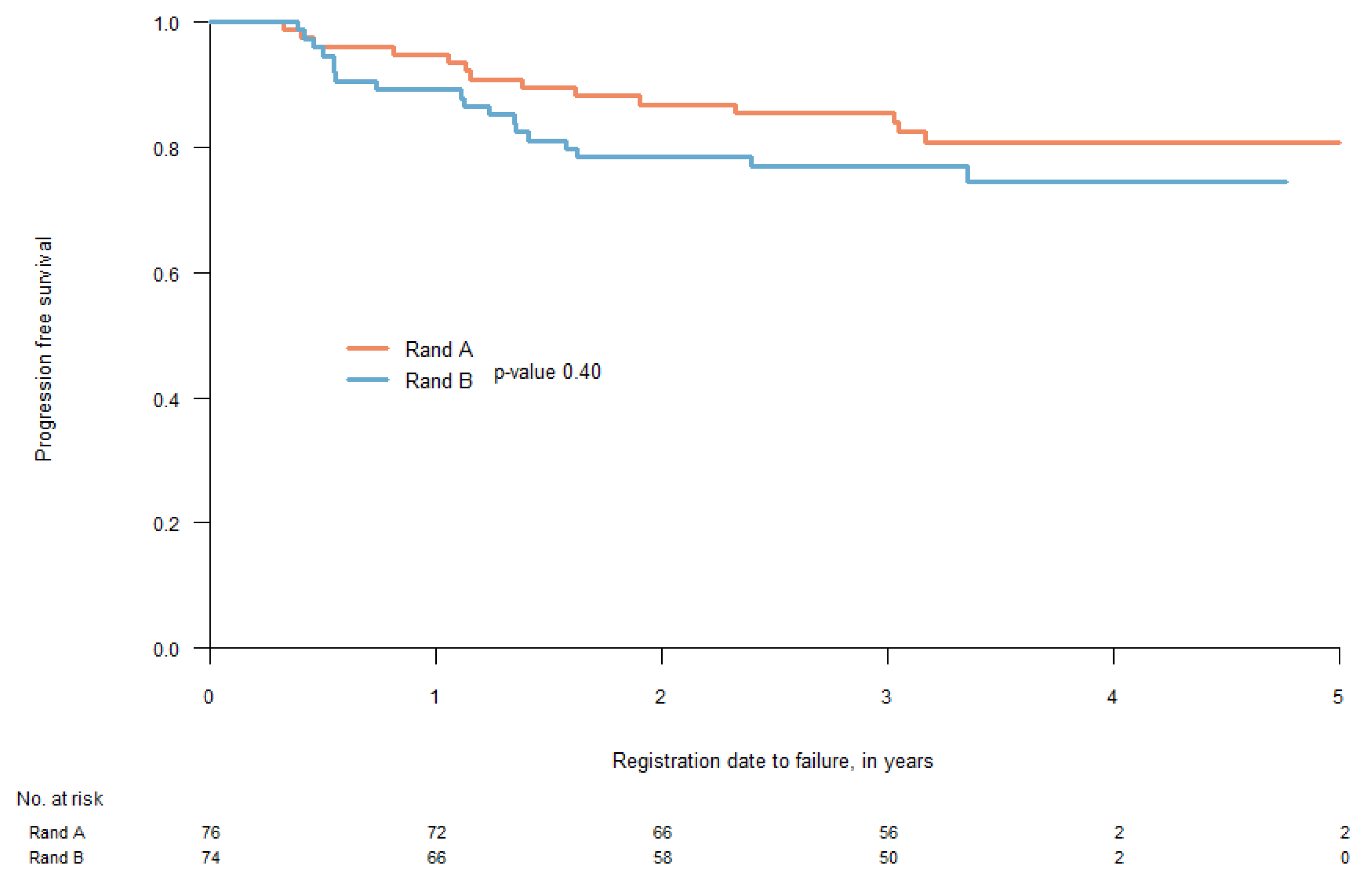
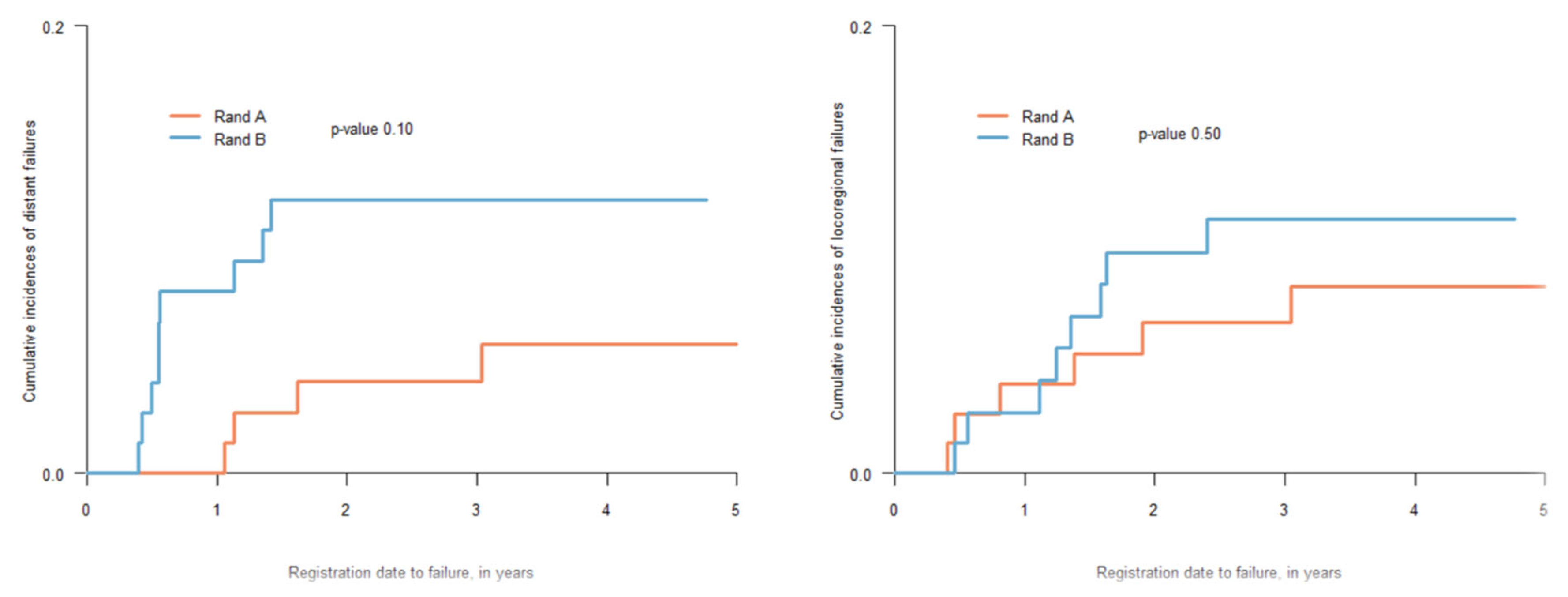
3.1. Treatment Outcome
3.2. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen-Tan, P.F.; Zhang, Q.; Ang, K.K.; Weber, R.S.; Rosenthal, D.; Soulieres, D.; Kim, H.; Silverman, C.; Raben, A.; Galloway, T.J.; et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long-term report of efficacy and toxicity. J. Clin. Oncol. 2014, 32, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, T.; Tribius, S.; Grob, T.J.; Meyer, F.; Busch, C.-J.; Petersen, C.; Dikomey, E.; Kriegs, M. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother. Oncol. 2013, 107, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Arenz, R.N.A.; Ziemann, F.; Mayer, C.; Wittig, A.; Dreffke, K.; Preising, S.; Wagner, S.; Klussmann, J.-P.; Engenhart-Cabillic, R.; Wittekindt, C. Increased radiosensitivity of HPV-positive head and neck cancer cell lines due to cell cycle dysregulation and induction of apoptosis. Strahlenther. Onkol. 2014, 190, 839–846. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Huang, S.H.; Siu, L.L.; Waldron, J.; Zhao, H.; Perez-Ordonez, B.; Weinreb, I.; Kim, J.; Ringash, J.; Bayley, A.; et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J. Clin. Oncol. 2013, 31, 543–550. [Google Scholar] [CrossRef]
- Sinha, P.; Thorstad, W.; Nussenbaum, B.; Haughey, B.; Adkins, D.; Kallogjeri, D.; Lewis, J.S., Jr. Distant metastasis in p16-positive oropharyngeal squamous cell carcinoma: A critical analysis of patterns and outcomes. Oral Oncol. 2014, 50, 45–51. [Google Scholar] [CrossRef]
- Rosenberg, A.J.; Agrawi, N.; Pearson, A.; Seiwert, T.Y.; Gooi, Z.; Blair, E.A.; Spiotto, M.T.; Jones, M. Low risk HPV-associated oropharyngeal squamous cell carcinoma treated with induction chemoimmunotherapy followed by TORS or radiotherapy. In Proceedings of the Multidisciplinary Head and Neck Cancers Symposium, Scottsdale, AZ, USA, 27–29 February 2020. [Google Scholar]
- Hall, S.F.; Griffiths, R.J.; O’Sullivan, B.; Liu, F.F. The addition of chemotherapy to radiotherapy did not reduce the rate of distant metastases in low-risk HPV-related oropharyngeal cancer in a real-world setting. Head Neck 2019, 41, 2271–2276. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Seymour, L.; Litiere, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef]
- Nickson, C.M.; Moori, P.; Carter, R.J.; Rubbi, C.P.; Parsons, J.L. Misregulation of DNA damage repair pathways i HPV-positive head and neck squamous cell carcinoma contributes to cellular radiosensitivity. Oncotarget 2017, 8, 29963–29975. [Google Scholar] [CrossRef]
- National Cancer Institute (US). Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE); Cancer Therapy Evaluation Program: Bethesda, MD, USA, 2003.
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncolgy Group (RTOG) and the European Organisation for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1345. [Google Scholar] [CrossRef]
- Adrian, G.; Carlsson, H.; Kjellén, E.; Sjövall, J.; Zackrisson, B.; Nilsson, P.; Gebre-Medhin, M. Primary tumor volume and prognosis for patients with p16-positive and p16-negative oropharyngeal squamous cell carcinoma treated with radiation therapy. Radiat. Oncol. 2022, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.R.; Lorch, J.H.; Goloubeva, O.; Tan, M.; Schumaker, L.M.; Sarlis, N.J.; Haddad, R.I.; Cullen, K.J. Survival and human papillomavirus in oropharynx cancer in TAX 324: A subset analysis from an international phase III trial. Ann. Oncol. 2011, 22, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Karrison, T.G.; Kocherginsky, M.; Mueller, J.; Egan, R.; Huang, C.H.; Brockstein, B.E.; Agulnik, M.B.; Mittal, B.B.; Yunus, F.; et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J. Clin. Oncol. 2014, 32, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; O’Neill, A.; Rabinowits, G.; Tishler, R.; Khuri, F.R.; Adkins, D.; Clark, J.; Sarlis, N.; Lorch, J.; Beitler, J.J.; et al. IInduction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Lacas, B.; Carmel, A.; Landais, C.; Wong, S.J.; Licitra, L.; Tobias, J.S.; Burtness, B.; Ghi, M.G.; Cohen, E.E.; Grau, C.; et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother. Oncol. 2021, 156, 281–293. [Google Scholar]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Nat. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Worden, F.P.; Kumar, B.; Lee, J.S.; Wolf, G.T.; Cordell, K.G.; Taylor, J.M.; Urba, S.G.; Eisbruch, A.; Teknos, T.N.; Chepeha, D.B.; et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: Response and survival positively associated with HPV 16 copy number. J. Clin. Oncol 2008, 26, 3138–3146. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W.F.; Chen, N.Y.; Zhang, N.; Hu, G.Q.; Xie, F.Y.; Sun, Y.; Chen, X.Z.; Li, J.G.; Zhu, X.D.; et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomized controlled trial. Lancet Oncol. 2016, 17, 1509–1520. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Gebre-Medhin, M.; Brun, E.; Engström, P.; Cange, H.H.; Hammarstedt-Nordenvall, L.; Reizenstein, J.; Nyman, J.; Abel, E.; Friesland, S.; Sjödin, H.; et al. ARTSCAN III: A Randomized Phase III Study Comparing Chemoradiotherapy with Cisplatin Versus Cetuximab in Patients with Locoregionally Advanced Head and Neck Squamous Cell Cancer. J. Clin. Oncol. 2021, 39, 38–47. [Google Scholar] [CrossRef]
- Chen, A.M.; Felix, C.; Wang, P.-C.; Hsu, S.; Basehart, V.; Garst, J.; Beron, P.; Wong, D.; Rosove, M.H.; Rao, S.; et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 2017, 18, 803–811. [Google Scholar] [CrossRef]
- Marur, S.; Li, S.; Cmelak, A.J.; Gillison, M.L.; Zhao, W.J.; Ferris, R.L.; Westra, W.H.; Gilbert, J.; Bauman, J.E.; Wagner, L.I.; et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients with HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2017, 35, 490–497. [Google Scholar] [CrossRef]
- Rosenberg, A.J.; Vokes, E.E. Optimizing Treatment De-Escalation in Head and Neck Cancer: Current and Future Perspectives. Oncologist 2021, 26, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Semrau, S.; Gostian, A.-O.; Traxdorf, M.; Eckstein, M.; Rutzner, S.; von der Grun, J.; Illmer, T.; Hautmann, M.; Klautke, G.; Laban, S.; et al. Implementation of double Immune Checkpoint Blockade increases response rate to induction chemotherapy in head and neck cancer. Cancers 2021, 13, 1959. [Google Scholar] [CrossRef] [PubMed]
| Total | Arm A | Arm B | ||
|---|---|---|---|---|
| Randomised | 150 | 76 | 74 | |
| Age: | 40.3–80.7 Median 59.8 | 40.3–80.7 Median 59.8 | 40.7–78.7 Median 59.7 | |
| Sex: | Female | 37 (24.6%) | 20 | 17 |
| Male | 113 (75.4%) | 56 | 57 | |
| Smoking | Never smoker Smoker/former smoker | 35.3% 64.7% | 22 54 | 31 43 |
| PS: | 0 | 143 (95.3%) | 72 | 71 |
| 1 | 7 (4.7%) | 4 | 3 | |
| Tumor site: | Oropharynx, tonsil | 95 (63.3%) | 51 | 44 |
| Oropharynx, base of tongue | 55 (36.7%) | 25 | 30 | |
| T-stage: | T1 | 34 (22.7%) | 16 | 18 |
| T2 | 68 (45.3%) | 37 | 31 | |
| T3 | 27 (18%) | 15 | 12 | |
| T4 | 21 (14%) | 8 | 13 | |
| N-stage: | N0 | 8 (5.3%) | 3 | 5 |
| N1 | 8 (5.3%) | 4 | 4 | |
| N2 | 134 (89.3%) | 69 | 65 | |
| T + N-stage: | T1N0 | 0 | 0 | 0 |
| T1N1 | 0 | 0 | 0 | |
| T1N2 | 36 | 17 | 19 | |
| T2N0 | 2 | 0 | 2 | |
| T2N1 | 1 | 0 | 1 | |
| T2N2 | 65 | 37 | 28 | |
| T3N0 | 3 | 2 | 1 | |
| T3N1 | 4 | 3 | 1 | |
| T3N2 | 18 | 9 | 9 | |
| T4N0 | 3 | 1 | 2 | |
| T4N1 | 3 | 1 | 2 | |
| T4N2 | 15 | 6 | 9 |
| Schedule | GTV | CTV1, Lymphnode Metastases | CTV2, Elective Volumes | Delivery |
|---|---|---|---|---|
| 1 | 68 (34) | 68 (34) | 46 (23) | Sequential |
| 2 | 68 (34) | 68 (34) | 51.7 or 54.4 (34) | SIB |
| 3 | 74.8 (34) | 68 (34) | 51.7 or 54.4 (34) | SIB |
| 4 | 68 (34) + 8-10 BT | 68 (34) | 51.7 or 54.4 (34) | SIB |
| 5 | 68 (34) + 8-10 BT | 68 (34) | 46 (23) | Sequential |
| Local | Regional | Distant | |
|---|---|---|---|
| ARM A | [n = 3] | [n = 2] | [n = 4] |
| T3N2b Base tongue | T2N2b Tonsil | T2N2b Tonsil | |
| T2N2c Tonsil | T2N2b Tonsil | T4N2c Base tongue | |
| T4N2b Tonsil | T2N2b Tonsil | ||
| T2N2c Tonsil | |||
| ARM B | [n = 4] | [n = 4] | [n = 9] |
| T2N2b Tonsil | T2N2b Tonsil | T3N2b Base tongue | |
| T2N2b Tonsil | T2N2b Tonsil | T4N2c Tonsil | |
| T4N2b Base tongue | T2N2b Base tongue | T4N2c Tonsil | |
| T2N2b Base tongue | T1N2b Base tongue | T2N2b Tonsil | |
| T3N2b Tonsil | |||
| T2N2b Base tongue | |||
| T1N2b Tonsil | |||
| T4 N2b Base tongue | |||
| T4N0 Tonsil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercke, C.; Wickart-Johansson, G.; Sjödin, H.; Farrajota Neves da Silva, P.; Alexandersson von Döbeln, G.; Margolin, G.; Jonmarker Jaraj, S.; Carstens, H.; Berglund, A.; Lax, I.; et al. Radiotherapy—Dose Escalated for Large Volume Primary Tumors—And Cetuximab with or without Induction Chemotherapy for HPV Associated Squamous Cell Carcinoma of the Head and Neck—A Randomized Phase II Trial. Cancers 2023, 15, 2543. https://doi.org/10.3390/cancers15092543
Mercke C, Wickart-Johansson G, Sjödin H, Farrajota Neves da Silva P, Alexandersson von Döbeln G, Margolin G, Jonmarker Jaraj S, Carstens H, Berglund A, Lax I, et al. Radiotherapy—Dose Escalated for Large Volume Primary Tumors—And Cetuximab with or without Induction Chemotherapy for HPV Associated Squamous Cell Carcinoma of the Head and Neck—A Randomized Phase II Trial. Cancers. 2023; 15(9):2543. https://doi.org/10.3390/cancers15092543
Chicago/Turabian StyleMercke, Claes, Gun Wickart-Johansson, Helena Sjödin, Pedro Farrajota Neves da Silva, Gabriella Alexandersson von Döbeln, Gregori Margolin, Sara Jonmarker Jaraj, Hanna Carstens, Anders Berglund, Ingmar Lax, and et al. 2023. "Radiotherapy—Dose Escalated for Large Volume Primary Tumors—And Cetuximab with or without Induction Chemotherapy for HPV Associated Squamous Cell Carcinoma of the Head and Neck—A Randomized Phase II Trial" Cancers 15, no. 9: 2543. https://doi.org/10.3390/cancers15092543
APA StyleMercke, C., Wickart-Johansson, G., Sjödin, H., Farrajota Neves da Silva, P., Alexandersson von Döbeln, G., Margolin, G., Jonmarker Jaraj, S., Carstens, H., Berglund, A., Lax, I., Hellström, M., Hammarstedt-Nordenvall, L., & Friesland, S. (2023). Radiotherapy—Dose Escalated for Large Volume Primary Tumors—And Cetuximab with or without Induction Chemotherapy for HPV Associated Squamous Cell Carcinoma of the Head and Neck—A Randomized Phase II Trial. Cancers, 15(9), 2543. https://doi.org/10.3390/cancers15092543








