Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use—Updated Outcome Report
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Disease Evaluations and Treatment Monitoring
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Dini, G.; Philip, T.; Hartmann, O.; Pinkerton, R.; Chauvin, F.; Garaventa, A.; Lanino, E.; Dallorso, S. Bone marrow transplantation for neuroblastoma: A review of 509 cases. Bone Marrow Transplant. 1989, 4, 42–46. [Google Scholar] [PubMed]
- Dini, G.; Lanino, E.; Garaventa, A.; Rogers, D.; Dallorso, S.; Viscoli, C.; Castagnola, E.; Manno, G.; Brisigotti, M.; Rosanda, C. Myeloablative therapy and unpurged autologous bone marrow transplantation for poor-prognosis neuroblastoma: Report of 34 cases. J. Clin. Oncol. 1991, 9, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Philip, T.; Zucker, J.M.; Bernard, J.L.; Lutz, P.; Bordigoni, P.; Plouvier, E.; Robert, A.; Roché, H.; Souillet, G.; Bouffet, E. Improved survival at 2 and 5 years in the LMCE1 unselected group of 72 children with stage IV neuroblastoma older than 1 year of age at diagnosis: Is cure possible in a small subgroup? J. Clin. Oncol. 1991, 9, 1037–1044. [Google Scholar] [CrossRef]
- Kushner, B.H.; O’Reilly, R.J.; Mandell, L.R.; Gulati, S.C.; LaQuaglia, M.; Cheung, N.K. Myeloablative combination chemo-therapy without total body irradiation for neuroblastoma. J. Clin. Oncol. 1991, 9, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Seeger, R.C.; Reynolds, C.P. Treatment of high-risk solid tumors of childhood with intensive therapy and autologous bone marrow transplantation. Pediatric Clin. N. Am. 1991, 38, 393–424. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Atkinson, J.B.; Stram, D.O.; Selch, M.; Reynolds, C.P.; Seeger, R.C. Patterns of relapse after autologous purged bone marrow transplantation for neuroblastoma: A Childrens Cancer Group pilot study. J. Clin. Oncol. 1993, 11, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, N.; Takahashi, H.; Kaneko, M.; Uchino, J.; Takeda, T.; Iwafuchi, M.; Ohhira, M.; Nishihira, H.; Mugishima, H.; Yokoyama, J.; et al. Treatment combined with bone marrow transplantation for advanced neuroblastoma: An analysis of patients who were pretreated intensively with the protocol of the Study Group of Japan. Med. Pediatric Oncol. 1995, 24, 181–187. [Google Scholar] [CrossRef]
- Garaventa, A.; Rondelli, R.; Lanino, E.; Dallorso, S.; Dini, G.; Bonetti, F.; Arrighini, A.; Santoro, N.; Rossetti, F.; Miniero, F. Myeloablative therapy and bone marrow rescue in advanced neuroblastoma: Report from the Italian Bone Marrow Transplant Registry. Bone Marrow Transplant. 1996, 18, 125–130. [Google Scholar]
- Stram, D.O.; Matthay, K.K.; O’Leary, M.; Reynolds, C.P.; Haase, G.M.; Atkinson, J.B.; Brodeur, G.M.; Seeger, R.C. Consolida-tion chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neu-roblastoma: A report of two concurrent Children’s Cancer Group studies. J. Clin. Oncol. 1996, 14, 2417–2426. [Google Scholar] [CrossRef]
- Kushner, B.H.; Gulati, S.C.; O’Reilly, R.J.; Heller, G.; Cheung, N.-K.V. Autografting with bone marrow exposed to multiple courses of very high dose cyclophosphamide in vivo and to 4-hydroperoxy-cyclophosphamide in vitro. Med. Pediatric Oncol. 1990, 18, 454–458. [Google Scholar] [CrossRef]
- Kushner, B.H.; Hadju, S.I.; Gulati, S.C.; Erlandson, R.A.; Exelby, P.R.; Lieberman, P.H. Extracranial primitive neuroectoder-mal tumors: The Memorial Sloan-Kettering Cancer Center experience. Cancer 1991, 67, 1825–1829. [Google Scholar] [CrossRef]
- Kushner, B.H.; Gulati, S.C.; Kwon, J.H.; O’Reilly, R.J.; Exelby, P.R.; Cheung, N.-K.V. High-dose melphalan with 6-hydroxydopamine-purged autologous bone marrow transplantation for poor-risk neuroblastoma. Cancer 1991, 68, 242–247. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, N.-K.V.; Kramer, K.; Dunkel, I.J.; Calleja, E.; Boulad, F. Topotecan combined with myeloablative doses of thiotepa and carboplatin for neuroblastoma, brain tumors, and other poor-risk solid tumors in children and young adults. Bone Marrow Transplant. 2001, 28, 551–556. [Google Scholar] [CrossRef]
- Kushner, B.H.; Kramer, K.; Modak, S.; Kernan, N.A.; Reich, L.M.; Danis, K.; Cheung, N.-K.V. Topotecan, thiotepa, and car-boplatin for neuroblastoma: Failure to prevent relapse in the central nervous system. Bone Marrow Transplant. 2006, 37, 271–276. [Google Scholar] [CrossRef]
- Fish, J.D.; Grupp, S.A. Stem cell transplantation for neuroblastoma. Bone Marrow Transplant. 2008, 41, 159–165. [Google Scholar] [CrossRef]
- Cheung, N.-K.V.; Heller, G. Chemotherapy Dose Intensity Correlates Strongly with Response, Median Survival, and Median Progression-Free Survival in Metastatic Neuroblastoma. J. Clin. Oncol. 1991, 9, 1050–1058. [Google Scholar] [CrossRef]
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N. Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef]
- Pritchard, J.; Cotterill, S.J.; Germond, S.M.; Imeson, J.; de Kraker, J.; Jones, D.R. High dose melphalan in the treatment of advanced neuroblastoma: Results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr. Blood Cancer 2005, 44, 348–357. [Google Scholar] [CrossRef]
- Berthold, F.; Boos, J.; Burdach, S.; Erttmann, R.; Henze, G.; Hermann, J.; Klingebiel, T.; Kremens, B.; Schilling, F.H.; Schrappe, M. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005, 6, 649–658. [Google Scholar] [CrossRef]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients with High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef]
- Yalçin, B.; Kremer, L.C.; van Dalen, E.C. High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma. Cochrane Database Syst. Rev. 2015, 5, CD006301. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.-K. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing with Granulocyte-Macrophage Colony-Stimulating Factor in Patients with Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol. 2018, 4, 1729–1735. [Google Scholar] [CrossRef]
- Mody, R.; Naranjo, A.; Van Ryn, C.; Yu, A.L.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.-E.-N.; Diccianni, M.B.; Sondel, P.M.; et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblas-toma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; London, W.B.; Tenney, S.C.; Diccianni, M.; Hank, J.A.; Parisi, M.T.; Shulkin, B.L.; et al. Outcomes Following GD2-Directed Postconsolidation Therapy for Neuroblastoma After Cessation of Random Assignment on ANBL0032: A Report from the Children’s Oncology Group. J Clin. Oncol. 2022, 40, 4107–4118. [Google Scholar] [CrossRef]
- Kushner, B.H.; Ostrovnaya, I.; Cheung, I.Y.; Kuk, D.; Modak, S.; Kramer, K.; Roberts, S.S.; Basu, E.M.; Yataghene, K.; Cheung, N.-K.V. Lack of survival advantage with autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy and isotretinoin. Oncotarget 2016, 7, 4155–4166. [Google Scholar] [CrossRef]
- Mora, J.; Castañeda, A.; Gorostegui, M.; Santa-María, V.; Garraus, M.; Muñoz, J.P.; Varo, A.; Perez-Jaume, S.; Mañe, S. Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission. Pediatr. Blood Cancer 2021, 68, e29121. [Google Scholar] [CrossRef]
- Kreissman, S.G.; Seeger, R.C.; Matthay, K.K.; London, W.B.; Sposto, R.; Grupp, S.A.; Haas-Kogan, D.A.; Laquaglia, M.P.; Yu, A.L.; Diller, L.; et al. Purged vs. non-purged peripheral blood stem cell transplantation for high-risk neuroblastoma (COG A3973): A randomized phase III trial. Lancet Oncol. 2013, 14, 999–1008. [Google Scholar] [CrossRef]
- Cheung, N.-K.V.; Cheung, I.Y.; Kushner, B.H.; Ostrovnaya, I.; Kramer, K.; Modak, S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol. 2012, 30, 3264–3270. [Google Scholar] [CrossRef]
- Kushner, B.H.; Ostrovnaya, I.; Cheung, I.Y.; Kuk, D.; Kramer, K.; Modak, S.; Yataghene, K.; Cheung, N.K. Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with Anti-GD2 immunotherapy and isotretinoin: A prospective Phase II study. Oncoimmunology 2015, 4, e1016704. [Google Scholar] [CrossRef]
- Kushner, B.H.; Modak, S.; Kramer, K.; LaQuaglia, M.P.; Yataghene, K.; Basu, E.M.; Roberts, S.S.; Cheung, N.K. Striking dichotomy in outcome of MYCN-amplified neuroblastoma in the contemporary era. Cancer 2014, 120, 2050–2059. [Google Scholar] [CrossRef]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement from the National Cancer Institute Clinical Trials Planning Meeting. J Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Dalton, C.F.; Reynolds, G.P. Phospholipid fatty acids and neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 2001, 309, 193–196. [Google Scholar] [CrossRef]
- Lindskog, M.; Gleissman, H.; Ponthan, F.; Castro, J.; Kogner, P.; Johnsen, J.I. Neuroblastoma cell death in response to docosahexaenoic acid: Sensitization to chemotherapy and arsenic-induced oxidative stress. Int. J. Cancer 2006, 118, 2584–2593. [Google Scholar] [CrossRef]
- Gleissman, H.; Segerström, L.; Hamberg, M.; Ponthan, F.; Lindskog, M.; Johnsen, J.I.; Kogner, P. Omega-3 fatty acid supplementation delays the progression of neuroblastoma in vivo. Int. J. Cancer 2011, 128, 1703–1711. [Google Scholar] [CrossRef]
- Mora, J.; Cruz, O.; Lavarino, C.; Rios, J.; Vancells, M.; Parareda, A.; Salvador, H.; Suñol, M.; Carrasco, R.; Guillen, A.; et al. Results of induction chemotherapy in children older than 18 months with stage-4 neuroblastoma treated with an adaptive-to-response modified N7 protocol (mN7). Clin. Transl. Oncol. 2015, 17, 521–529. [Google Scholar] [CrossRef]
- Kaplan, E.; Meier, P. Non parametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Contal, C.; O’Quigley, J. An application of change point methods in studying the effect of age on survival in breast cancer. Comput. Stat. Data Anal. 1999, 30, 253–270. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Ser. B Methodol. 1972, 2, 187–220. [Google Scholar]
- Matthay, K.K.; Edeline, V.; Lumbroso, J.; Tanguy, M.L.; Asselain, B.; Zucker, J.M.; Valteau-Couanet, D.; Hartmann, O.; Michon, J. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J. Clin. Oncol. 2003, 21, 2486–2491. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, H.M.; Cohn, S.L.; Shore, R.M.; Bardo, D.M.; Haut, P.R.; Olszewski, M.; Schmoldt, J.; Liu, D.; Rademaker, A.W.; Kletzel, M. Scintigraphic response by 123Imetaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J. Clin. Oncol. 2004, 22, 3909–3915. [Google Scholar] [CrossRef]
- Schmidt, M.; Simon, T.; Hero, B.; Schicha, H.; Berthold, F. The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: Results of the German Neuroblastoma Trial NB97. Eur. J. Cancer. 2008, 44, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.K.; Heller, G.; Kushner, B.H.; Burch, L.; O’Reilly, R.J. Stage IV neuroblastoma more than 1 year of age at diagnosis: Major response to chemotherapy and survival durations correlated strongly with dose intensity. Prog. Clin. Biol. Res. 1991, 366, 567–573. [Google Scholar]
- La Quaglia, M.P.; Kushner, B.H.; Su, W.; Heller, G.; Kramer, K.; Abramson, S.; Rosen, N.; Wolden, S.; Cheung, N.K. The Impact of Gross Total Resection on Local Control and Survival in High-Risk Neuroblastoma. J. Pediatr. Surg. 2004, 39, 412–417. [Google Scholar] [CrossRef] [PubMed]
- London, W.B.; Bagatell, R.; Weigel, B.J.; Fox, E.; Guo, D.; Van Ryn, C.; Naranjo, A.; Park, J.R. Historical time-to-progression (TTP) and progression-free survival (PFS) in relapsed/refractory neuroblastoma modern-era (2002-14) patients from Children’s Oncology Group (COG) early-phase trials. Cancer 2017, 123, 4914–4923. [Google Scholar] [CrossRef]
- Moreno, L.; Rubie, H.; Varo, A.; LeDeley, M.C.; Amoroso, L.; Chevance, A.; Garaventa, A.; Gambart, M.; Bautista, F.; Valteau-Couanet, D.; et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr. Blood Cancer 2017, 64, 25–31. [Google Scholar] [CrossRef]
- Castañeda, A.; Gorostegui, M.; Miralles, S.L.; Chamizo, A.; Patiño, S.C.; Flores, M.A.; Garraus, M.; Lazaro, J.J.; Santa-Maria, V.; Varo, A.; et al. How we approach the treatment of patients with high-risk neuroblastoma with naxitamab: Experience from the Hospital Sant Joan de Déu in Barcelona, Spain. ESMO Open 2022, 7, 100462. [Google Scholar] [CrossRef]
- Mora, J.; Castañeda, A.; Flores, M.A.; Santa-María, V.; Garraus, M.; Gorostegui, M.; Simao, M.; Perez-Jaume, S.; Mañe, S. The Role of Autologous Stem-Cell Transplantation in High-Risk Neuroblastoma Consolidated by anti-GD2 Immunotherapy. Results of Two Consecutive Studies. Front Pharmacol. 2020, 11, 575009. [Google Scholar] [CrossRef]
- Cheung, I.Y.; Cheung, N.V.; Modak, S.; Mauguen, A.; Feng, Y.; Basu, E.; Roberts, S.S.; Ragupathi, G.; Kushner, B.H. Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients with High-Risk Neuroblastoma with Prior Disease Progression. J. Clin. Oncol. 2021, 39, 215–226. [Google Scholar] [CrossRef]
- Suzuki, M.; Cheung, N.K.V. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin. Ther. Targets 2015, 3, 349–362. [Google Scholar] [CrossRef] [PubMed]

| n = 82 | n | |
|---|---|---|
| MYCN: | 82 | |
| A | 21 (25.6%) | |
| NA | 61 (74.4%) | |
| Chemotherapy cycles: | 82 | |
| 5 | 13 (15.9%) | |
| >5 | 69 (84.1%) | |
| ASCT: | 82 | |
| No | 71 (86.6%) | |
| Yes | 11 (13.4%) | |
| RDT: | 82 | |
| No | 56 (68.3%) | |
| Yes | 26 (31.7%) | |
| MRD: | 82 | |
| No | 70 (85.4%) | |
| Yes | 12 (14.6%) | |
| Age at diagnosis (y) | 3.0 [0.6; 13.0] | 82 |
| Age at treatment initiation (y) | 3.9 [1.4; 13.8] | 82 |
| Stage: | 82 | |
| 3 | 1 (1.2%) | |
| 4 | 81 (98.8%) | |
| Induction Regimen: | 82 | |
| mN7 | 16 (19.5%) | |
| GPOH NB2004 | 6 (7.3%) | |
| SIOPEN HR-NB-01 | 10 (12.2%) | |
| COG 3973 | 5 (6.1%) | |
| Chinese protocols | 40 (48.8%) | |
| Russian HR-NB protocols | 1 (1.2%) | |
| GALOP protocol | 2 (2.4%) | |
| CCG | 2 (2.4%) |
| EFS | OS | |||||
|---|---|---|---|---|---|---|
| 3-Year Survival [95% CI] | 5-Year Survival [95% CI] | p-Value | 3-Year Survival [95% CI] | 5-Year Survival [95% CI] | p-Value | |
| All patients | 57.9 [47.2; 70.9] | 57.9 [47.2; 70.9] | - | 81.3 [72.4; 91.3] | 78.6 [68.7; 89.8] | - |
| MYCN: A | 71.4 [54.5; 93.6] | 71.4 [54.5; 93.6] | 0.38 | 81.0 [65.8; 99.6] | 81.0 [65.8; 99.6] | 0.60 |
| NA | 53.3 [41.0; 69.2] | 53.3 [41.0; 69.2] | 81.7 [71.3; 93.5] | 78.4 [67.0; 91.7] | ||
| Chemotherapy cycles: 5 | 59.8 [37.8; 94.7] | 59.8 [37.8; 94.7] | 0.93 | 76.9 [57.1; 100.0] | 76.9 [57.1; 100.0] | 0.49 |
| >5 | 57.6 [46.0; 72.1] | 57.6 [46.0; 72.1] | 82.0 [72.3; 93.1] | 78.8 [67.8; 91.5] | ||
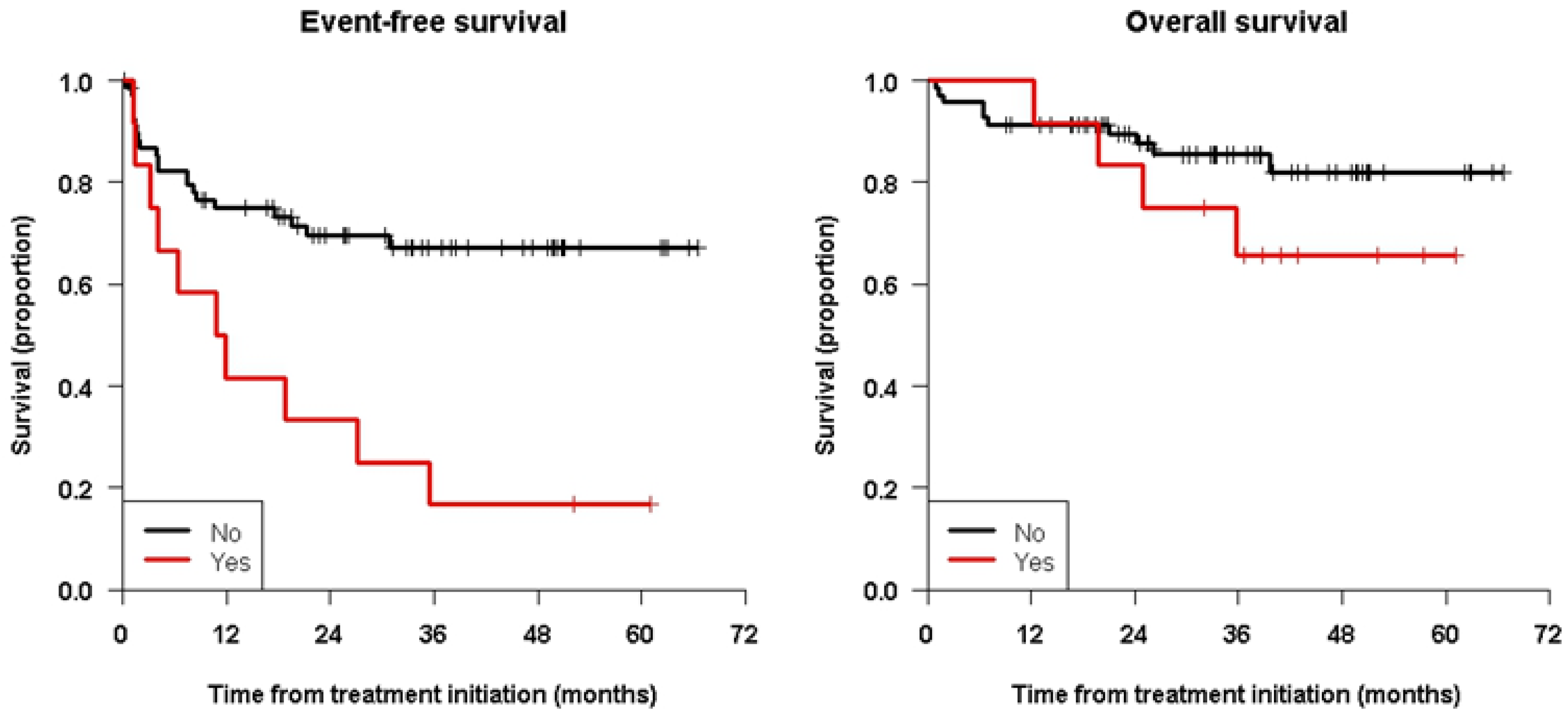
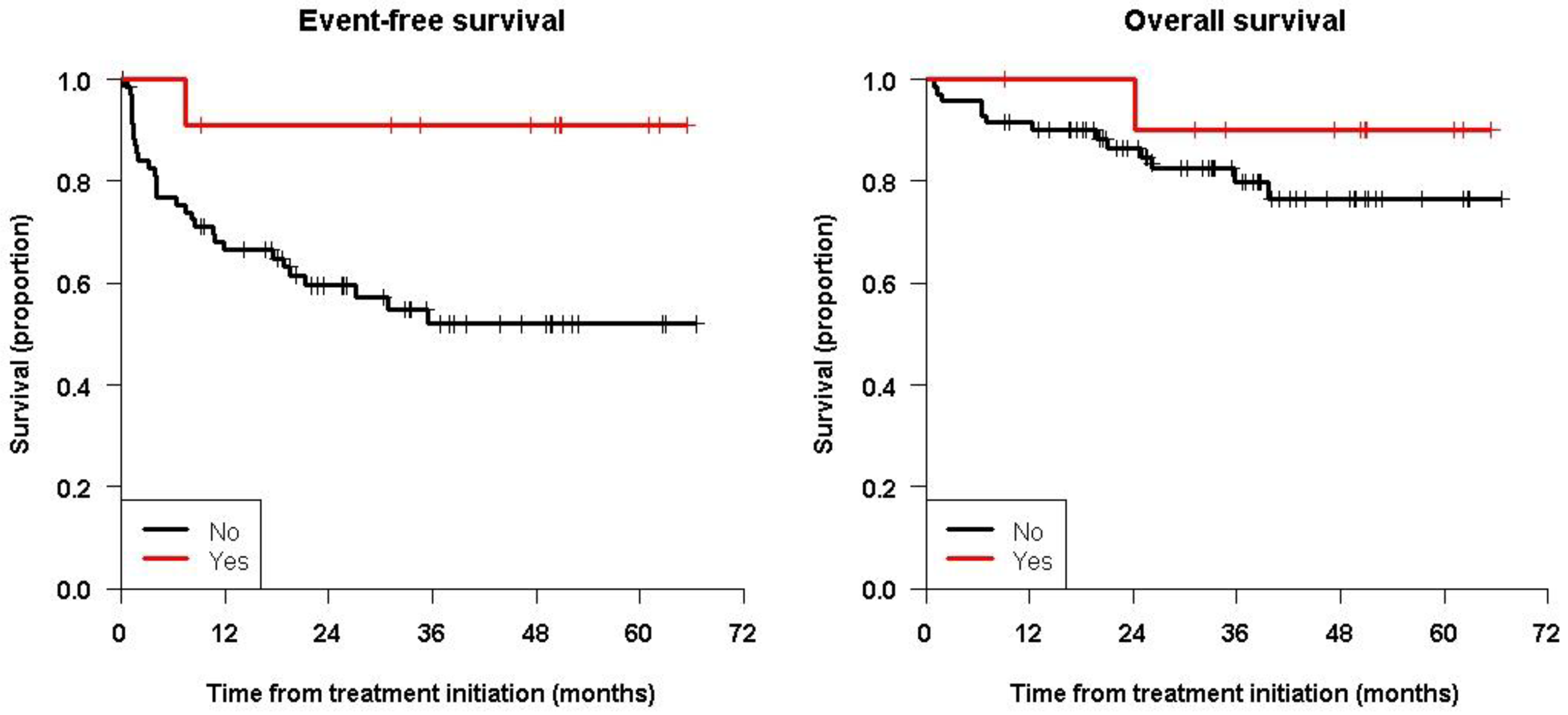
| ASCT: No | 51.9 [40.3; 66.9] | 51.9 [40.3; 66.9] | 0.037 | 79.9 [70.0; 91.2] | 76.4 [65.2; 89.5] | 0.36 |
| Yes | 90.9 [75.4; 100.0] | 90.9 [75.4; 100.0] | 90.0 [73.2; 100.0] | 90.0 [73.2; 100.0] | ||
| RDT: No | 53.4 [40.1; 71.0] | 53.4 [40.1; 71.0] | 0.62 | 81.7 [71.3; 93.5] | 81.7 [71.3; 93.5] | 0.96 |
| Yes | 65.2 [49.1; 86.4] | 65.2 [49.1; 86.4] | 81.0 [65.4; 100.0] | 74.7 [57.4; 97.4] | ||
| MRD: No | 67.2 [56.5; 80.1] | 67.2 [56.5; 80.1] | 0.0011 | 85.4 [76.9; 94.9] | 82.0 [71.9; 93.6] | 0.23 |
| Yes | 16.7 [4.7; 59.1] | 16.7 [4.7; 59.1] | 65.6 [43.2; 99.7] | 65.6 [43.2; 99.7] | ||
| Age at diagnosis (y): <2.6 | 77.6 [63.3; 95.1] | 77.6 [63.3; 95.1] | 0.044 | 88.8 [77.6; 100.0] | 88.8 [77.6; 100.0] | 0.27 |
| ≥2.6 | 47.1 [34.2; 65.0] | 47.1 [34.2; 65.0] | 77.0 [65.1; 91.1] | 73.3 [60.4; 89.0] | ||
| Age at initiation (y): < 5 | 62.3 [50.3; 77.1] | 62.3 [50.3; 77.1] | 0.14 | 87.2 [78.7; 96.7] | 83.2 [72.5; 95.5] | 0.086 |
| ≥5 | 43.4 [24.9; 75.6] | 43.4 [24.9; 75.6] | 63.9 [43.9; 93.1] | 63.9 [43.9; 93.1] | ||
| EFS | OS | |||
|---|---|---|---|---|
| HR [95% CI] | p-Value | HR [95% CI] | p-Value | |
| MYCN: A | Ref. | Ref. | Ref. | Ref. |
| NA | 1.49 [0.61; 3.63] | 0.38 | 0.73 [0.23; 2.35] | 0.60 |
| Chemotherapy cycles: 5 | Ref. | Ref. | Ref. | Ref. |
| >5 | 0.96 [0.37; 2.50] | 0.93 | 0.64 [0.18; 2.29] | 0.49 |
| ASCT: No | Ref. | Ref. | Ref. | Ref. |
| Yes | 0.16 [0.02; 1.16] | 0.070 | 0.40 [0.05; 3.08] | 0.38 |
| RDT: No | Ref. | Ref. | Ref. | Ref. |
| Yes | 0.82 [0.38; 1.78] | 0.62 | 1.03 [0.34; 3.08] | 0.96 |
| MRD: No | Ref. | Ref. | Ref. | Ref. |
| Yes | 3.29 [1.54; 7.00] | 0.0020 | 1.99 [0.62; 6.37] | 0.24 |
| Age at diagnosis (y) | 1.13 [0.96; 1.32] | 0.13 | 1.08 [0.82; 1.41] | 0.59 |
| Age at initiation (y) | 1.12 [0.95; 1.31] | 0.18 | 1.05 [0.79; 1.40] | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, J.; Castañeda, A.; Gorostegui, M.; Varo, A.; Perez-Jaume, S.; Simao, M.; Muñoz, J.P.; Garraus, M.; Larrosa, C.; Salvador, N.; et al. Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use—Updated Outcome Report. Cancers 2023, 15, 2535. https://doi.org/10.3390/cancers15092535
Mora J, Castañeda A, Gorostegui M, Varo A, Perez-Jaume S, Simao M, Muñoz JP, Garraus M, Larrosa C, Salvador N, et al. Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use—Updated Outcome Report. Cancers. 2023; 15(9):2535. https://doi.org/10.3390/cancers15092535
Chicago/Turabian StyleMora, Jaume, Alicia Castañeda, Maite Gorostegui, Amalia Varo, Sara Perez-Jaume, Margarida Simao, Juan Pablo Muñoz, Moira Garraus, Cristina Larrosa, Noelia Salvador, and et al. 2023. "Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use—Updated Outcome Report" Cancers 15, no. 9: 2535. https://doi.org/10.3390/cancers15092535
APA StyleMora, J., Castañeda, A., Gorostegui, M., Varo, A., Perez-Jaume, S., Simao, M., Muñoz, J. P., Garraus, M., Larrosa, C., Salvador, N., Lavarino, C., Krauel, L., & Mañe, S. (2023). Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use—Updated Outcome Report. Cancers, 15(9), 2535. https://doi.org/10.3390/cancers15092535







