Role of Hemidesmosomes in Oral Carcinogenesis: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
- Are alterations to hemidesmosomes or their components correlated with particular stages of oral cancer?
- Which cellular pathways do hemidesmosomes contribute to during oral carcinogenesis?
- Can alterations in hemidesmosomal components be used as stage-specific biomarkers for oral cancer progression as well as prognosis?
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Selection Criteria
2.2.1. Inclusion Criteria
- -
- Article types: in-vivo and in-vitro studies;
- -
- Hemidesmosomes and components (not all adherens junction);
- -
- Oral cancer and/or precancerous conditions (human and/or animal);
- -
- Published in English;
- -
- No restriction on publication date.
2.2.2. Exclusion Criteria
- -
- Article types: Review articles, conference abstracts, letters to the editors, personal communications and opinion articles, and case reports;
- -
- Retracted studies;
- -
- No full text available.
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Literature Selection
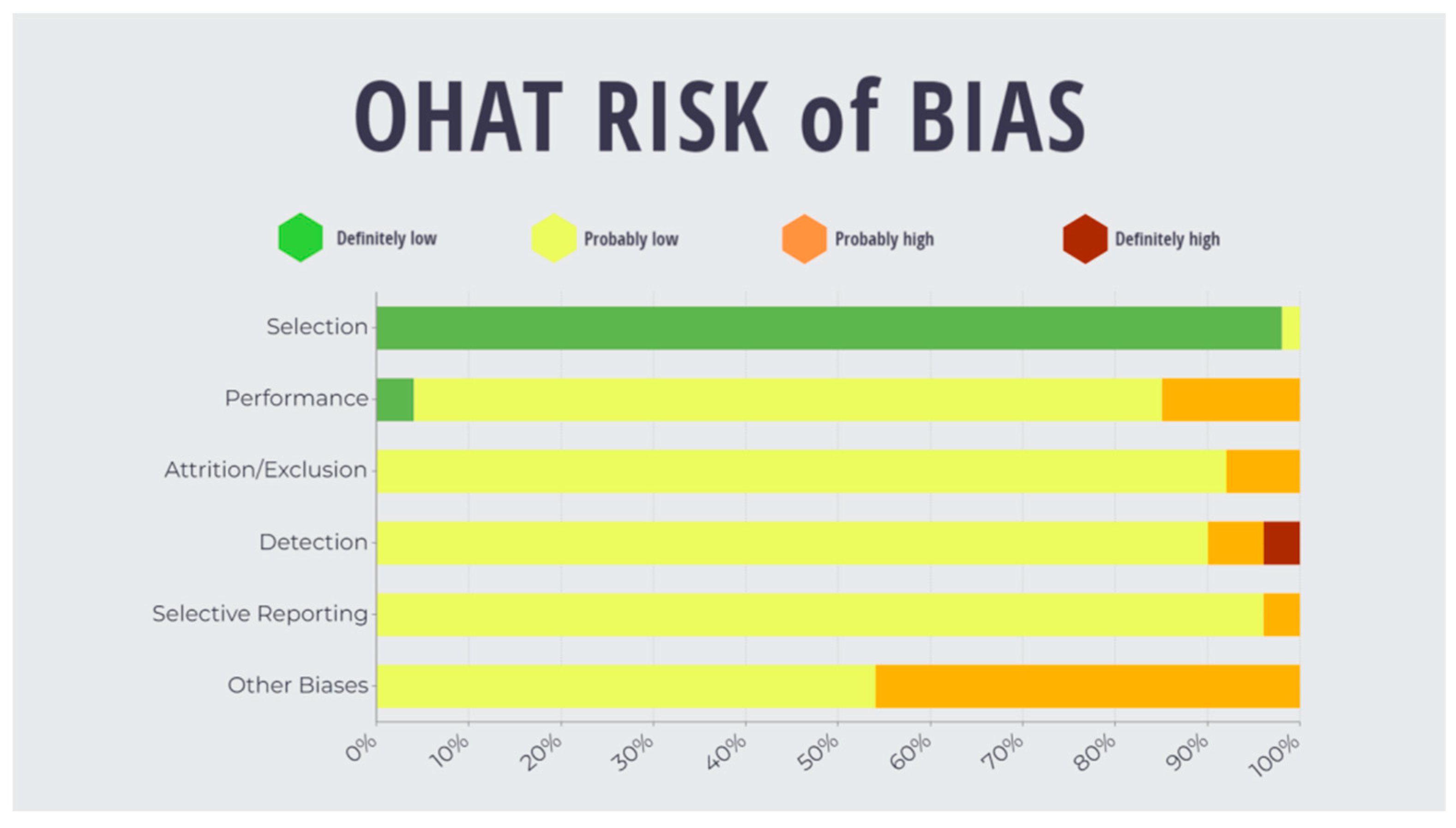
3.2. Risk of Bias
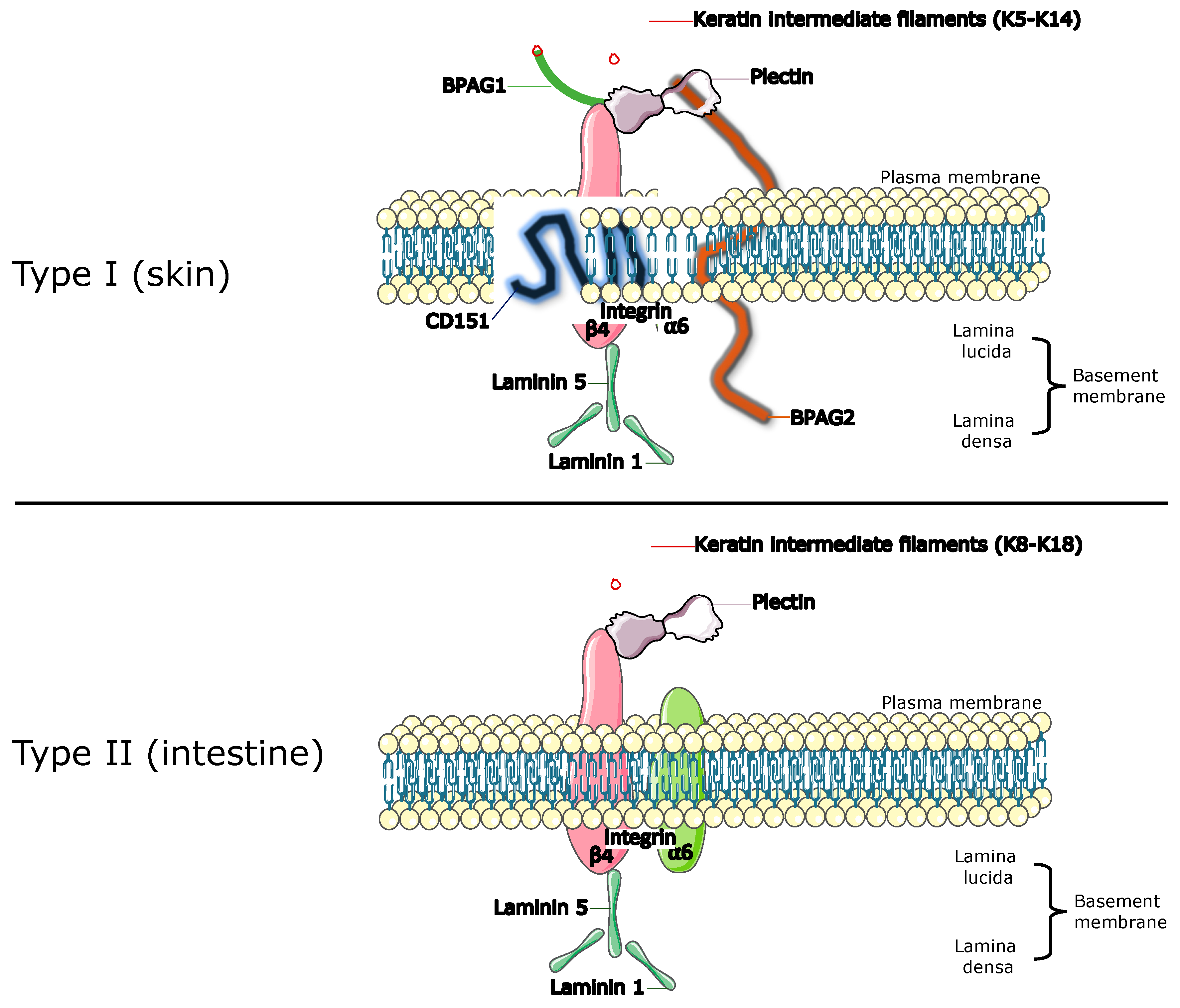
3.3. Hemidesmosome Complex
3.3.1. α6β4. Heterodimer
3.3.2. Plectin
3.3.3. BPA1/BPAG1e/BP230/Dystonin
3.3.4. BPAG2/Col17/BP180/COL17A1
3.3.5. CD151
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| #1 | Hemidesmosom* or half-desmosom* or hemi-desmosom |
| #2 | Oral or mouth or tongue or mucosa* or palate or lip or gum* or gingiva* or alveolar or buccal or retromolar or floor of* |
| #3 | Cancer or carcinogenesis or neoplasm or malignant or cancer stage or oral potentially malignant dis* or OPMD or squamous cell carcinoma or neoplastic cell transformation or OSCC or dysplas* |
| #4 | Leukoplakia or submucous fibrosis or erythroplakia or lichen planus or erythroleukoplakia or proliferative verrucous leukoplakia or lupus erythematosus or dyskeratosis congenita or actinic cheilitis or chronic hyperplastic candidosis or exophytic verrucous hyperplasia or graft vs. host disease or actinic keratosis or actinic cheilitis or palatal lesions in reverse smokers or lichenoid lesions |
| #5 | #1 and #2 and #3 |
| #6 | #1 and #2 and #4 |
| #7 | #5 or #6 |
| Potential Bias | First Author Surname, Year | ||||||||||
| Chaudhari et al. (2017), India [38] | Cheng et al. (2002), UK [16] | Dmello et al. (2016), India, Norway, USA [39] | Downer et al. (1993), UK [24] | Frithiof (1972), Sweden [17] | Garzino-Demo et al. (1998), Italy [25] | Haapalainen et al. (1995), Finland, Canada [26] | Herold-Mende et al. (2001), Germany [27] | Jones et al. (1996), Netherlands [28] | Jones et al. (1993), United Kingdom [29] | ||
| Selection | Q1. Was administered dose or exposure level adequately randomised? | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ |
| Q2. Was allocation to study groups adequately concealed? | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | |
| Performance | Q3. Were experimental conditions identical across study groups? | ↓ | ↓ | ↑ | ↓ | ↑ | ↓ | ↓ | ↓ | ↑ | ↓ |
| Q4. Were experimental conditions identical across study groups? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| Attrition/exclusion | Q5. Were outcome data complete without attrition or exclusion from the analysis? | ↓ | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ |
| Q6. Can we be confident in the exposure characterisation? | ↓ | ↑↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| Detection | Q7. Can we be confident in the outcome assessment? | ↓ | ↑↑ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ |
| Selective reportion | Q8. Were all measured outcomes reported? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ |
| Other biases | Q9. Statistics: were statistical methods appropriate? | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ |
| Potential bias | First Author Surname, Year | ||||||||||
| Kanojia et al. (2012), India [30] | Meng et al. (2021), Japan [42] | Nagata et al. (2013), Japan [31] | Parikka et al. (2003), Finland [40] | Parikka et al. (2006), Finland [41] | Sawant et al. (2017), India [32] | Sawant et al. (2000), India [18] | Schreurs et al. (2020), Norway [33] | Shklar, Flynn & Szabo (1978), USA [19] | Sugiyama et al. (1993), UK [34] | ||
| Selection | Q1. Was administered dose or exposure level adequately randomised? | ↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ |
| Q2. Was allocation to study groups adequately concealed? | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | |
| Performance | Q3. Were experimental conditions identical across study groups? | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | ↑ |
| Q4. Were experimental conditions identical across study groups? | ↓ | ↓↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| Attrition/exclusion | Q5. Were outcome data complete without attrition or exclusion from the analysis? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Q6. Can we be confident in the exposure characterisation? | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| Detection | Q7. Can we be confident in the outcome assessment? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Selective reportion | Q8. Were all measured outcomes reported? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Other biases | Q9. Statistics: were statistical methods appropriate? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ |
| Potential bias | First Author Surname, Year | ||||||||||
| Takkunen et al. (2008), Finland [35] | Takkunen et al. (2006), Finland [36] | White et al. (1984), UK [21] | White et al. (1981), UK [20] | Witjes et al. (1995), Netherland [37] | Xin et al. (2014), Japan [6] | ||||||
| Selection | Q1. Was administered dose or exposure level adequately randomised? | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ||||
| Q2. Was allocation to study groups adequately concealed? | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | |||||
| Performance | Q3. Were experimental conditions identical across study groups? | ↑ | ↓ | ↓↓ | ↓ | ↑ | ↓ | ||||
| Q4. Were experimental conditions identical across study groups? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||
| Attrition/exclusion | Q5. Were outcome data complete without attrition or exclusion from the analysis? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||
| Q6. Can we be confident in the exposure characterisation? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||
| Detection | Q7. Can we be confident in the outcome assessment? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||
| Selective reportion | Q8. Were all measured outcomes reported? | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||
| Other biases | Q9. Statistics: were statistical methods appropriate? | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ||||
| Progressive Number | Author, Year, Country | Hemidesmosomal Component | Anatomical Location | Cell + Experimental Model | OPMD | Dysplasia | OSCC Characteristics | Main Outcomes/Findings Reported |
|---|---|---|---|---|---|---|---|---|
| 1 | Chaudhari et al. (2017), India [38] | Plectin, BPAG1e | Tongue | OSCC cell lines and mice In vitro: qRT-PCR, WB, IFL, SA, TMIC and BCCIA, SAA, MTT cell viability assay, CGA, GZG, RhoA/Rac1/Cdc42 activation assay, fractionation and quantification of F-Actin, SWATH analysis In vivo: Tumourigenicity assay | N/A | N/A | TNM Staging Classification 50-year-old male—T3N0M0 35-year-old male—T4N1M0 Unspecified Grading System 50-year-old male—PD 35-year-old male—PD to MD |
|
| 2 | Cheng et al. (2002), UK [16] | Entire hemidesmosomes | Oral mucosa | Normal, leukoplakia, OSCC and normal margins adjacent to OSCC specimens In vitro: TEM | Leukoplakia | Severe | Unspecified Stage and Staging Classification Unspecified Grading System WD |
|
| 3 | Dmello et al. (2016), India, Norway, USA [39] | β4, plectin | Oral mucosa | In vitro: RT-PCR, WB, IP, SWMA SAA, ZG, IFL, CA, MISA, EMT induction and stimulation of β4 signalling, IEM, IHC | N/A | N/A | Unspecified Staging Classification n = 20 < Stage III, n = 54 ≥ III Unspecified Grading Classification n = 67 PD and MD, n = 7 WD |
|
| 4 | Downer et al. (1993), UK [24] | α6, β4 | Retromolar Pad Oral mucosa | Normal, hyperproliferative and OSCC specimens In vitro: IHC, IPX, double label IFL | N/A | N/A | Unspecified Staging System Grading based on differentiation n = 10 MD or WD, n = 5 PD |
|
| 5 | Frithiof (1972), Sweden [17] | Entire hemidesmosomes | Gingival tissues Oral mucosa | Normal, hyperplastic, pre-invasive and invasive OSCC specimens In vitro: EM | N/A | N/A | Staging by pre-invasive vs. invasive n = 10 pre-invasive OSCC, n = 13 invasive OSCC Unspecified Grading Classification |
|
| 6 | Garzino-Demo et al. (1998), Italy [25] | α6β4 | Retromolar Pad Oral mucosa | Normal, OL and OSCC samples In vitro: IPX, WB | Leukoplakia | Mild, moderate or severe | Unspecified Stage and Staging Classification Grading by Differentiation n = 1 PD, n = 5 MD, n = 2 WD |
|
| 7 | Haapalainen et al. (1995), Finland, Canada [26] | α6β4 | BM | Reticular OLP and bullous OLP samples In vitro: IHC, IFL | OLP | N/A | N/A |
|
| 8 | Herold-Mende et al. (2001), Germany [27] | α6β4 BPA1 | FoM Uvula | H&N epithelial tissues for tumour patients In vitro: IIF, ISH, EM | N/A | 6 cases of mild to moderate dysplasia 2 cases of severe dysplasia | TNM Staging System n = 2 T1 to T3 n = 1 T3N1M0 to T4N3M1 Unspecified Grading Classification MD FoM Carcinoma WD Tongue Carcinoma |
|
| 9 | Jones et al. (1996), Netherlands [28] | α6β4 | FoM | OSCC Cell Lines (H376) In vitro: CC, SAA, CT, CE, MAD, FACS, FC, CA assays, IB, IFL | N/A | N/A | OSCC present Unspecified Stage and Staging Classification Unspecified Grade and Grading System |
|
| 10 | Jones et al. (1993), United Kingdom [29] | α6β4 | Cheek Hard palate Lateral tongue FoM | Normal and OSCC specimens In vitro: IHC | N/A | Mild or moderate | Unspecified Stage and Staging System Unspecified Grading System 10 MD and 7 PD specimens |
|
| 11 | Kanojia et al. (2012), India [30] | α6, β4 | Dorsal tongue Buccal mucosa | Dissected tissues that were exposed to 4NQO In vivo: Tumour induction via 4NQO exposure. SDS PAGE. RT-PCR, IHC, IFL | N/A | Rats treated with 4NQO for 80/120 days | OSCC present Unspecified Stage and Staging Classification Unspecified Grade and Grading System and Grading |
|
| 12 | Meng et al. (2021), Japan [42] | BP180 | Tongue FoM Gingiva | FFPE tumour specimens In vitro: TMAC, IHC Cohort Study | N/A | N/A | UICC TNM Staging Classification (7th edition) n = 70 N0, n = 28 N1-N3, n = 59 T1 + T2, n = 39 T3 + T4, n = 43 Clinical stage I + II, n = 55 Clinical stage III + IV Unspecified Grade and Grading System |
|
| 13 | Nagata et al. (2013), Japan [31] | β4 | Lips and Oral Cavity | OSCC biopsies In vitro: RT-qPCR Cohort Study | N/A | N/A | UICC TNM Staging Classification (7th edition) n = 83 T1, n = 123 T2, n = 5 T3, n = 59 T4, n = 149 N0, n = 41 N1, n = 80 N2, n = 14 M1 YK Grading Classification (Yamamoto et al. 1983) n = 115 1–3, n = 155 4c-d |
|
| 14 | Parikka et al. (2003), Finland [40] | Collagen XVII | Tongue, BM | Normal and OSCC cell lines In vitro: IHC, WB, IP, ISH, RT-PCR | N/A | WHO 3-tier grading of oral dysplasia | Unspecified Stage and Staging Classifications Unspecified Grading Classifications n = 15 MD, n = 5 PD |
|
| 15 | Parikka et al. (2006), Finland [41] | Collagen XVII | Tongue, BM | Buccal mucosa tissue, SCC tumours, human tongue SCC cell line HSC-3 In vitro: IHC, FC, IP, Transmigration Assay | N/A | N/A | OSCC present Unspecified Stage and Staging Classification Unspecified Grade and Grading System |
|
| 16 | Sawant et al. (2017), India [2] | β4 | BM, tongue | FFPE, glutaraldehyde/osmium tetroxide-fixed and araldite embedded In vitro: IHC, TEM | N/A | N/A | TMN Staging System n = 27 < T3, n = 48 ≥ T3 Unspecified Grading System n = 24 PD, n = 44 MD, n = 7 WD |
|
| 17 | Sawant et al. (2000), India [18] | Entire hemidesmosome | Cheek pouch | Hamster cheek pouch tumours In vivo: TEM, Optical | N/A | Marked to massive | OSCC present Unspecified Stage and Staging Classification Unspecified Grade and Grading System |
|
| 18 | Schreurs et al. (2020), Norway [33] | α6β4, CD151, Plectin 1a, Col17A1, Dystonin | Oral buccal mucosa | OLP biopsy In vitro: Biopsy, FFPE and cryogenic freezing, IFL staining | OLP | N/A | N/A |
|
| 19 | Shklar, Flynn & Szabo (1978), USA [19] | Entire hemidesmosome | Tongue and buccal mucosa | OLP biopsy In vitro: Biopsy, EM | OLP | N/A | N/A |
|
| 20 | Sugiyama et al. (1993), UK [34] | α6, β4 | Tongue, BM, FoM, AP | Normal and SCC human keratinocyte cell lines In vitro: FC, IHC, CA, IFL | N | N/A | Unspecified Staging System I: tumour ≤ 2 cm in dimension; no regional lymph node involvement, no metastases II: tumour > 2 cm, ≤ 4 cm, no regional lymph node involvement, no metastases III: tumour ≤ 4 cm, regional lymph node involvement, no distant metastases Unspecified Grading System WD and MD |
|
| 21 | Takkunen et al. (2008), Finland [35] | α6β4 | Gingiva | OSCC UT-SCC-43A (43A), OSCC UT-SCC-43B (43B), Snail-transfected 43A-SNA In vitro: IFL, IP, WB | N/A | N/A | TMN Staging System T4N1M0 Unspecified Grade and Grading System |
|
| 22 | Takkunen et al. (2006), Finland [36] | α6β4 | Mandibular gingiva | Primary tumour (43A) and recurrent tumour (43B) In vitro: IFL, IP, WB, NB | N/A | N/A | N/A |
|
| 23 | White et al. (1984), UK [21] | Entire Hemidesmosome | Cheek pouches | Cheek pouch epithelium administered with 0.5% DMBA In vivo: Stereology | N/A | Mild to severe | N/A |
|
| 24 | White et al. (1981), UK [20] | Entire Hemidesmosome | Cheek pouch | In vivo: Cheek pouch epithelium administered with 0.5% DMBA | Y | Unspecified | N/A |
|
| 25 | Witjes et al. (1995), Netherland [37] | α6β4 | Palate | UHG-RaC’93 In vitro: IFL, Gel electrophoresis | Not specified | EAI | N/A |
|
| 26 | Xin et al. (2014), Japan [6] | β4, COL17A1 | Tongue, FoM, BM | OSCC tissues fixed in 10% formalin and placed in paraffin In vitro: TMAC, IHC | N/A | N/A | OSCC present Unspecified Stage and Staging Classification Unspecified Grade and Grading System and Grading |
|
| Reference Number/Progressive Number | First Author Surname et al. (Year), Country | Hemidesmosomal Component | Anatomical Location | Cell + Experimental Model | OPMD Present | Dysplasia | OSCC Characteristics | Main Outcomes/Findings Reported |
|---|---|---|---|---|---|---|---|---|
| 3 | Dmello et al. (2016), India, Norway, USA [39] | β4, plectin | Oral mucosa | In vitro: RT-PCR, WB, IP, SWMA SAA, ZG, IFL, CA, MISA, EMT induction and stimulation of β4 integrin signalling, IEM, IHC | N/A | N/A | Unspecified Staging Classification n = 20 < Stage III, n = 54 ≥ III Unspecified Grading Classification n = 67 PD and MD, n = 7 WD |
|
| 11 | Meng et al. (2021), Japan [42] | BP180 | Tongue FoM Gingiva | FFPE tumour specimens In vitro: TMAC, IHC Cohort Study | N/A | N/A | UICC TNM Staging Classification (7th edition) n = 70 N0, n = 28 N1-N3, n = 59 T1 + T2, n = 39 T3 + T4, n = 43 Clinical stage I + II, n = 55 Clinical stage III + IV Unspecified Grade and Grading System |
|
| 12 | Nagata et al. (2013), Japan [31] | β4 | Lips and Oral Cavity | OSCC biopsies In vitro: RT-qPCR Cohort Study | N/A | N/A | UICC TNM Staging Classification (7th edition) n = 83 T1, n = 123 T2, n = 5 T3, n = 59 T4, n = 149 N0, n = 41 N1, n = 80 N2, n = 14 M1 YK Grading Classification (Yamamoto et al. 1983) n = 115 1–3, n = 155 4c-d |
|
| N. of Studies with Increased Expression (Refs) | N. of Studies with Decreased Expression (Refs) | N. of Studies with Loss of Expression (Refs) | ||||
|---|---|---|---|---|---|---|
| OPMDs | OSCC | OPMDs | OSCC | OPMDs | OSCC | |
| Hemidesmosomal complex | 0 | 0 | 1 (19) | 3 (18; 20; 21) | 0 | 2 (16; 17) |
| α6 | 1 (25) | 2 (25; 30) | 0 | 0 | 0 | 1 (29) |
| β4 | 1 (33) | 1 (25) | 1 (25) | 1 (32) | 0 | 1 (29) |
| α6β4 | 1 (29; 33) | 1 (26) | 1 (26) | 1 (34) | 0 | 1 (36) |
| Plectin | 1 (33) | 0 | 0 | 0 | 0 | 0 |
| BPAG1e | 0 | 2 (26; 33) | 0 | 0 | 0 | 0 |
| BPAG2 | 3 (33; 40;42) | 2 (6; 40) | 0 | 0 | 0 | 0 |
| CD151 | 1 (33) | 0 | 0 | 0 | 0 | |
Appendix B. Summary of Abbreviations
| Abbreviations: | Full form: |
| 4NQO | 4 Nitroquinoline 1 Oxide |
| Arp | Actin Related Proteins |
| ATR-OLP | Atrophic Oral Lichen Planus |
| BCCIA | Boyden Chamber Cell Invasion Assay |
| BM | Buccal Mucosa |
| BP180 | Bullous Pemphigoid Antigen II/Collagen XVII/COL17A1/BPA 2/BPAg2 |
| BPA1 | Bullous Pemphigoid Antigen 1 |
| BPAG1e | Bullous Pemphigoid Antigen 1e |
| BPM | Basal Plasma Membrane |
| CA | Cell Adhesion |
| CC | Cell Culture |
| CE | Clonal Expansion |
| COL17A1 | Collagen Type 17 Alpha 1 |
| DFS | Disease-Free Survival |
| DMBA | 7,12 Dimethylbenz(α)anthracene |
| EAI | Epithelial Atypia Index |
| EGFR | Epidermal Growth Factor Receptor |
| EM | Electron Microscopy |
| EMT | Epithelial Mesenchymal Transition |
| FACS | Fluorescence-Activated Cell Sorting |
| FC | Flow Cytometry |
| FFPE | Formalin Fixed Paraffin Embedded tissue |
| FoM | Floor of Mouth |
| GZG | Gelatin Zymography |
| H&N | Head and Neck |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| H376t+ | H376 line cells transfected with an α6β4 vector |
| IEM | Immune-Electron Microscopy |
| IF | Intermediate Filament |
| IFL | Immunofluorescence |
| IHC | Immunohistochemistry |
| IIF | Indirect Immunofluorescence |
| IP | Immunoprecipitation |
| IPX | Immunoperoxidase |
| ISH | In Situ Hybridisation |
| JUP | Plakoglobin |
| L1 | Laminin 1 |
| L5 | Laminin 5 |
| LMN | Lymph Node Metastasis |
| MAD | Monoclonal Antibody Detection |
| MAPK | Mitogen-Activated Protein Kinase |
| MD | Moderately Differentiated |
| MISA | Migration, Invasion and Spreading Assays |
| MMP | Matrix Metallopeptidase |
| MTT Cell Viability Assay | 3 (4,5 dimethylthiazol 2 yl) 2,5 diphenyl 2H tetrazolium bromide cell viability assay |
| NB | Northern Blotting |
| NDRG1 | N Myc downstream-regulated gene 1 |
| OL | Oral Leukoplakia |
| OLP | Oral Lichen Planus |
| OSCC | Oral Squamous Cell Carcinomas |
| PD | Poorly Differentiated |
| qRT- PCR | Quantitative Real-Time Polymerase Chain Reaction |
| RT-qPCR | Quantitative Real-Time Polymerase Chain Reaction |
| SCC | Squamous Cell Carcinoma |
| SDS PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| SWATH | Sequential Window Acquisition Of All Theoretical Fragment Ion Spectra |
| SWMA | Scratch Wound Migration Assay |
| TEM | Transmission Electron Microscopy |
| TMAC | Tissue Microassay Construction |
| UICC | Union For International Cancer Control |
| WB | Western Blot |
| WD | Well Differentiated |
| YK | Yamamoto Kohama |
| ZG | Zymography |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, D.; Sreenivasan, P.; Öhman, J.; Wallström, M.; Braz-Silva, P.H.; Giglio, D.; Kjeller, G.; Hasséus, B. Potentially Malignant Oral Disorders and Cancer Transformation. Anticancer Res. 2018, 38, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Woo, S.-B.; Zain, R.B.; Sklavounou, A.; McCullough, M.J.; Lingen, M. Oral cancer and oral potentially malignant disorders. Int. J. Dent. 2014, 2014, 853479. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ramadas, K.; Amarasinghe, H.; Subramanian, S.; Johnson, N. Oral Cancer: Prevention, Early Detection, and Treatment. In Cancer: Disease Control Priorities, 3rd ed.; Gelband, H., Jha, P., Sankaranarayanan, R., Horton, S., Eds.; The World Bank: Washington, DC, USA, 2015; Volume 3. [Google Scholar]
- Xin, Z.; Yamaguchi, A.; Sakamoto, K. Aberrant expression and altered cellular localization of desmosomal and hemidesmosomal proteins are associated with aggressive clinicopathological features of oral squamous cell carcinoma. Virchows Arch. 2014, 465, 35–47. [Google Scholar] [CrossRef]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 363–378. [Google Scholar] [CrossRef]
- Fontao, L.; Stutzmann, J.; Gendry, P.; Launay, J.F. Regulation of the Type II Hemidesmosomal Plaque Assembly in Intestinal Epithelial Cells. Exp. Cell Res. 1999, 250, 298–312. [Google Scholar] [CrossRef]
- Alroy, J.; Gould, V.E. Epithelial-stromal interface in normal and neoplastic human bladder epithelium. Ultrastruct. Pathol. 1980, 1, 201–210. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yamanishi, Y.; Dabbous, M.K. Electron microscopic observations of collagenolytic activity of basal cell epithelioma of the skin in vivo and in vitro. Cancer Res. 1972, 32, 2561–2567. [Google Scholar]
- Deporter, D.A.; ten Cate, A.R. Fine structural localization of acid and alkaline phosphatase in collagen-containing vesicles of fibroblasts. J. Anat. 1973, 114, 457–461. [Google Scholar]
- Lazarus, G.S.; Daniels, J.R.; Brown, R.S.; Bladen, H.A.; Fullmer, H.M. Degradation of collagen by a human granulocyte collagenolytic system. J. Clin. Investig. 1968, 47, 2622–2629. [Google Scholar] [CrossRef]
- Werb, Z.; Gordon, S. Secretion of a specific collagenase by stimulated macrophages. J. Exp. Med. 1975, 142, 346–360. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Ng, Y.Y.; Tarleton, K.; Tran, A.; Tran, T.; Xue, W.Y.; Youssef, P.; Yuan, P.; Zhang, D.; et al. Milk-Derived Proteins and Peptides in Head and Neck Carcinoma Treatment. Biomolecules 2022, 12, 290. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Cheng, L.H.; Hudson, J. Ultrastructural changes in malignant transformation of oral mucosa. Br. J. Oral Maxillofac. Surg. 2002, 40, 207–212. [Google Scholar] [CrossRef]
- Frithiof, L. Electron Microscopic Observations on Structures Related to the Epithelial Basement Membrane in Squamous Cell Carcinoma. Acta Oto-Laryngol. 1972, 73, 323–334. [Google Scholar] [CrossRef]
- Sawant, S.S.; Kandarkar, S.V. Role of vitamins C and E as chemopreventive agents in the hamster cheek pouch treated with the oral carcinogen-DMBA. Oral Dis. 2000, 6, 241–247. [Google Scholar] [CrossRef]
- Shklar, G.; Flynn, E.; Szabo, G. Basement membrane alterations in oral lichen planus. J. Investig. Dermatol. 1978, 70, 45–50. [Google Scholar] [CrossRef]
- White, F.H.; Gohari, K. Quantitative studies of hemidesmosomes during progressive DMBA carcinogenesis in hamster cheek-pouch mucosa. Br. J. Cancer 1981, 44, 440–450. [Google Scholar] [CrossRef]
- White, F.H.; Gohari, K. Hemidesmosomal dimensions and frequency in experimental oral carcinogenesis: A stereological investigation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1984, 45, 1–13. [Google Scholar] [CrossRef]
- Green, K.J.; Jones, J.C. Desmosomes and hemidesmosomes: Structure and function of molecular components. FASEB J. 1996, 10, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Degenstein, L.; Dowling, J.; Yu, Q.-C.; Wollmann, R.; Perman, B.; Fuchs, E. Gene targeting of BPAG1: Abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell 1995, 81, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Downer, C.S.; Watt, F.M.; Speight, P.M. Loss of alpha 6 and beta 4 integrin subunits coincides with loss of basement membrane components in oral squamous cell carcinomas. J. Pathol. 1993, 171, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Garzino-Demo, P.; Carrozzo, M.; Trusolino, L.; Savoia, P.; Gandolfo, S.; Marchisio, P.C. Altered expression of alpha 6 integrin subunit in oral squamous cell carcinoma and oral potentially malignant lesions. Oral Oncol. 1998, 34, 204–210. [Google Scholar] [CrossRef]
- Haapalainen, T.; Oksala, O.; Kallioinen, M.; Oikarinen, A.; Larjava, H.; Salo, T. Destruction of the epithelial anchoring system in lichen planus. J. Investig. Dermatol. 1995, 105, 100–103. [Google Scholar] [CrossRef]
- Herold-Mende, C.; Kartenbeck, J.; Tomakidi, P.; Bosch, F.X. Metastatic growth of squamous cell carcinomas is correlated with upregulation and redistribution of hemidesmosomal components. Cell Tissue Res. 2001, 306, 399–408. [Google Scholar] [CrossRef]
- Jones, J.; Sugiyama, M.; Giancotti, F.; Speight, P.M.; Watt, F.M. Transfection of beta 4 integrin subunit into a neoplastic keratinocyte line fails to restore terminal differentiation capacity or influence proliferation. Cell Adhes. Commun. 1996, 4, 307–316. [Google Scholar] [CrossRef]
- Jones, J.; Sugiyama, M.; Watt, F.M.; Speight, P.M. Integrin expression in normal, hyperplastic, dysplastic, and malignant oral epithelium. J. Pathol. 1993, 169, 235–243. [Google Scholar] [CrossRef]
- Kanojia, D.; Sawant, S.S.; Borges, A.M.; Ingle, A.D.; Vaidya, M.M. Alterations in keratins and associated proteins during 4- Nitroquinoline-1-oxide induced rat oral carcinogenesis. J. Carcinog. 2012, 11, 14. [Google Scholar]
- Nagata, M.; Noman, A.A.; Suzuki, K.; Kurita, H.; Ohnishi, M.; Ohyama, T.; Kitamura, N.; Kobayashi, T.; Uematsu, K.; Takahashi, K.; et al. ITGA3 and ITGB4 expression biomarkers estimate the risks of locoregional and hematogenous dissemination of oral squamous cell carcinoma. BMC Cancer 2013, 13, 410. [Google Scholar] [CrossRef]
- Sawant, S.; Dongre, H.; Ahire, C.; Sharma, S.; Kannan, S.; Mahadik, S.; Chaukar, D.; Lukmani, F.; Patil, A.; D’Cruz, A.; et al. A nomogram for predicting the risk of neck node metastasis in pathologically node-negative oral cavity carcinoma. Oral Dis. 2017, 23, 1087–1098. [Google Scholar] [CrossRef]
- Schreurs, O.; Balta, M.G.; Karatsaidis, A.; Schenck, K. Composition of hemidesmosomes in basal keratinocytes of normal buccal mucosa and oral lichen planus. Eur. J. Oral Sci. 2020, 128, 369–378. [Google Scholar] [CrossRef]
- Sugiyama, M.; Speight, P.M.; Prime, S.S.; Watt, F.M. Comparison of integrin expression and terminal differentiation capacity in cell lines derived from oral squamous cell carcinomas. Carcinogenesis 1993, 14, 2171–2176. [Google Scholar] [CrossRef]
- Takkunen, M.; Ainola, M.; Vainionpää, N.; Grenman, R.; Patarroyo, M.; de Herreros, A.G.; Konttinen, Y.T.; Virtanen, I. Epithelial-mesenchymal transition downregulates laminin alpha5 chain and upregulates laminin alpha4 chain in oral squamous carcinoma cells. Histochem. Cell Biol. 2008, 130, 509–525. [Google Scholar] [CrossRef]
- Takkunen, M.; Grenman, R.; Hukkanen, M.; Korhonen, M.; García de Herreros, A.; Virtanen, I. Snail-dependent and -independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J. Histochem. Cytochem. 2006, 54, 1263–1275. [Google Scholar] [CrossRef]
- Witjes, M.; Scholma, J.; van Drunen, E.; Roodenburg, J.L.; Mesander, G.; Hagemeijer, A.; Tomson, A.M. Characterization of a rat oral squamous cell carcinoma cell line UHG-RaC ’93 induced by 4-nitroquinoline-1-oxide in vivo. Carcinogenesis 1995, 16, 2825–2832. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Charles, S.E.; D’Souza, Z.C.; Vaidya, M.M. Hemidesmosomal linker proteins regulate cell motility, invasion and tumorigenicity in oral squamous cell carcinoma derived cells. Exp. Cell Res. 2017, 360, 125–137. [Google Scholar] [CrossRef]
- Dmello, C.; Sawant, S.; Alam, H.; Gangadaran, P.; Tiwari, R.; Dongre, H.; Rana, N.; Barve, S.; Costea, D.E.; Chaukar, D.; et al. Vimentin-mediated regulation of cell motility through modulation of beta4 integrin protein levels in oral tumor derived cells. Int. J. Biochem. Cell Biol. 2016, 70, 161–172. [Google Scholar] [CrossRef]
- Parikka, M.; Kainulainen, T.; Tasanen, K.; Väänänen, A.; Bruckner-Tuderman, L.; Salo, T. Alterations of collagen XVII expression during transformation of oral epithelium to dysplasia and carcinoma. J. Histochem. Cytochem. 2003, 51, 921–929. [Google Scholar] [CrossRef]
- Parikka, M.; Nissinen, L.; Kainulainen, T.; Bruckner-Tuderman, L.; Salo, T.; Heino, J.; Tasanen, K. Collagen XVII promotes integrin-mediated squamous cell carcinoma transmigration—A novel role for alphaIIb integrin and tirofiban. Exp. Cell Res. 2006, 312, 1431–1438. [Google Scholar] [CrossRef]
- Meng, X.; Matsumoto, F.; Mori, T.; Miura, N.; Ino, Y.; Onidani, K.; Kobayashi, K.; Matsuzaki, Y.; Yoshimoto, S.; Ikeda, K.; et al. BP180 Is a Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2021, 41, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Te Molder, L.; Juksar, J.; Harkes, R.; Wang, W.; Kreft, M.; Sonnenberg, A. Tetraspanin CD151 and integrin α3β1 contribute to the stabilization of integrin α6β4-containing cell-matrix adhesions. J. Cell Sci. 2019, 132, jcs235366. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, X.; Wang, H.; Li, L.; Zhang, C.; Xiang, R.; Tan, X.; Li, Z.; Jiang, C.; Zheng, L.; et al. High TSPAN8 expression in epithelial cancer cell-derived small extracellular vesicles promote confined diffusion and pronounced uptake. J. Extracell. Vesicles 2021, 10, e12167. [Google Scholar] [CrossRef]
- Kannan, S.; Balaram, P.; Chandran, G.J.; Pillai, M.R.; Pillai, K.R.; Nalinakumari, K.; Nair, M.K.; Kartha, C. Ultrastructural analysis of the adjacent epithelium of oral squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 1996, 34, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bouameur, J.E.; Schneider, Y.; Begré, N.; Hobbs, R.P.; Lingasamy, P.; Fontao, L.; Green, K.J.; Favre, B.; Borradori, L. Phosphorylation of serine 4,642 in the C-terminus of plectin by MNK2 and PKA modulates its interaction with intermediate filaments. J. Cell Sci. 2013, 126, 4195–4207. [Google Scholar]
- de Vicente, J.C.; Fresno, M.F.; Villalain, L.; Vega, J.A.; Vallejo, G.H. Expression and clinical significance of matrix metalloproteinase-2 and matrix metalloproteinase-9 in oral squamous cell carcinoma. Oral Oncol. 2005, 41, 283–293. [Google Scholar] [CrossRef]
- Georgopoulou, M.; Tosios, K.; Goutas, N.; Kouloukoussa, M. Arp2/3 Complex Is Expressed in Oral Squamous Cell Carcinoma: An Immunohistochemical Study of 88 Cases. Open J. Stomatol. 2019, 9, 29–38. [Google Scholar] [CrossRef]
- Liu, S.; Liu, L.; Ye, W.; Ye, D.; Wang, T.; Guo, W.; Liao, Y.; Xu, D.; Song, H.; Zhang, L.; et al. High Vimentin Expression Associated with Lymph Node Metastasis and Predicated a Poor Prognosis in Oral Squamous Cell Carcinoma. Sci. Rep. 2016, 6, 38834. [Google Scholar] [CrossRef]
- Jones, J.C.; Hopkinson, S.B.; Goldfinger, L.E. Structure and assembly of hemidesmosomes. Bioessays 1998, 20, 488–494. [Google Scholar] [CrossRef]
- Mogilner, A.; Oster, G. Cell motility driven by actin polymerization. Biophys. J. 1996, 71, 3030–3045. [Google Scholar] [CrossRef]
- Geuijen, C.A.; Sonnenberg, A. Dynamics of the α6β4 integrin in keratinocytes. Mol. Biol. Cell 2002, 13, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- te Molder, L.; de Pereda, J.M.; Sonnenberg, A. Regulation of hemidesmosome dynamics and cell signaling by integrin α6β4. J. Cell Sci. 2021, 134, jcs259004. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Mainiero, F.; Murgia, C.; Wary, K.K.; Curatola, A.M.; Pepe, A.; Blumemberg, M.; Westwick, J.K.; Der, C.J.; Giancotti, F.G. The coupling of α6β4 integrin to Ras–MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997, 16, 2365–2375. [Google Scholar] [CrossRef]
- O’Connor, K.L.; Chen, M.; Towers, L.N. Integrin α6β4 cooperates with LPA signaling to stimulate Rac through AKAP-Lbc-mediated RhoA activation. Am. J. Physiol. Cell Physiol. 2012, 302, C605–C614. [Google Scholar] [CrossRef]
- O’Connor, K.L.; Nguyen, B.-K.; Mercurio, A.M. RhoA function in lamellae formation and migration is regulated by the α6β4 integrin and cAMP metabolism. J. Cell Biol. 2000, 148, 253–258. [Google Scholar] [CrossRef]
- Shaw, L.M.; Rabinovitz, I.; Wang, H.H.-F.; Toker, A.; Mercurio, A.M. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell 1997, 91, 949–960. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, J.; Chong, T.W.; Elmi, H.; Ma, J.; Madi, J.; Mamgain, A.; Melendez, E.; Messina, J.; Mongia, N.; Nambiar, S.; et al. Role of Hemidesmosomes in Oral Carcinogenesis: A Systematic Review. Cancers 2023, 15, 2533. https://doi.org/10.3390/cancers15092533
Nguyen J, Chong TW, Elmi H, Ma J, Madi J, Mamgain A, Melendez E, Messina J, Mongia N, Nambiar S, et al. Role of Hemidesmosomes in Oral Carcinogenesis: A Systematic Review. Cancers. 2023; 15(9):2533. https://doi.org/10.3390/cancers15092533
Chicago/Turabian StyleNguyen, Jordan, Tze Wei Chong, Hafsa Elmi, Jiani Ma, John Madi, Asha Mamgain, Eileen Melendez, Julian Messina, Nikhil Mongia, Sanjana Nambiar, and et al. 2023. "Role of Hemidesmosomes in Oral Carcinogenesis: A Systematic Review" Cancers 15, no. 9: 2533. https://doi.org/10.3390/cancers15092533
APA StyleNguyen, J., Chong, T. W., Elmi, H., Ma, J., Madi, J., Mamgain, A., Melendez, E., Messina, J., Mongia, N., Nambiar, S., Ng, T. J., Nguyen, H., McCullough, M., Canfora, F., O’Reilly, L. A., Cirillo, N., Paolini, R., & Celentano, A. (2023). Role of Hemidesmosomes in Oral Carcinogenesis: A Systematic Review. Cancers, 15(9), 2533. https://doi.org/10.3390/cancers15092533









