Tumor Organoid and Spheroid Models for Cervical Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. 2D Cancer Models and Importance of TME
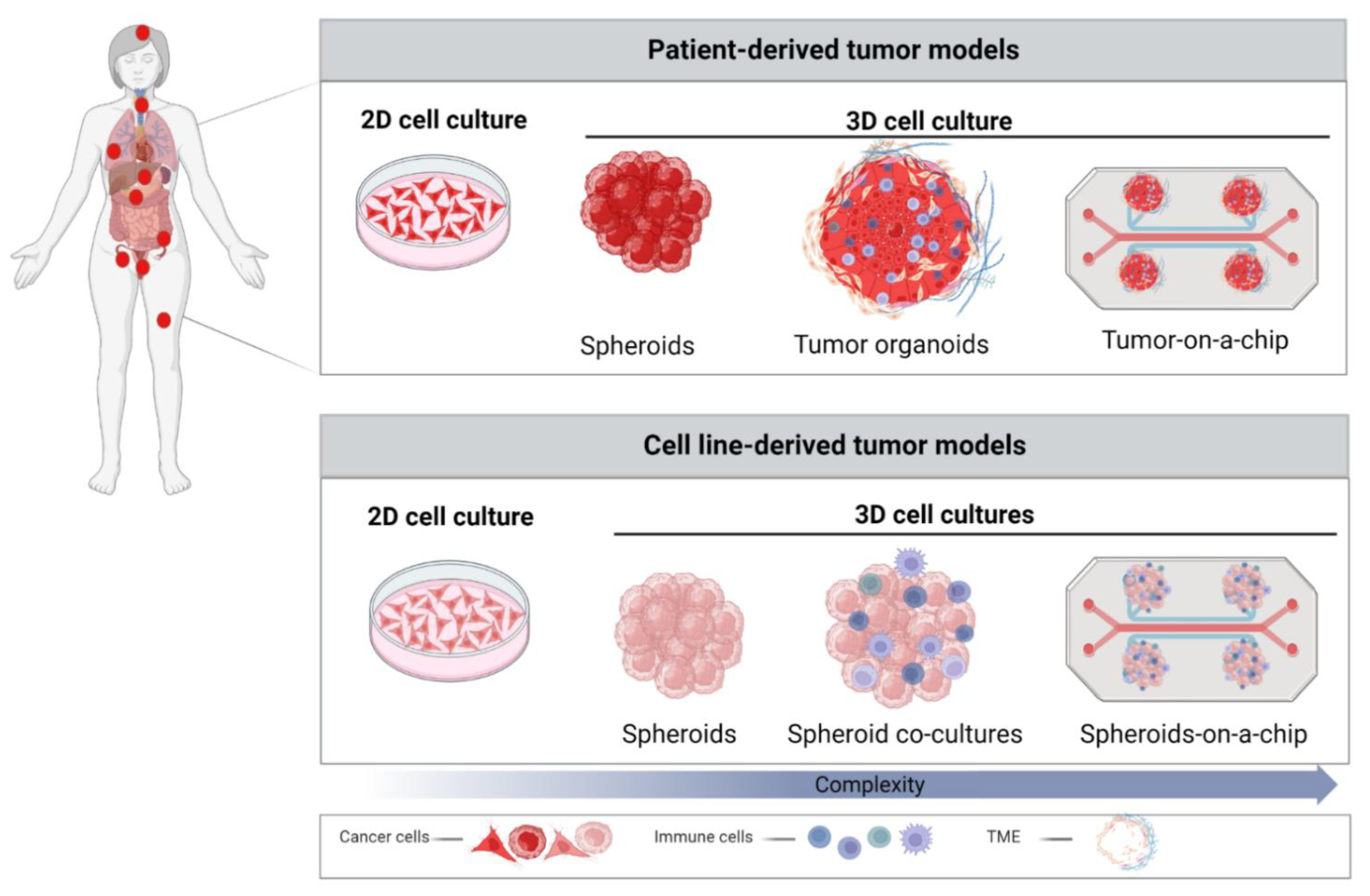
3. Role of 3D Models in Cancer Research
3.1. Spheroids
3.2. Patient-Derived Organoids (PDOs)
| Cervical Cancer In Vitro Models | Cell Origin | Application | Advantage | Limitation |
|---|---|---|---|---|
 | CC cell lines CaSki HeLa SiHa C-33-A | Disease modeling [90] Drug screening [91] Immunotherapy [91] Anti-HPV vaccine [92] | Simple and low-cost maintenance Well-established Simple analysis Long-term cultures Reproducible High-throughput potential | Lack of cell–cell and cell–ECM interaction Lack of natural structures Altered cellular functions Higher sensitivity to drugs Unpredictable for clinical trials |
 | CC cell lines CaSki HeLa SiHa | Disease modeling [52,90,93,94,95] Drug response [96,97,98,99] Immunotherapy [100,101,102] Anti-HPV vaccine [92] | Simple maintenance Moderate costs Cell–cell interaction Preserved cellular functions Mimicking tumor structure Flexible to increase the complexity High-throughput potential | Lack of cell–ECM interactions Lack of heterogeneity Variation in uniformity and reproducibility Challenging analysis |
 | Cancer tissue (SCC, AdCC, neuroendocrine CC) | Disease modeling [88,89,103,104] Personalized therapy [24,89,103] NCT04278326 | Natural tumor cell functions Mimicking tumor structure and TME More predictable drug responses High-throughput potential Biobanks establishment Personalized therapy | Difficult to maintain Patient-dependent variation ECM matrix variations High costs Challenging analysis Ethical concern |
4. 3D Cervical Cancer Spheroids and Organoids in Translational Research
4.1. Drug Discovery
4.2. Pre-Clinical Testing of Immunotherapies
4.3. 3D Cultures as a Platform to Test Combinatorial Treatment Strategies against Cervical Cancer
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- RKI. Krebs in Deutschland für 2015/2016; Robert Koch-Institut: Berlin, Germany, 2019. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Stübs, F.A.; Koch, M.C.; Mallmann, P.; Dannecker, C.; Dietl, A.; Sevnina, A.; Mergel, F.; Lotz, L.; Hack, C.C.; et al. Diagnosis, Therapy and Follow-up of Cervical CancerGuideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021)—Part 2 with Recommendations on Psycho-oncology, Rehabilitation, Follow-up, Recurrence, Palliative Therapy and Healthc. Geburtshilfe Frauenheilkd. 2022, 82, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.W.; Stübs, F.A.; Koch, M.C.; Mallmann, P.; Dannecker, C.; Dietl, A.; Sevnina, A.; Mergel, F.; Lotz, L.; Hack, C.C.; et al. Diagnosis, Therapy and Follow-up of Cervical CancerGuideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021)—Part 1 with Recommendations on Epidemiology, Screening, Diagnostics and Therapy. Geburtshilfe Frauenheilkd. 2022, 82, 139–180. [Google Scholar] [CrossRef] [PubMed]
- Hirte, H.W.; Clark, D.A.; Mazurka, J.; O’Connell, G.; Rusthoven, J. A rapid and simple method for the purification of tumor cells from ascitic fluid of ovarian carcinoma. Gynecol. Oncol. 1992, 44, 223–226. [Google Scholar] [CrossRef]
- Shepherd, T.G.; Thériault, B.L.; Campbell, E.J.; Nachtigal, M.W. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat. Protoc. 2006, 1, 2643–2649. [Google Scholar] [CrossRef]
- Bongso, A.; Gajra, B.; Lian, N.P.; Wong, P.C.; Soon-Chye, N.; Ratnam, S. Establishment of human endometrial cell cultures. Hum. Reprod. 1988, 3, 705–713. [Google Scholar] [CrossRef]
- Karst, A.M.; Drapkin, R. Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat. Protoc. 2012, 7, 1755–1764. [Google Scholar] [CrossRef]
- Inada, K.; Hayashi, S.; Iguchi, T.; Sato, T. Establishment of a Primary Culture Model of Mouse Uterine and Vaginal Stroma for Studying In Vitro Estrogen Effects. Exp. Biol. Med. 2006, 231, 303–310. [Google Scholar] [CrossRef]
- Stanley, M.A.; Parkinson, E.K. Growth requirements of human cervical epithelial cells in culture. Int. J. Cancer 1979, 24, 407–414. [Google Scholar] [CrossRef]
- Fan, T.; Li, X.; Li, Y.; Zhi, Y.; Rong, S.; Cheng, G.; Zhang, X. An improved method for primary culture of normal cervical epithelial cells and establishment of cell model in vitro with HPV-16 E6 gene by lentivirus. J. Cell. Physiol. 2018, 233, 2773–2780. [Google Scholar] [CrossRef]
- Zuñiga Martinez, M.D.L.; López Mendoza, C.M.; Tenorio Salazar, J.; García Carrancá, A.M.; Cerbón Cervantes, M.A.; Alcántara-Quintana, L.E. Establishment, authenticity, and characterization of cervical cancer cell lines. Mol. Cell. Oncol. 2022, 9, 2078628. [Google Scholar] [CrossRef] [PubMed]
- Skok, K.; Gradišnik, L.; Maver, U.; Kozar, N.; Sobočan, M.; Takač, I.; Arko, D.; Kavalar, R. Gynaecological cancers and their cell lines. J. Cell. Mol. Med. 2021, 25, 3680–3698. [Google Scholar] [CrossRef] [PubMed]
- Ghoshdastider, U.; Rohatgi, N.; Naeini, M.M.; Baruah, P.; Revkov, E.; Guo, Y.A.; Rizzetto, S.; Wong, A.M.L.; Solai, S.; Nguyen, T.T.; et al. Pan-cancer analysis of ligand-receptor cross-talk in the tumor microenvironment. Cancer Res. 2021, 81, 1802–1812. [Google Scholar] [CrossRef]
- Korneev, K.V.; Atretkhany, K.-S.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef]
- NCI. NCI Dictionary of Cancer Terms; National Institutes of Health: Bethesda, MD, USA, 2018.
- Alfarouk, K.O.; Muddathir, A.K.; Shayoub, M.E.A. Tumor acidity as evolutionary spite. Cancers 2011, 3, 408–414. [Google Scholar] [CrossRef]
- Pickl, M.; Ries, C.H. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 2009, 28, 461–468. [Google Scholar] [CrossRef]
- El Feky, S.; Megahed, M.; Abd El Moneim, N.; Zaher, E.; Khamis, S.; Ali, L. Cytotoxic, chemosensitizing and radiosensitizing effects of curcumin based on thioredoxin system inhibition in breast cancer cells: 2D vs. 3D cell culture system. Exp. Ther. Med. 2021, 21, 506. [Google Scholar] [CrossRef] [PubMed]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells cultured in 2D vs 3D reveals differences in AKT/mTOR/S6-kinase signaling and drug response. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef]
- Weaver, V.M.; Petersen, O.W.; Wang, F.; Larabell, C.A.; Briand, P.; Damsky, C.; Bissell, M.J. Reversion of the Malignant Phenotype of Human Breast Cells in Three-Dimensional Culture and In Vivo by Integrin Blocking Antibodies. J. Cell Biol. 1997, 137, 231–245. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Brady, L.; Gil da Costa, R.M.; Coleman, I.M.; Matson, C.K.; Risk, M.C.; Coleman, R.T.; Nelson, P.S. A comparison of prostate cancer cell transcriptomes in 2D monoculture vs 3D xenografts identify consistent gene expression alterations associated with tumor microenvironments. Prostate 2020, 80, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nishie, R.; Ueda, S.; Miyamoto, S.; Hashida, S.; Konishi, H.; Terada, S.; Kogata, Y.; Sasaki, H.; Tsunetoh, S.; et al. Patient-Derived Xenograft Models in Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9369. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Bachran, C.; Stanke, J.; Elezkurtaj, S.; Kaufmann, A.M.; Fuchs, H.; Loddenkemper, C.; Schneider, A.; Cichon, G. Creation and characterization of a xenograft model for human cervical cancer. Gynecol. Oncol. 2010, 118, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.N.; Zheng, J.; Foltz, W.D.; Weersink, R.; Chaudary, N.; Jaffray, D.A.; Allen, C. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release 2014, 178, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Kim, S.; Choi, Y.-L.; Cho, Y.J.; Oh, E.; Choi, J.-J.; Jung, K.; Song, J.-Y.; Ahn, S.E.; Kim, B.-G.; et al. HER2 as a novel therapeutic target for cervical cancer. Oncotarget 2015, 6, 36219–36230. [Google Scholar] [CrossRef] [PubMed]
- Larmour, L.I.; Cousins, F.L.; Teague, J.A.; Deane, J.A.; Jobling, T.W.; Gargett, C.E. A patient derived xenograft model of cervical cancer and cervical dysplasia. PLoS ONE 2018, 13, e0206539. [Google Scholar] [CrossRef]
- Chaudary, N.; Pintilie, M.; Hedley, D.; Fyles, A.W.; Milosevic, M.; Clarke, B.; Hill, R.P.; Mackay, H. Hedgehog pathway signaling in cervical carcinoma and outcome after chemoradiation. Cancer 2012, 118, 3105–3115. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Zhang, Y.; Zhang, N.; Maawy, A.; Mii, S.; Yamamoto, M.; Uehara, F.; Miwa, S.; Yano, S.; Murakami, T.; et al. Establishment of a Patient-Derived Orthotopic Xenograft (PDOX) Model of HER-2-Positive Cervical Cancer Expressing the Clinical Metastatic Pattern. PLoS ONE 2015, 10, e0117417. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, K.; Walton, Z.; Wang, Y.; Ebi, H.; Shimamura, T.; Liu, Y.; Tupper, T.; Ouyang, J.; Li, J.; et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 2012, 483, 613–617. [Google Scholar] [CrossRef]
- Mueller-Klieser, W. Multicellular spheroids. J. Cancer Res. Clin. Oncol. 1987, 113, 101–122. [Google Scholar] [CrossRef]
- Achilli, T.-M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, R.L.; Gey, G.O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J. Natl. Cancer Inst. 1956, 16, 1375–1403. [Google Scholar] [PubMed]
- Bissell, M.J. The Differentiated State of Normal and Malignant Cells or How to Define a “Normal” Cell in Culture. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 1981; pp. 27–100. [Google Scholar]
- Emerman, J.T.; Burwen, S.J.; Pitelka, D.R. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell 1979, 11, 109–119. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef]
- Sutherland, R.M.; McCredie, J.A.; Inch, W.R. Growth of Multicell Spheroids in Tissue Culture as a Model of Nodular Carcinomas2. J. Natl. Cancer Inst. 1971, 46, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Groebe, K.; Mueller-Klieser, W. Distributions of oxygen, nutrient, and metabolic waste concentrations in multicellular spheroids and their dependence on spheroid parameters. Eur. Biophys. J. 1991, 19, 169–181. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef]
- Sivakumar, H.; Devarasetty, M.; Kram, D.E.; Strowd, R.E.; Skardal, A. Multi-Cell Type Glioblastoma Tumor Spheroids for Evaluating Sub-Population-Specific Drug Response. Front. Bioeng. Biotechnol. 2020, 8, 538663. [Google Scholar] [CrossRef]
- Gheytanchi, E.; Naseri, M.; Karimi-Busheri, F.; Atyabi, F.; Mirsharif, E.S.; Bozorgmehr, M.; Ghods, R.; Madjd, Z. Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int. 2021, 21, 204. [Google Scholar] [CrossRef]
- Pant, T.; Gaikwad, G.; Jain, D.; Dandekar, P.; Jain, R. Establishment and characterization of lung co-culture spheroids for paclitaxel loaded Eudragit® RL 100 nanoparticle evaluation. Biotechnol. Prog. 2021, 37, e3203. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Li, X.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Establishment and Characterization of a Recurrent Osteosarcoma Cell Line: OSA 1777. J. Orthop. Res. 2020, 38, 902–910. [Google Scholar] [CrossRef]
- Cavaco, M.; Fraga, P.; Valle, J.; Andreu, D.; Castanho, M.A.R.B.; Neves, V. Development of Breast Cancer Spheroids to Evaluate Cytotoxic Response to an Anticancer Peptide. Pharmaceutics 2021, 13, 1863. [Google Scholar] [CrossRef]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Sterzynska, K.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. Drug resistance evaluation in novel 3D in vitro model. Biomed. Pharmacother. 2021, 138, 111536. [Google Scholar] [CrossRef] [PubMed]
- Chitcholtan, K.; Asselin, E.; Parent, S.; Sykes, P.H.; Evans, J.J. Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp. Cell Res. 2013, 319, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, K.; Asra Ahmad, Z.; Annabel Dass, S.; Shamsuddin, S.; Mohana Kumaran, N.; Balakrishnan, V. Growth and Invasion of 3D Spheroid Tumor of HeLa and CasKi Cervical Cancer Cells. Oncologie 2021, 23, 279–291. [Google Scholar] [CrossRef]
- López, J.; Poitevin, A.; Mendoza-Martínez, V.; Pérez-Plasencia, C.; García-Carrancá, A. Cancer-initiating cells derived from established cervical cell lines exhibit stem-cell markers and increased radioresistance. BMC Cancer 2012, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, T.; Shibata, K.; Takahashi, J.; Watanabe, M.; Nakata, S.; Nakamura, K.; Yamaguchi, T. Glycolytic oscillations in HeLa cervical cancer cell spheroids. FEBS J. 2022, 289, 5551–5570. [Google Scholar] [CrossRef]
- Daum, A.-K.; Dittmann, J.; Jansen, L.; Peters, S.; Dahmen, U.; Heger, J.I.; Hoppe-Seyler, F.; Gille, A.; Clement, J.H.; Runnebaum, I.B.; et al. ITIH5 shows tumor suppressive properties in cervical cancer cells grown as multicellular tumor spheroids. Am. J. Transl. Res. 2021, 13, 10298–10314. [Google Scholar]
- Gottfried, E.; Kunz-Schughart, L.A.; Andreesen, R.; Kreutz, M. Brave Little World: Spheroids as an in vitro Model to Study Tumor-Immune-Cell Interactions. Cell Cycle 2006, 5, 691–695. [Google Scholar] [CrossRef]
- Courau, T.; Bonnereau, J.; Chicoteau, J.; Bottois, H.; Remark, R.; Assante Miranda, L.; Toubert, A.; Blery, M.; Aparicio, T.; Allez, M.; et al. Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment. J. Immunother. Cancer 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.E. Invited review Multicell spheroids as a model for cell kinetic studies. Cell Prolif. 1990, 23, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.M. Cell and Environment Interactions in Tumor Microregions: The Multicell Spheroid Model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef]
- Kaur, G.; Doroshow, J.H.; Teicher, B.A. Format (2D vs 3D) and media effect target expression and response of patient-derived and standard NSCLC lines to EGFR inhibitors. Cancer Treat. Res. Commun. 2021, 29, 100463. [Google Scholar] [CrossRef]
- Tamaki, M.; McDonald, W.; Amberger, V.R.; Moore, E.; Del Maestro, R.F. Implantation of C6 astrocytoma spheroid into collagen type I gels: Invasive, proliferative, and enzymatic characterizations. J. Neurosurg. 1997, 87, 602–609. [Google Scholar] [CrossRef]
- Gunay, G.; Kirit, H.A.; Kamatar, A.; Baghdasaryan, O.; Hamsici, S.; Acar, H. The effects of size and shape of the ovarian cancer spheroids on the drug resistance and migration. Gynecol. Oncol. 2020, 159, 563–572. [Google Scholar] [CrossRef]
- Vinci, M.; Box, C.; Eccles, S.A. Three-dimensional (3D) tumor spheroid invasion assay. J. Vis. Exp. 2015, 99, e52686. [Google Scholar] [CrossRef]
- Timmins, N.; Dietmair, S.; Nielsen, L. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis 2004, 7, 97–103. [Google Scholar] [CrossRef]
- Jadhav, U.; Chigurupati, S.; Lakka, S.S.; Mohanam, S. Inhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cells. Int. J. Oncol. 2004, 25, 1407–1414. [Google Scholar] [CrossRef]
- Upreti, M.; Jamshidi-Parsian, A.; Koonce, N.A.; Webber, J.S.; Sharma, S.K.; Asea, A.A.A.; Mader, M.J.; Griffin, R.J. Tumor-Endothelial Cell Three-dimensional Spheroids: New Aspects to Enhance Radiation and Drug Therapeutics. Transl. Oncol. 2011, 4, 365-IN3. [Google Scholar] [CrossRef] [PubMed]
- Brassard-Jollive, N.; Monnot, C.; Muller, L.; Germain, S. In vitro 3D Systems to Model Tumor Angiogenesis and Interactions With Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 594903. [Google Scholar] [CrossRef] [PubMed]
- KORFF, T.; KIMMINA, S.; MARTINY-BARON, G.; AUGUSTIN, H.G. Blood vessel maturation in a 3-dimensional spheroidal coculture model: Direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, S.M.; Welch-Reardon, K.M.; Waterman, M.L.; Hughes, C.C.W.; George, S.C. A three-dimensional in vitro model of tumor cell intravasation. Integr. Biol. 2014, 6, 603. [Google Scholar] [CrossRef] [PubMed]
- Krishnapriya, S.; Sidhanth, C.; Manasa, P.; Sneha, S.; Bindhya, S.; Nagare, R.P.; Ramachandran, B.; Vishwanathan, P.; Murhekar, K.; Shirley, S.; et al. Cancer stem cells contribute to angiogenesis and lymphangiogenesis in serous adenocarcinoma of the ovary. Angiogenesis 2019, 22, 441–455. [Google Scholar] [CrossRef]
- Kwak, T.J.; Lee, E. In vitro modeling of solid tumor interactions with perfused blood vessels. Sci. Rep. 2020, 10, 20142. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Rosenbluth, J.M.; Schackmann, R.C.J.; Gray, G.K.; Selfors, L.M.; Li, C.M.-C.; Boedicker, M.; Kuiken, H.J.; Richardson, A.; Brock, J.; Garber, J.; et al. Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat. Commun. 2020, 11, 1711. [Google Scholar] [CrossRef]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef]
- Karthaus, W.R.; Iaquinta, P.J.; Drost, J.; Gracanin, A.; van Boxtel, R.; Wongvipat, J.; Dowling, C.M.; Gao, D.; Begthel, H.; Sachs, N.; et al. Identification of Multipotent Luminal Progenitor Cells in Human Prostate Organoid Cultures. Cell 2014, 159, 163–175. [Google Scholar] [CrossRef]
- Harper, K.L.; Sosa, M.S.; Entenberg, D.; Hosseini, H.; Cheung, J.F.; Nobre, R.; Avivar-Valderas, A.; Nagi, C.; Girnius, N.; Davis, R.J.; et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 2016, 540, 588–592. [Google Scholar] [CrossRef]
- Nuciforo, S.; Fofana, I.; Matter, M.S.; Blumer, T.; Calabrese, D.; Boldanova, T.; Piscuoglio, S.; Wieland, S.; Ringnalda, F.; Schwank, G.; et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep. 2018, 24, 1363–1376. [Google Scholar] [CrossRef]
- Wang, K.; Yuen, S.T.; Xu, J.; Lee, S.P.; Yan, H.H.N.; Shi, S.T.; Siu, H.C.; Deng, S.; Chu, K.M.; Law, S.; et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014, 46, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Matano, M.; Date, S.; Shimokawa, M.; Takano, A.; Fujii, M.; Ohta, Y.; Watanabe, T.; Kanai, T.; Sato, T. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. Nat. Med. 2015, 21, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Holtzinger, A.; Jagan, I.; BeGora, M.; Lohse, I.; Ngai, N.; Nostro, C.; Wang, R.; Muthuswamy, L.B.; Crawford, H.C.; et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell– and patient-derived tumor organoids. Nat. Med. 2015, 21, 1364–1371. [Google Scholar] [CrossRef]
- Grassi, L.; Alfonsi, R.; Francescangeli, F.; Signore, M.; De Angelis, M.L.; Addario, A.; Costantini, M.; Flex, E.; Ciolfi, A.; Pizzi, S.; et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 2019, 10, 201. [Google Scholar] [CrossRef]
- Mullenders, J.; de Jongh, E.; Brousali, A.; Roosen, M.; Blom, J.P.A.; Begthel, H.; Korving, J.; Jonges, T.; Kranenburg, O.; Meijer, R.; et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc. Natl. Acad. Sci. USA 2019, 116, 4567–4574. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Kopper, O.; de Witte, C.J.; Lõhmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Itami, M.; Hippo, Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol. Oncol. 2019, 154, 189–198. [Google Scholar] [CrossRef]
- Hill, S.J.; Decker, B.; Roberts, E.A.; Horowitz, N.S.; Muto, M.G.; Worley, M.J.; Feltmate, C.M.; Nucci, M.R.; Swisher, E.M.; Nguyen, H.; et al. Prediction of DNA Repair Inhibitor Response in Short-Term Patient-Derived Ovarian Cancer Organoids. Cancer Discov. 2018, 8, 1404–1421. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef] [PubMed]
- Campaner, E.; Zannini, A.; Santorsola, M.; Bonazza, D.; Bottin, C.; Cancila, V.; Tripodo, C.; Bortul, M.; Zanconati, F.; Schoeftner, S.; et al. Breast Cancer Organoids Model Patient-Specific Response to Drug Treatment. Cancers 2020, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, S.; Piccotti, F.; Allevi, R.; Truffi, M.; Sorrentino, L.; Russo, L.; Agozzino, M.; Signati, L.; Bonizzi, A.; Villani, L.; et al. Establishment and Morphological Characterization of Patient-Derived Organoids from Breast Cancer. Biol. Proced. Online 2019, 21, 12. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Ebisawa, K.; Odaka, A.; Sugiyama, T.; Itami, M.; Hippo, Y. Establishment and characterization of patient-derived organoids from a young patient with cervical clear cell carcinoma. Cancer Sci. 2019, 110, 2992–3005. [Google Scholar] [CrossRef]
- Lõhmussaar, K.; Oka, R.; Espejo Valle-Inclan, J.; Smits, M.H.H.; Wardak, H.; Korving, J.; Begthel, H.; Proost, N.; van de Ven, M.; Kranenburg, O.W.; et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell 2021, 28, 1380–1396.e6. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.S.; Oh, J.H.; Choi, E.; Kim, S.; Kim, H.; Nam, E.J. Preclinical investigation of patient-derived cervical cancer organoids for precision medicine. J. Gynecol. Oncol. 2023, 34, e35. [Google Scholar] [CrossRef]
- Wan, P.K.-T.; Leung, T.H.-Y.; Siu, M.K.-Y.; Mo, X.-T.; Tang, H.W.-M.; Chan, K.K.-L.; Cheung, A.N.-Y.; Ngan, H.Y.-S. HPV-induced Nurr1 promotes cancer aggressiveness, self-renewal, and radioresistance via ERK and AKT signaling in cervical cancer. Cancer Lett. 2021, 497, 14–27. [Google Scholar] [CrossRef]
- González-Torres, A.; Bañuelos-Villegas, E.G.; Martínez-Acuña, N.; Sulpice, E.; Gidrol, X.; Alvarez-Salas, L.M. MYPT1 is targeted by miR-145 inhibiting viability, migration and invasion in 2D and 3D HeLa cultures. Biochem. Biophys. Res. Commun. 2018, 507, 348–354. [Google Scholar] [CrossRef]
- Wang, Y.T.; Li, W.; Liu, Q.; Guan, X.; Hu, J. Dendritic cells treated with HPV16mE7 in a three-dimensional model promote the secretion of IL-12p70 and IFN-γ. Exp. Mol. Pathol. 2011, 91, 325–330. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 35001. [Google Scholar] [CrossRef]
- Thippabhotla, S.; Zhong, C.; He, M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci. Rep. 2019, 9, 13012. [Google Scholar] [CrossRef]
- De Gregorio, V.; La Rocca, A.; Urciuolo, F.; Annunziata, C.; Tornesello, M.L.; Buonaguro, F.M.; Netti, P.A.; Imparato, G. Modeling the epithelial-mesenchymal transition process in a 3D organotypic cervical neoplasia. Acta Biomater. 2020, 116, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, O.P.; González-Torres, A.; Álvarez-Salas, L.M.; Hernández-Sánchez, H.; García-Pérez, B.E.; del Rocío Thompson-Bonilla, M.; Jaramillo-Flores, M.E. Effect of naringenin and its combination with cisplatin in cell death, proliferation and invasion of cervical cancer spheroids. RSC Adv. 2021, 11, 129–141. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J. Mixed hydrogel bead-based tumor spheroid formation and anticancer drug testing. Analyst 2014, 139, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.; Seo, O.W.; Kim, M.; Hulme, J.; An, S.S.A. Monitoring the effects of doxorubicin on 3D-spheroid tumor cells in real-time. Onco Targets Ther. 2016, 9, 7207–7218. [Google Scholar] [CrossRef]
- Casagrande, N.; De Paoli, M.; Celegato, M.; Borghese, C.; Mongiat, M.; Colombatti, A.; Aldinucci, D. Preclinical evaluation of a new liposomal formulation of cisplatin, lipoplatin, to treat cisplatin-resistant cervical cancer. Gynecol. Oncol. 2013, 131, 744–752. [Google Scholar] [CrossRef]
- Giannattasio, A.; Weil, S.; Kloess, S.; Ansari, N.; Stelzer, E.H.K.; Cerwenka, A.; Steinle, A.; Koehl, U.; Koch, J. Cytotoxicity and infiltration of human NK cells in in vivo-like tumor spheroids. BMC Cancer 2015, 15, 351. [Google Scholar] [CrossRef]
- Yuti, P.; Wutti-in, Y.; Sawasdee, N.; Kongkhla, K.; Phanthaphol, N.; Choomee, K.; Chieochansin, T.; Panya, A.; Junking, M.; Yenchitsomanus, P.; et al. Anti-CD19 chimeric antigen receptor T cells secreting anti-PD-L1 single-chain variable fragment attenuate PD-L1 mediated T cell inhibition. Int. Immunopharmacol. 2022, 113, 109442. [Google Scholar] [CrossRef]
- Park, D.; Son, K.; Hwang, Y.; Ko, J.; Lee, Y.; Doh, J.; Jeon, N.L. High-Throughput Microfluidic 3D Cytotoxicity Assay for Cancer Immunotherapy (CACI-IMPACT Platform). Front. Immunol. 2019, 10, 1133. [Google Scholar] [CrossRef]
- Maru, Y.; Kawata, A.; Taguchi, A.; Ishii, Y.; Baba, S.; Mori, M.; Nagamatsu, T.; Oda, K.; Kukimoto, I.; Osuga, Y.; et al. Establishment and Molecular Phenotyping of Organoids from the Squamocolumnar Junction Region of the Uterine Cervix. Cancers 2020, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Nakamura, K.; Takeda, T.; Chiwaki, F.; Banno, K.; Aoki, D.; Takeshita, F.; Sasaki, H. Aurora kinase blockade drives de novo addiction of cervical squamous cell carcinoma to druggable EGFR signalling. Oncogene 2022, 41, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Costa, D.; Sousa, Â. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, B.; Dong, X.; Zhou, Y.; He, Y.; Zhang, T.; Bao, L. Enhancement of cisplatin-induced cytotoxicity against cervical cancer spheroid cells by targeting long non-coding RNAs. Pathol. Res. Pract. 2019, 215, 152653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rashmi, R.; Inkman, M.; Jayachandran, K.; Ruiz, F.; Waters, M.R.; Grigsby, P.W.; Markovina, S.; Schwarz, J.K. Integrating imaging and RNA-seq improves outcome prediction in cervical cancer. J. Clin. Investig. 2021, 131, e139232. [Google Scholar] [CrossRef]
- Sethi, T.; Rintoul, R.C.; Moore, S.M.; MacKinnon, A.C.; Salter, D.; Choo, C.; Chilvers, E.R.; Dransfield, I.; Donnelly, S.C.; Strieter, R.; et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999, 5, 662–668. [Google Scholar] [CrossRef]
- Rozzo, C.; Chiesa, V.; Caridi, G.; Pagnan, G.; Ponzoni, M. Induction of apoptosis in human neuroblastoma cells by abrogation of integrin-mediated cell adhesion. Int. J. Cancer 1997, 70, 688–698. [Google Scholar] [CrossRef]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell Adhesion Mediated Drug Resistance (CAM-DR): Role of Integrins and Resistance to Apoptosis in Human Myeloma Cell Lines. Blood 1999, 93, 1658–1667. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Li, W.; Chen, W.; He, Q.; Zhang, X.; Tang, J.; Wang, Y.; Liu, B.; Liu, J. 3D bioprinted tumor model with extracellular matrix enhanced bioinks for nanoparticle evaluation. Biofabrication 2022, 14, 025002. [Google Scholar] [CrossRef]
- Pirola, L.; Ciesielski, O.; Balcerczyk, A. The Methylation Status of the Epigenome: Its Emerging Role in the Regulation of Tumor Angiogenesis and Tumor Growth, and Potential for Drug Targeting. Cancers 2018, 10, 268. [Google Scholar] [CrossRef]
- Heredia-Mendez, A.J.; Sánchez-Sánchez, G.; López-Camarillo, C. Reprogramming of the Genome-Wide DNA Methylation Landscape in Three-Dimensional Cancer Cell Cultures. Cancers 2023, 15, 1991. [Google Scholar] [CrossRef]
- Amatangelo, M.; Garipov, A.; Li, H.; Conejo-Garcia, J.R.; Speicher, D.; Zhang, R. Three-dimensional culture sensitizes epithelial ovarian cancer cells to EZH2 methyltransferase inhibition. Cell Cycle 2013, 12, 2113–2119. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Sheraton, M.V.; Chiew, G.G.Y.; Melnikov, V.; Tan, E.Y.; Luo, K.Q.; Verma, N.; Sloot, P.M.A. Emergence of spatio-temporal variations in chemotherapeutic drug efficacy: In-vitro and in-Silico 3D tumour spheroid studies. BMC Cancer 2020, 20, 1201. [Google Scholar] [CrossRef]
- Sarisozen, C.; Abouzeid, A.H.; Torchilin, V.P. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur. J. Pharm. Biopharm. 2014, 88, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, A.S.; Eetezadi, S.; Allen, C. Multicellular Tumor Spheroids for Evaluation of Cytotoxicity and Tumor Growth Inhibitory Effects of Nanomedicines In Vitro: A Comparison of Docetaxel-Loaded Block Copolymer Micelles and Taxotere®. PLoS ONE 2013, 8, e62630. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Patel, N.R.; Torchilin, V.P. Accumulation and toxicity of antibody-targeted doxorubicin-loaded PEG–PE micelles in ovarian cancer cell spheroid model. J. Control. Release 2012, 164, 95–102. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Nocelo, M.; Abuín, C.; López-López, R.; de la Fuente, M. Development and characterization of a three-dimensional co-culture model of tumor T cell infiltration. Biofabrication 2016, 8, 025002. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.S.; Cany, J.; van den Brand, D.; Oudenampsen, M.; Brock, R.; Torensma, R.; Bekkers, R.L.; Jansen, J.H.; Massuger, L.F.; Dolstra, H. Umbilical cord blood CD34+ progenitor-derived NK cells efficiently kill ovarian cancer spheroids and intraperitoneal tumors in NOD/SCID/IL2Rgnull mice. Oncoimmunology 2017, 6, e1320630. [Google Scholar] [CrossRef]
- Lanuza, P.M.; Vigueras, A.; Olivan, S.; Prats, A.C.; Costas, S.; Llamazares, G.; Sanchez-Martinez, D.; Ayuso, J.M.; Fernandez, L.; Ochoa, I.; et al. Activated human primary NK cells efficiently kill colorectal cancer cells in 3D spheroid cultures irrespectively of the level of PD-L1 expression. Oncoimmunology 2018, 7, e1395123. [Google Scholar] [CrossRef]
- Sherman, H.; Gitschier, H.J.; Rossi, A.E. A Novel Three-Dimensional Immune Oncology Model for High-Throughput Testing of Tumoricidal Activity. Front. Immunol. 2018, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Xenia Elena, B.; Nicoleta Gales, L.; Florina Zgura, A.; Iliescu, L.; Maricela Anghel, R.; Haineala, B. Assessment of Immune Status in Dynamics for Patients with Cancer Undergoing Immunotherapy. J. Oncol. 2021, 2021, 6698969. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Ros, W.; Delord, J.-P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Santin, A.D.; Deng, W.; Frumovitz, M.; Buza, N.; Bellone, S.; Huh, W.; Khleif, S.; Lankes, H.A.; Ratner, E.S.; O’Cearbhaill, R.E.; et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol. Oncol. 2020, 157, 161–166. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chester, C.; Melero, I.; Kohrt, H. Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann. Oncol. 2016, 27, 1190–1198. [Google Scholar] [CrossRef]
- Boucherit, N.; Gorvel, L.; Olive, D. 3D Tumor Models and Their Use for the Testing of Immunotherapies. Front. Immunol. 2020, 11, 603640. [Google Scholar] [CrossRef]
- Schnalzger, T.E.; Groot, M.H.; Zhang, C.; Mosa, M.H.; Michels, B.E.; Röder, J.; Darvishi, T.; Wels, W.S.; Farin, H.F. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019, 38, e100928. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e12. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Takeshita, R.; Mukaida, R.; Takatori, E.; Nagasawa, T.; Omi, H.; Sugiyama, T. Safe administration of bevacizumab combination chemotherapy for the patients with recurrent cervical cancer after pelvic radiotherapy: Two case reports. Mol. Clin. Oncol. 2018, 9, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Long, H.J.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Salani, R.; Brady, W.E.; Lankes, H.A.; Cohn, D.E.; Mutch, D.G.; Mannel, R.S.; Bell-McGuinn, K.M.; Di Silvestro, P.A.; Jelovac, D.; et al. A phase I trial of paclitaxel, cisplatin, and veliparib in the treatment of persistent or recurrent carcinoma of the cervix: An NRG Oncology Study (NCT#01281852). Ann. Oncol. 2017, 28, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, N.; Wattanapanitch, M.; Thongsin, N.; Phanthaphol, N.; Chiawpanit, C.; Thuwajit, C.; Yenchitsomanus, P.-T.; Panya, A. Doxorubicin sensitizes breast cancer cells to natural killer cells in connection with increased Fas receptors. Int. J. Mol. Med. 2022, 49, 40. [Google Scholar] [CrossRef]
- De Witte, C.J.; Espejo Valle-Inclan, J.; Hami, N.; Lõhmussaar, K.; Kopper, O.; Vreuls, C.P.H.; Jonges, G.N.; van Diest, P.; Nguyen, L.; Clevers, H.; et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep. 2020, 31, 107762. [Google Scholar] [CrossRef]
- Gopal, S.; Kwon, S.-J.; Ku, B.; Lee, D.W.; Kim, J.; Dordick, J.S. 3D tumor spheroid microarray for high-throughput, high-content natural killer cell-mediated cytotoxicity. Commun. Biol. 2021, 4, 893. [Google Scholar] [CrossRef]
- Kusakabe, M.; Taguchi, A.; Tanikawa, M.; Hoshi, D.; Tsuchimochi, S.; Qian, X.; Toyohara, Y.; Kawata, A.; Wagatsuma, R.; Yamaguchi, K.; et al. Application of organoid culture from HPV18-positive small cell carcinoma of the uterine cervix for precision medicine. Cancer Med. 2023, 12, 8476–8489. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.; Wang, Z.; Liu, Y.; Yu, J.; Wang, W.; Chen, S.; Wu, W.; Wang, J.; Qian, G.; et al. Standardization of Organoid Culture in Cancer Research. Cancer Med. 2023; early view. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, Z.; Li, Q.; Yang, S.; Jiang, L.; Yang, Y.; Li, Y.; Gu, Z. Organoids revealed: Morphological analysis of the profound next generation in-vitro model with artificial intelligence. Bio Des. Manuf. 2023, 1–21. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, N.; Sun, X.; Li, Q.; Zeng, Y.; Chen, F.; Sun, S.; Xu, J.; Zhang, J.; Ye, H.; et al. Automated evaluation of tumor spheroid behavior in 3D culture using deep learning-based recognition. Biomaterials 2021, 272, 120770. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Creighton, C.J.; Zhang, Y.; Chen, F.; Thrall, M.J.; Kim, M.P. Ex Vivo Four-Dimensional Lung Cancer Model Mimics Metastasis. Ann. Thorac. Surg. 2015, 99, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, R.; Zhang, J.; Zhao, Y.; Qiao, S.; Crouzier, T.; Yan, H.; Tian, W. A novel 4D cell culture mimicking stomach peristalsis altered gastric cancer spheroids growth and malignance. Biofabrication 2021, 13, 035034. [Google Scholar] [CrossRef]
- Murphy, R.J.; Browning, A.P.; Gunasingh, G.; Haass, N.K.; Simpson, M.J. Designing and interpreting 4D tumour spheroid experiments. Commun. Biol. 2022, 5, 91. [Google Scholar] [CrossRef]
- Wadman, M. FDA no longer has to require animal testing for new drugs. Science 2023, 379, 127–128. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutle, I.; Polten, R.; Hachenberg, J.; Klapdor, R.; Morgan, M.; Schambach, A. Tumor Organoid and Spheroid Models for Cervical Cancer. Cancers 2023, 15, 2518. https://doi.org/10.3390/cancers15092518
Kutle I, Polten R, Hachenberg J, Klapdor R, Morgan M, Schambach A. Tumor Organoid and Spheroid Models for Cervical Cancer. Cancers. 2023; 15(9):2518. https://doi.org/10.3390/cancers15092518
Chicago/Turabian StyleKutle, Ivana, Robert Polten, Jens Hachenberg, Rüdiger Klapdor, Michael Morgan, and Axel Schambach. 2023. "Tumor Organoid and Spheroid Models for Cervical Cancer" Cancers 15, no. 9: 2518. https://doi.org/10.3390/cancers15092518
APA StyleKutle, I., Polten, R., Hachenberg, J., Klapdor, R., Morgan, M., & Schambach, A. (2023). Tumor Organoid and Spheroid Models for Cervical Cancer. Cancers, 15(9), 2518. https://doi.org/10.3390/cancers15092518







