Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocols and Registration
2.2. Inclusion/Exclusion Criteria
2.3. Search Strategy
2.4. Studies Selection
- Study characteristics: authors, publication year, country, study design, and time.
- Population characteristics: total sample, sample rhTSH, sample THW.
- Outcome evaluation.
- Initial response to 131I therapy after THW or rhTSH preparation.
- Progression disease.
- Onset of side-effects.
2.5. Risk of Bias (ROB)
2.6. Statistical Analysis
3. Results
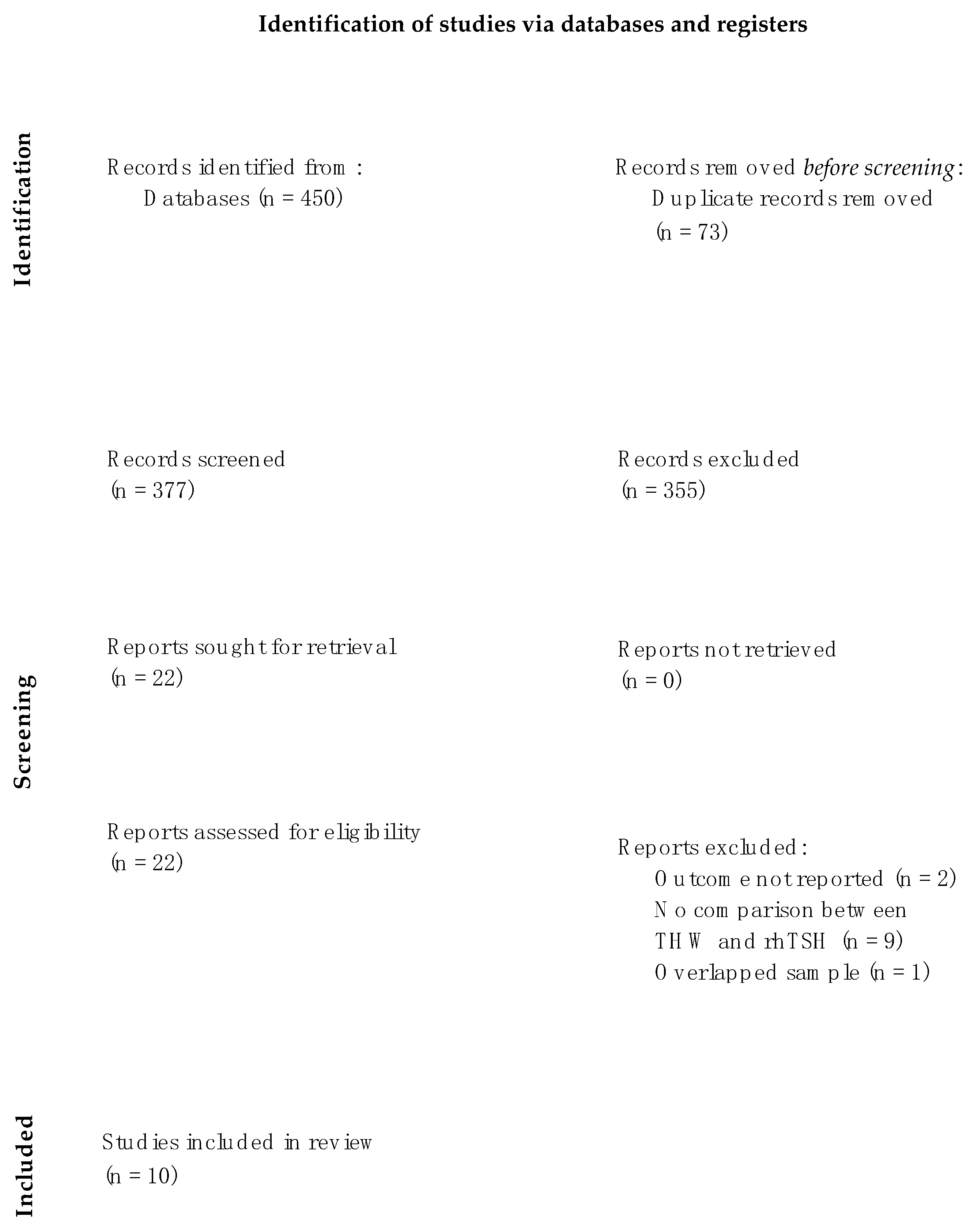
3.1. Search Results and Characteristics of the Studies
3.2. Study Characteristics
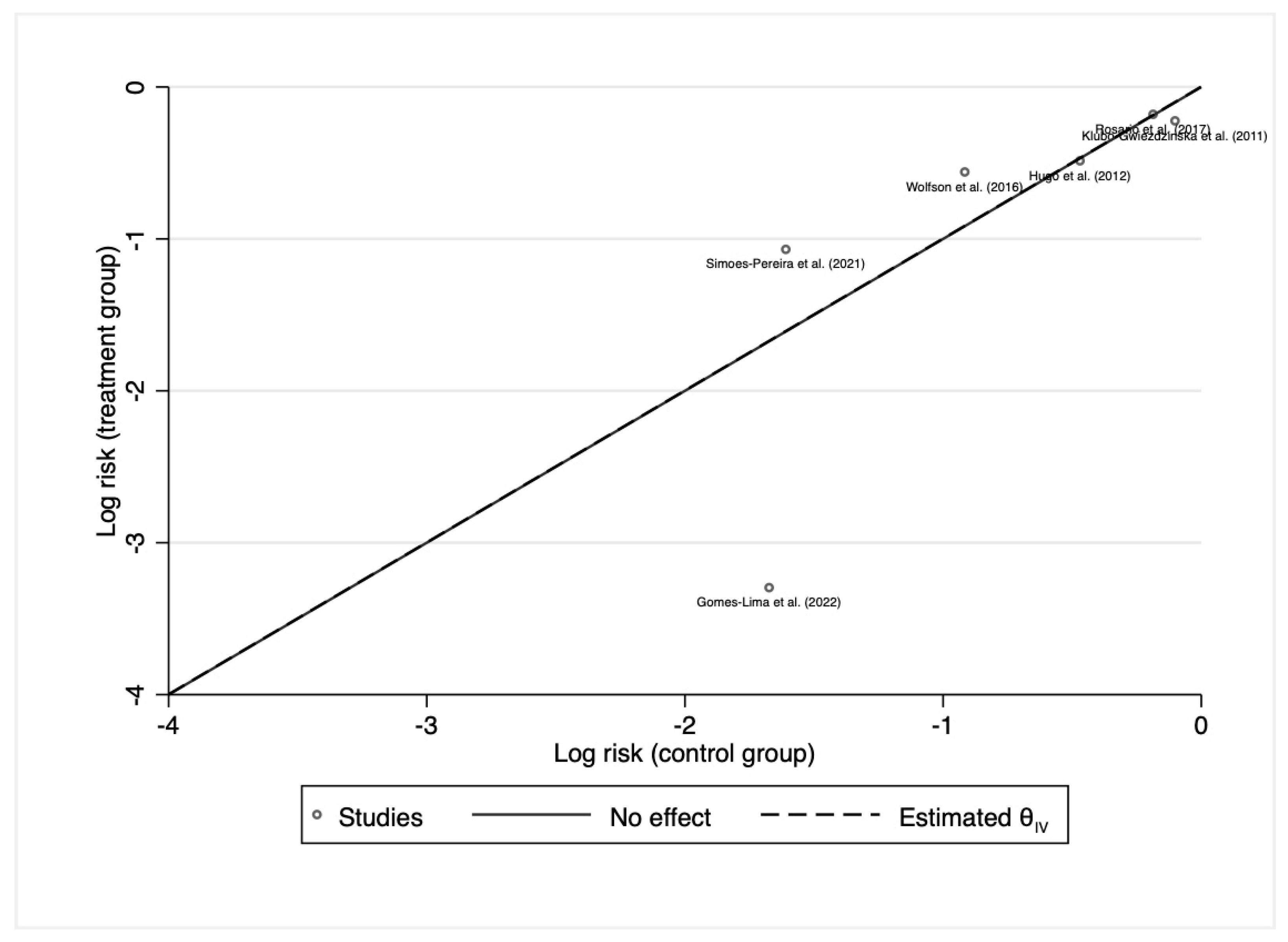
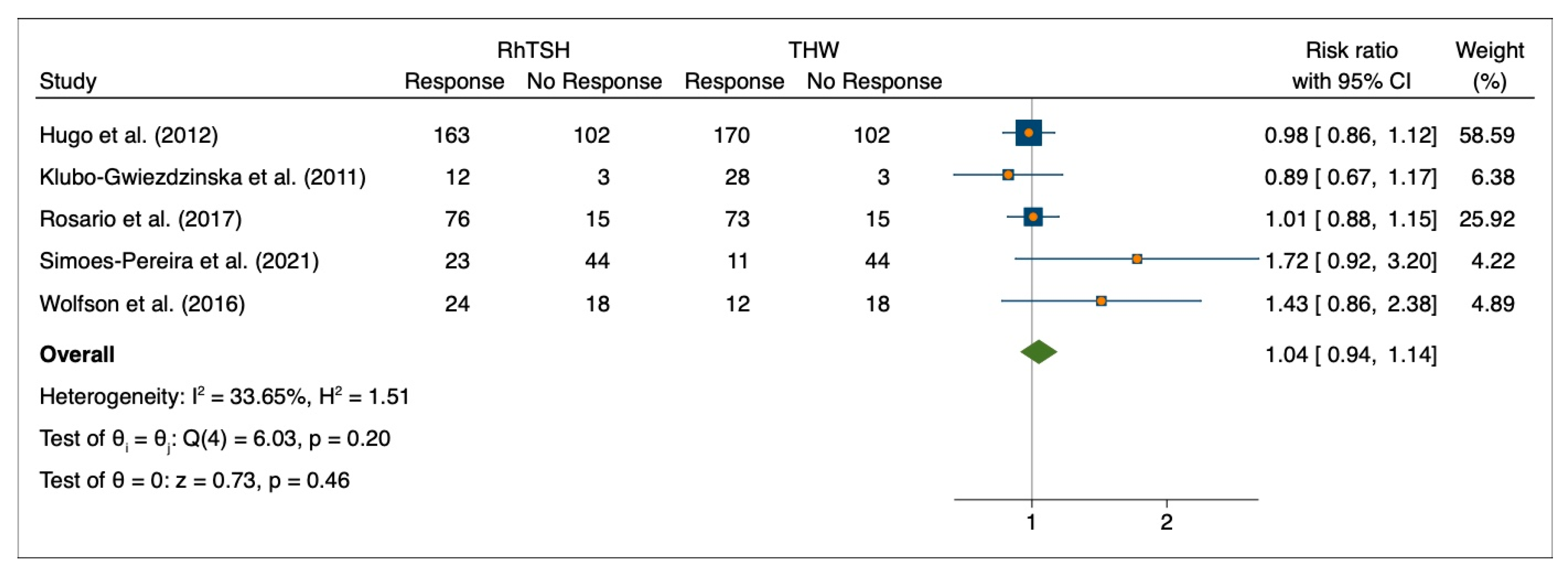
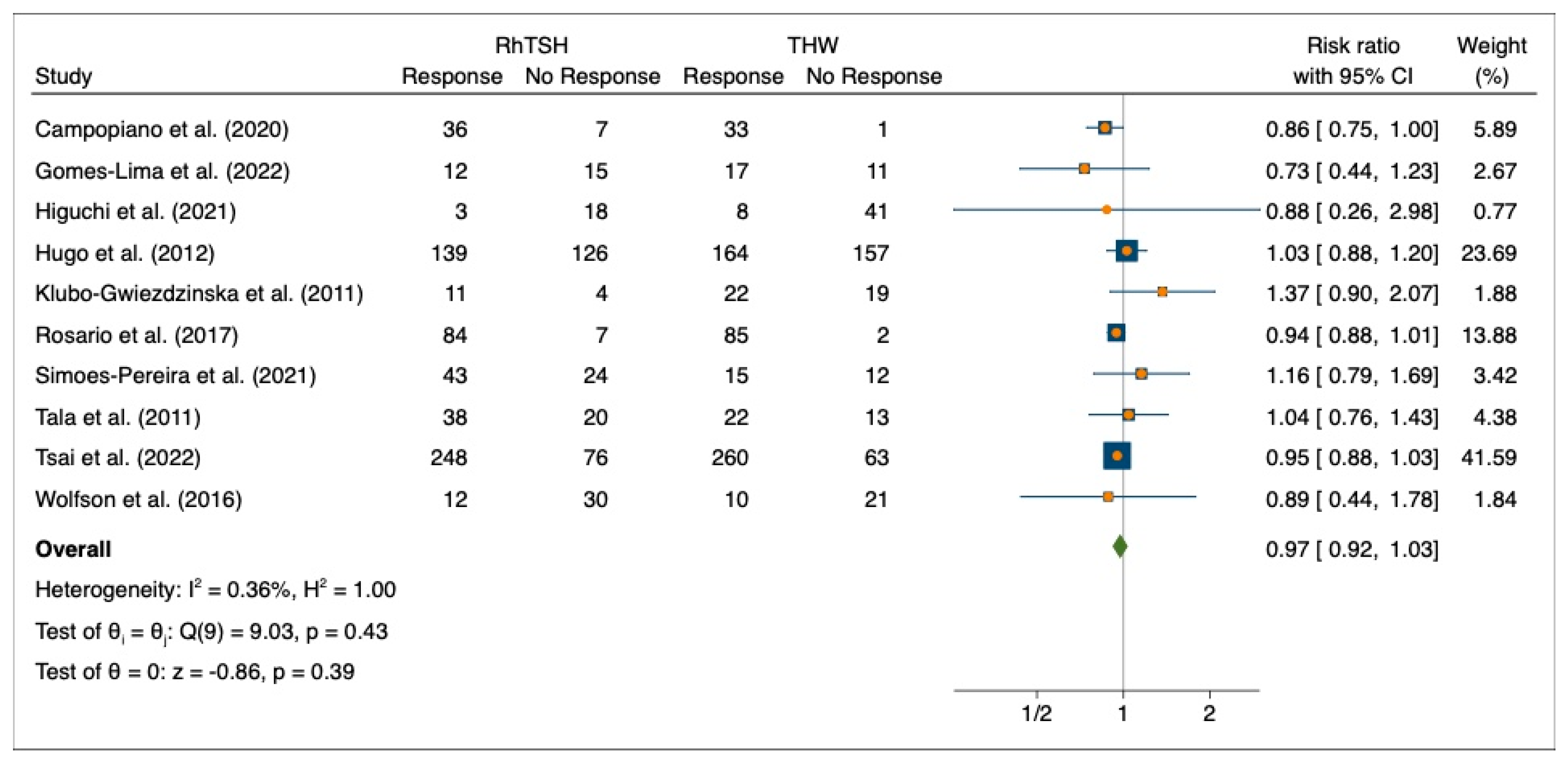
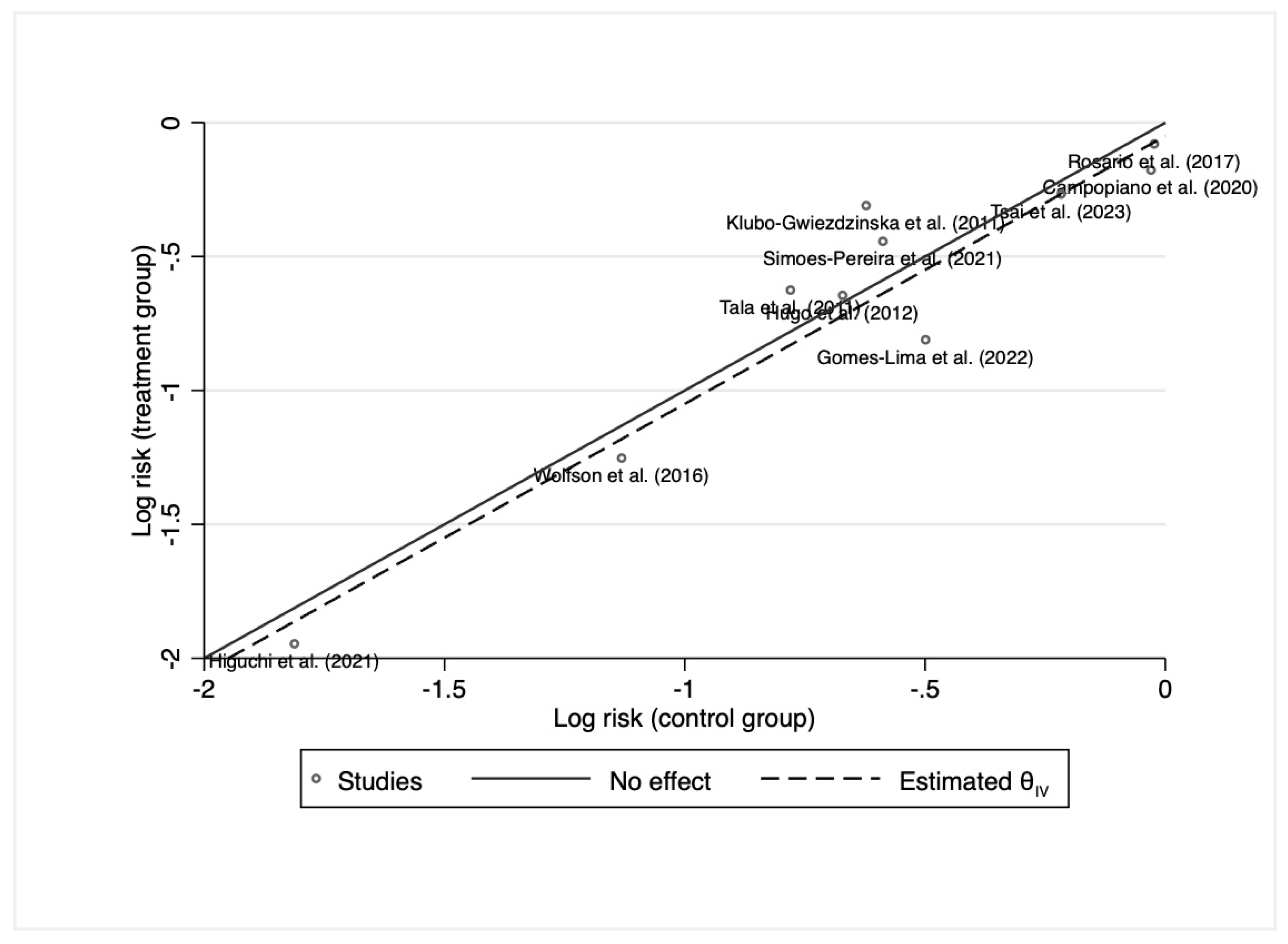
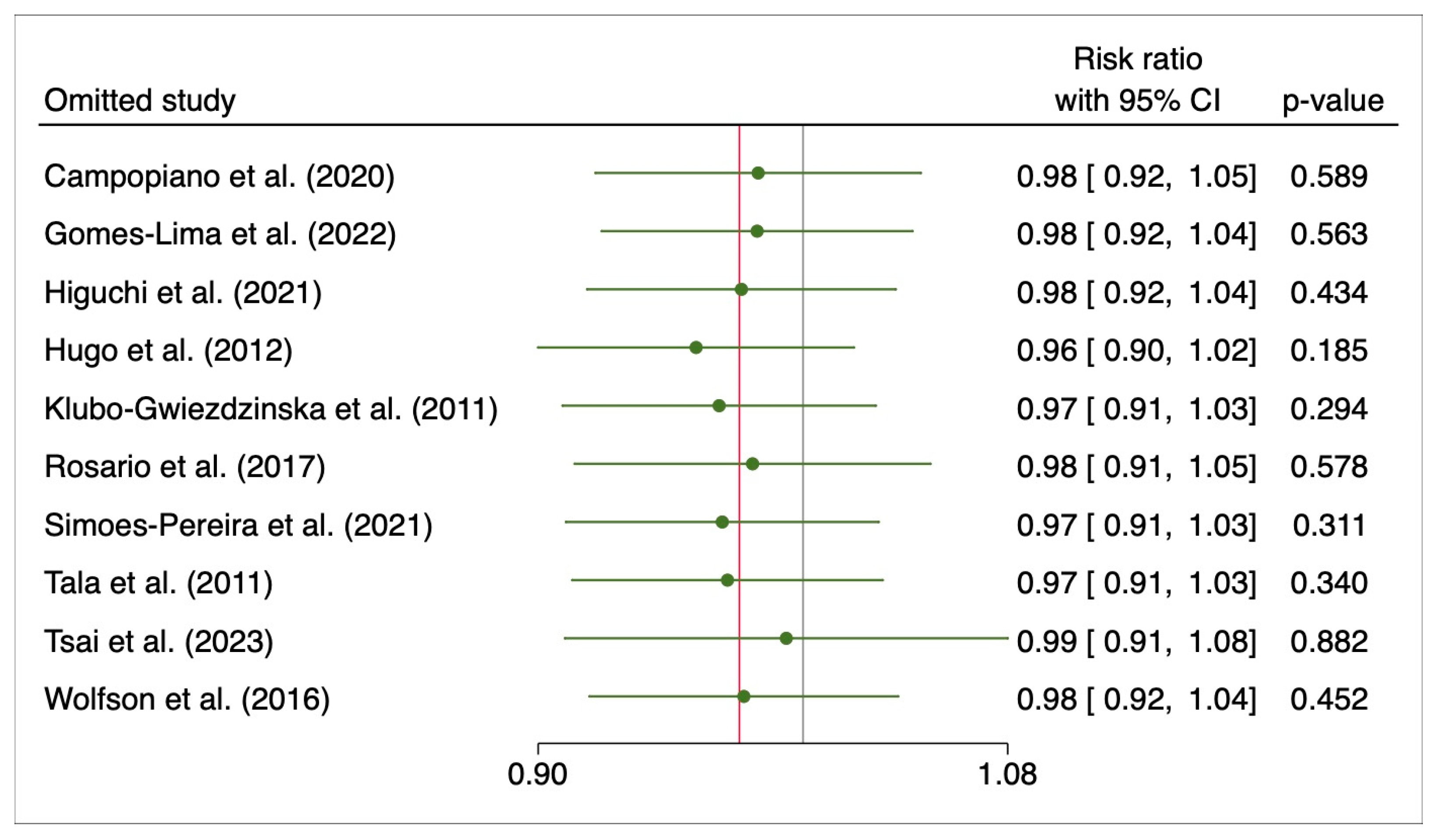
3.3. Initial Response to 131I Therapy after Preparation with rhTSH or THW
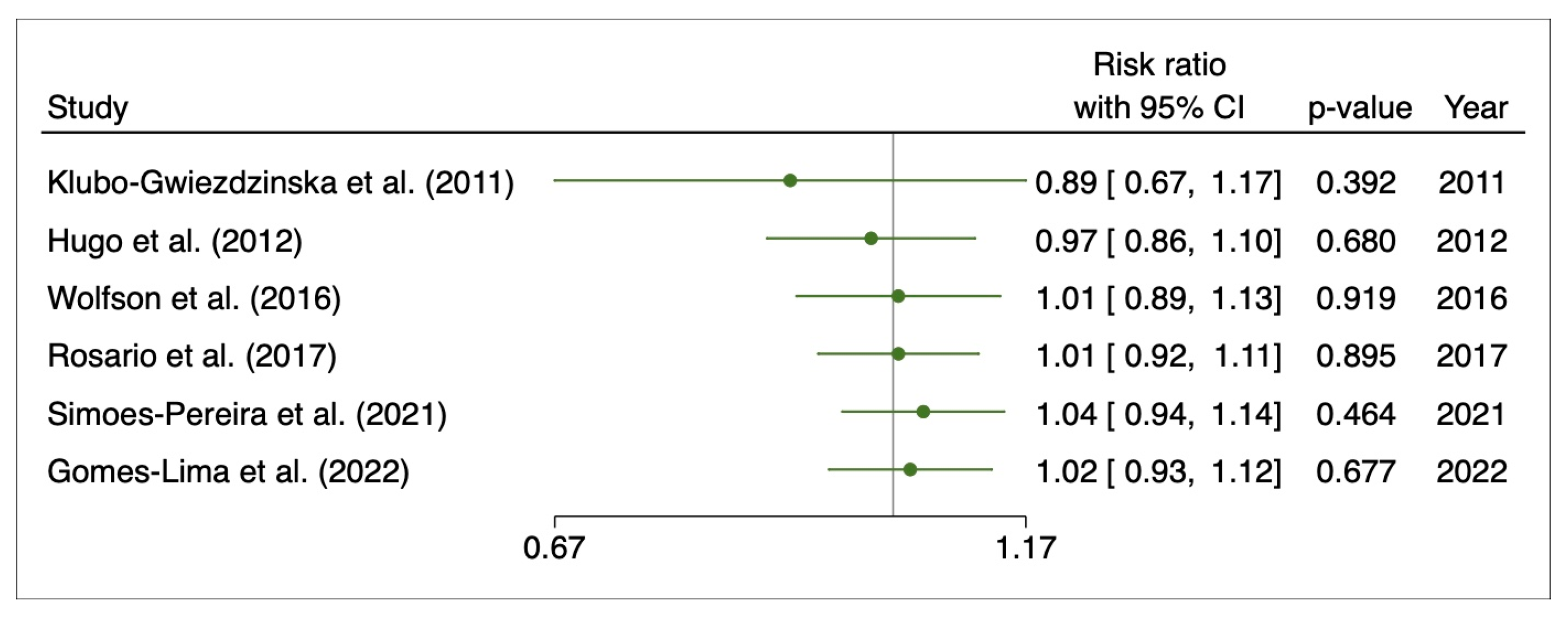
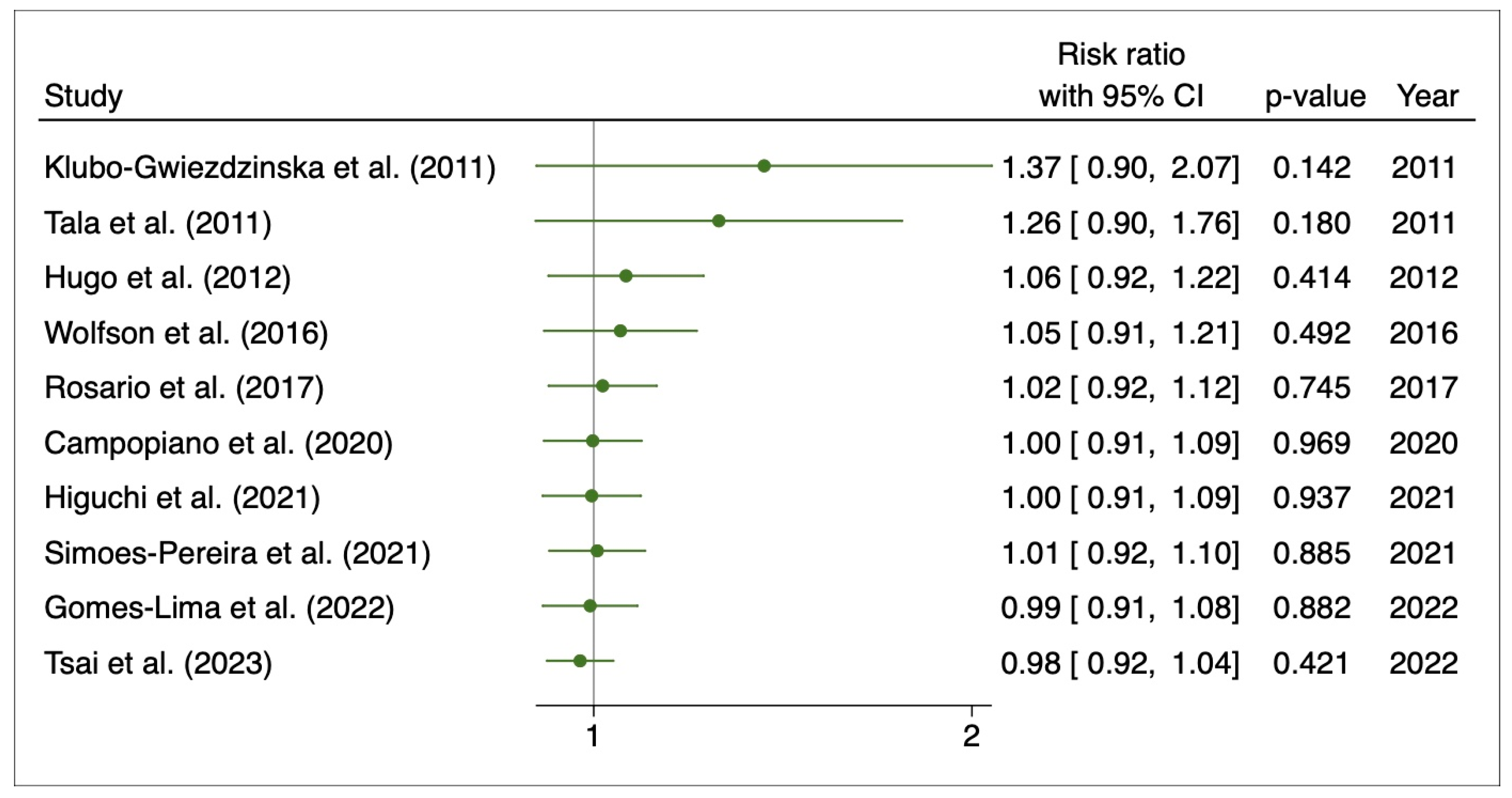
3.4. Disease Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surveillance, Epidemiology, and End Results Program. Thyroid Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/thyro.html (accessed on 23 March 2023).
- Carhill, A.A.; Litofsky, D.R.; Ross, D.S.; Jonklaas, J.; Cooper, D.S.; Brierley, J.D.; Ladenson, P.W.; Ain, K.B.; Fein, H.G.; Haugen, B.R.; et al. Long-Term Outcomes Following Therapy in Differentiated Thyroid Carcinoma: NTCTCS Registry Analysis 1987-2012. J. Clin. Endocrinol. Metab. 2015, 100, 3270–3279. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Al-Attar, N.C.; Brown, O.H.; Shaughness, G.G.; Rosculet, N.P.; Avram, A.M.; Hughes, D.T. Location and Causation of Residual Lymph Node Metastasis After Surgical Treatment of Regionally Advanced Differentiated Thyroid Cancer. Thyroid 2018, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Palmer, F.L.; Nixon, I.J.; Thomas, D.; Patel, S.G.; Shaha, A.R.; Shah, J.P.; Tuttle, R.M.; Ganly, I. Multi-Organ Distant Metastases Confer Worse Disease-Specific Survival in Differentiated Thyroid Cancer. Thyroid 2014, 24, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Ylli, D.; Van Nostrand, D.; Wartofsky, L. Conventional Radioiodine Therapy for Differentiated Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.I.; Kim, S.W.; Jung, J.; Jeon, M.J.; Kim, W.G.; Kim, T.Y.; Kim, H.K.; Kang, H.C.; Han, J.M.; et al. Prognosis of Differentiated Thyroid Carcinoma with Initial Distant Metastasis: A Multicenter Study in Korea. Endocrinol. Metab. 2018, 33, 287–295. [Google Scholar] [CrossRef]
- Higashi, T.; Nishii, R.; Yamada, S.; Nakamoto, Y.; Ishizu, K.; Kawase, S.; Togashi, K.; Itasaka, S.; Hiraoka, M.; Misaki, T.; et al. Delayed Initial Radioactive Iodine Therapy Resulted in Poor Survival in Patients with Metastatic Differentiated Thyroid Carcinoma: A Retrospective Statistical Analysis of 198 Cases. J. Nucl. Med. 2011, 52, 683–689. [Google Scholar] [CrossRef]
- Diessl, S.; Holzberger, B.; Mäder, U.; Grelle, I.; Smit, J.W.A.; Buck, A.K.; Reiners, C.; Verburg, F.A. Impact of Moderate vs Stringent TSH Suppression on Survival in Advanced Differentiated Thyroid Carcinoma. Clin. Endocrinol. 2012, 76, 586–592. [Google Scholar] [CrossRef]
- Van Nostrand, D. 131I Treatment of Distant Metastases. In Thyroid Cancer: A Comprehensive Guide to Clinical Management; Springer: New York, NY, USA, 2016; pp. 595–627. [Google Scholar] [CrossRef]
- Giovanella, L.; Van Nostrand, D. Advanced Differentiated Thyroid Cancer: When to Stop Radioiodine? Q. J. Nucl. Med. Mol. Imaging 2019, 63, 267–270. [Google Scholar] [CrossRef]
- Avram, A.M.; Giovanella, L.; Greenspan, B.; Lawson, S.A.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Ovčariček, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Nuclear Medicine Evaluation and Therapy of Differentiated Thyroid Cancer: Abbreviated Version. J. Nucl. Med. 2022, 63, 15N–35N. [Google Scholar]
- Chung, J.K.; Kim, H.W.; Youn, H.; Cheon, G.J. Sodium Iodide Symporter (NIS) in the Management of Patients with Thyroid Carcinoma. Nucl. Med. Mol. Imaging 2018, 52, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Duntas, L.H. Management of Endocrine Disease: The Role of RhTSH in the Management of Differentiated Thyroid Cancer: Pros and Cons. Eur. J. Endocrinol. 2019, 181, R133–R145. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.; Sherman, S.I.; Skarulis, M.C.; Reynolds, J.R.; Lassmann, M.; Hänscheid, H.; Reiners, C. Comparison of Radioiodine Biokinetics Following the Administration of Recombinant Human Thyroid Stimulating Hormone and after Thyroid Hormone Withdrawal in Thyroid Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Hänscheid, H.; Lassmann, M.; Luster, M.; Thomas, S.R.; Pacini, F.; Ceccarelli, C.; Ladenson, P.W.; Wahl, R.L.; Schlumberger, M.; Ricard, M.; et al. Iodine Biokinetics and Dosimetry in Radioiodine Therapy of Thyroid Cancer: Procedures and Results of a Prospective International Controlled Study of Ablation after RhTSH or Hormone Withdrawal. J. Nucl. Med. 2006, 47, 648–654. [Google Scholar]
- Taïeb, D.; Jacob, T.; Zotian, E.; Mundler, O. Lack of Efficacy of Recombinant Human Thyrotropin versus Thyroid Hormone Withdrawal for Radioiodine Therapy Imaging in a Patient with Differentiated Thyroid Carcinoma Lung Metastases. Thyroid 2004, 14, 465–467. [Google Scholar] [CrossRef]
- Freudenberg, L.S.; Jentzen, W.; Petrich, T.; Frömke, C.; Marlowe, R.J.; Heusner, T.; Brandau, W.; Knapp, W.H.; Bockisch, A. Lesion Dose in Differentiated Thyroid Carcinoma Metastases after RhTSH or Thyroid Hormone Withdrawal: 124I PET/CT Dosimetric Comparisons. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2267–2276. [Google Scholar] [CrossRef]
- Tsai, H.C.; Ho, K.C.; Chen, S.H.; Tseng, J.R.; Yang, L.Y.; Lin, K.J.; Cheng, J.C.; Liou, M.J. Feasibility of Recombinant Human TSH as a Preparation for Radioiodine Therapy in Patients with Distant Metastases from Papillary Thyroid Cancer: Comparison of Long-Term Survival Outcomes with Thyroid Hormone Withdrawal. Diagnostics 2022, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pereira, J.; Ferreira, T.C.; Limbert, E.; Cavaco, B.M.; Leite, V. Outcomes of Thyrotropin Alfa Versus Levothyroxine Withdrawal-Aided Radioiodine Therapy for Distant Metastasis of Papillary Thyroid Cancer. Thyroid 2021, 31, 1514–1522. [Google Scholar] [CrossRef]
- Hong, C.M.; Kim, C.Y.; Son, S.H.; Jung, J.H.; Lee, C.H.; Jeong, J.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. I-131 Biokinetics of Remnant Normal Thyroid Tissue and Residual Thyroid Cancer in Patients with Differentiated Thyroid Cancer: Comparison between Recombinant Human TSH Administration and Thyroid Hormone Withdrawal. Ann. Nucl. Med. 2017, 31, 582–589. [Google Scholar] [CrossRef]
- Gomes-Lima, C.J.; Chittimoju, S.; Wehbeh, L.; Dia, S.; Pagadala, P.; Al-Jundi, M.; Jhawar, S.; Tefera, E.; Mete, M.; Klubo-Gwiezdzinska, J.; et al. Metastatic Differentiated Thyroid Cancer Survival Is Unaffected by Mode of Preparation for 131I Administration. J. Endocr. Soc. 2022, 6, bvac032. [Google Scholar] [CrossRef]
- Hugo, J.; Robenshtok, E.; Grewal, R.; Larson, S.; Tuttle, R.M. Recombinant Human Thyroid Stimulating Hormone-Assisted Radioactive Iodine Remnant Ablation in Thyroid Cancer Patients at Intermediate to High Risk of Recurrence. Thyroid 2012, 22, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J.; Burman, K.D.; Van Nostrand, D.; Mete, M.; Jonklaas, J.; Wartofsky, L. Radioiodine Treatment of Metastatic Thyroid Cancer: Relative Efficacy and Side Effect Profile of Preparation by Thyroid Hormone Withdrawal versus Recombinant Human Thyrotropin. Thyroid 2012, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tala, H.; Robbins, R.; Fagin, J.A.; Larson, S.M.; Tuttle, R.M. Five-Year Survival Is Similar in Thyroid Cancer Patients with Distant Metastases Prepared for Radioactive Iodine Therapy with Either Thyroid Hormone Withdrawal or Recombinant Human TSH. J. Clin. Endocrinol. Metab. 2011, 96, 2105–2111. [Google Scholar] [CrossRef]
- Higuchi, C.R.S.; Fernanda, P.; Jurnior, P.A.; Andrade, F.A.; Corbo, R.; Vaisman, M.; Vaisman, F.; Bulzico, D. Clinical Outcomes After Radioiodine Therapy, according to the Method of Preparation by Recombinant TSH vs. Endogenous Hypothyroidism, in Thyroid Cancer Patients at Intermediate-High Risk of Recurrence. Front. Nucl. Med. 2021, 1, 785768. [Google Scholar] [CrossRef]
- Rosario, P.W.; Mourão, G.F.; Calsolari, M.R. Recombinant Human TSH versus Thyroid Hormone Withdrawal in Adjuvant Therapy with Radioactive Iodine of Patients with Papillary Thyroid Carcinoma and Clinically Apparent Lymph Node Metastases Not Limited to the Central Compartment (CN1b). Arch. Endocrinol. Metab. 2017, 61, 167–172. [Google Scholar] [CrossRef]
- Campopiano, M.C.; Podestà, D.; Bianchi, F.; Giani, C.; Agate, L.; Bottici, V.; Cappagli, V.; Lorusso, L.; Matrone, A.; Puleo, L.; et al. No Difference in the Outcome of Metastatic Thyroid Cancer Patients When Using Recombinant or Endogenous TSH. Eur. J. Endocrinol. 2020, 183, 411–417. [Google Scholar] [CrossRef]
- Tsai, J.R.; Wu, S.T.; Chi, S.Y.; Yang, Y.T.; Chan, Y.C.; Lim, L.S.; Chiew, Y.E.W.; Chen, W.C.; Chen, Y.N.; Chou, C.K. Recombinant Human Thyrotropin versus Thyroid Hormone Withdrawal Preparation for Radioiodine Ablation in Differentiated Thyroid Cancer: Experience in a South Taiwanese Medical Center. Kaohsiung J. Med. Sci. 2023, 39, 175–181. [Google Scholar] [CrossRef]
- Wolfson, R.M.; Rachinsky, I.; Morrison, D.; Driedger, A.; Spaic, T.; Van Uum, S.H.M. Recombinant Human Thyroid Stimulating Hormone versus Thyroid Hormone Withdrawal for Radioactive Iodine Treatment of Differentiated Thyroid Cancer with Nodal Metastatic Disease. J. Oncol. 2016, 2016, 6496750. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.M.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. ESMO Clinical Practice Guideline Update on the Use of Systemic Therapy in Advanced Thyroid Cancer. Ann. Oncol. 2022, 33, 674–684. [Google Scholar] [CrossRef]
- Lee, J.; Yun, M.J.; Nam, K.H.; Chung, W.Y.; Soh, E.Y.; Park, C.S. Quality of Life and Effectiveness Comparisons of Thyroxine Withdrawal, Triiodothyronine Withdrawal, and Recombinant Thyroid-Stimulating Hormone Administration for Low-Dose Radioiodine Remnant Ablation of Differentiated Thyroid Carcinoma. Thyroid 2010, 20, 173–179. [Google Scholar] [CrossRef]
- Botella-Carretero, J.I.; Galán, J.M.; Caballero, C.; Sancho, J.; Escobar-Morreale, H.F. Quality of Life and Psychometric Functionality in Patients with Differentiated Thyroid Carcinoma. Endocr. Relat. Cancer 2003, 10, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Tagay, S.; Herpertz, S.; Langkafel, M.; Erim, Y.; Freudenberg, L.; Schöpper, N.; Bockisch, A.; Senf, W.; Görges, R. Health-Related Quality of Life, Anxiety and Depression in Thyroid Cancer Patients under Short-Term Hypothyroidism and TSH-Suppressive Levothyroxine Treatment. Eur. J. Endocrinol. 2005, 153, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.R.; Haugen, B.R.; Pacini, F.; Reiners, C.; Schlumberger, M.; Sherman, S.I.; Cooper, D.S.; Schuff, K.G.; Braverman, L.E.; Skarulis, M.C.; et al. A Comparison of Short-Term Changes in Health-Related Quality of Life in Thyroid Carcinoma Patients Undergoing Diagnostic Evaluation with Recombinant Human Thyrotropin Compared with Thyroid Hormone Withdrawal. J. Clin. Endocrinol. Metab. 2006, 91, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Tagay, S.; Herpertz, S.; Langkafel, M.; Erim, Y.; Bockisch, A.; Senf, W.; Görges, R. Health-Related Quality of Life, Depression and Anxiety in Thyroid Cancer Patients. Qual. Life Res. 2006, 15, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Taïeb, D.; Sebag, F.; Cherenko, M.; Baumstarck-Barrau, K.; Fortanier, C.; Farman-Ara, B.; De Micco, C.; Vaillant, J.; Thomas, S.; Conte-Devolx, B.; et al. Quality of Life Changes and Clinical Outcomes in Thyroid Cancer Patients Undergoing Radioiodine Remnant Ablation (RRA) with Recombinant Human TSH (RhTSH): A Randomized Controlled Study. Clin. Endocrinol. 2009, 71, 115–123. [Google Scholar] [CrossRef]
- Badihian, S.; Jalalpour, P.; Mirdamadi, M.; Moslehi, M. Quality of Life, Anxiety and Depression in Patients with Differentiated Thyroid Cancer under Short Term Hypothyroidism Induced by Levothyroxine Withdrawal. Klin. Onkol. 2016, 29, 439–444. [Google Scholar] [CrossRef]
- Smith, C.D.; Grondin, R.; LeMaster, W.; Martin, B.; Gold, B.T.; Ain, K.B. Reversible Cognitive, Motor, and Driving Impairments in Severe Hypothyroidism. Thyroid 2015, 25, 28–36. [Google Scholar] [CrossRef]
- Wu, S.Q.; Feng, F.; Zou, R.J.; Fu, H.L.; Sun, J.W.; Jia, X.Z.; Yin, Y.F.; Wang, H. Abnormal Brain Glucose Metabolism in Papillary Thyroid Cancer Patients 4 Weeks After Withdrawal of Levothyroxine: A Cross-Sectional Study Using 18F-FDG PET/CT. Front. Endocrinol. 2021, 12, 595933. [Google Scholar] [CrossRef]
- Duntas, L.H.; Biondi, B. Short-Term Hypothyroidism after Levothyroxine-Withdrawal in Patients with Differentiated Thyroid Cancer: Clinical and Quality of Life Consequences. Eur. J. Endocrinol. 2007, 156, 13–19. [Google Scholar] [CrossRef]
- Münte, T.F.; Lill, C.; Ötting, G.; Brabant, G. Cognitive Changes in Short-Term Hypothyroidism Assessed with Event-Related Brain Potentials. Psychoneuroendocrinology 2004, 29, 1109–1118. [Google Scholar] [CrossRef]
- Constant, E.L.; De Volder, A.G.; Ivanoiu, A.; Bol, A.; Labar, D.; Seghers, A.; Cosnard, G.; Melin, J.; Daumerie, C. Cerebral Blood Flow and Glucose Metabolism in Hypothyroidism: A Positron Emission Tomography Study. J. Clin. Endocrinol. Metab. 2001, 86, 3864–3870. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Choi, E.K.; Song, I.U.; Chung, Y.A.; Park, J.S.; Oh, J.K. Differences in Brain Glucose Metabolism During Preparation for 131I Ablation in Thyroid Cancer Patients: Thyroid Hormone Withdrawal Versus Recombinant Human Thyrotropin. Thyroid 2017, 27, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lodemann, E.; Bockisch, A.; Görges, R. Short-Term Hypothyroidism in Thyroid Cancer Patients and Cognitive-Motor Performance Relevant for Driving. Psychoneuroendocrinology 2012, 37, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Dueren, C.; Dietlein, M.; Luster, M.; Plenzig, F.; Steinke, R.; Grimm, J.; Groth, P.; Eichhorn, W.; Reiners, C. The Use of Thyrogen in the Treatment of Differentiated Thyroid Carcinoma: An Intraindividual Comparison of Clinical Effects and Implications of Daily Life. Exp. Clin. Endocrinol. Diabetes 2010, 118, 513–519. [Google Scholar] [CrossRef]
- Wang, T.S.; Cheung, K.; Mehta, P.; Roman, S.A.; Walker, H.D.; Sosa, J.A. To Stimulate or Withdraw? A Cost-Utility Analysis of Recombinant Human Thyrotropin versus Thyroxine Withdrawal for Radioiodine Ablation in Patients with Low-Risk Differentiated Thyroid Cancer in the United States. J. Clin. Endocrinol. Metab. 2010, 95, 1672–1680. [Google Scholar] [CrossRef]
- Mernagh, P.; Campbell, S.; Dietein, M.; Luster, M.; Mazzaferri, E.; Weston, A.R. Cost-Effectiveness of Using Recombinant Human TSH Prior to Radioiodine Ablation for Thyroid Cancer, Compared with Treating Patients in a Hypothyroid State: The German Perspective. Eur. J. Endocrinol. 2006, 155, 405–414. [Google Scholar] [CrossRef]
- Mernagh, P.; Suebwongpat, A.; Silverberg, J.; Weston, A. Cost-Effectiveness of Using Recombinant Human Thyroid-Stimulating Hormone before Radioiodine Ablation for Thyroid Cancer: The Canadian Perspective. Value Health 2010, 13, 180–187. [Google Scholar] [CrossRef]
- Dietlein, M.; Busemeyer, S.; Kobe, C.; Schmidt, M.; Theissen, P.; Schicha, H. Recombinant Human TSH versus Hypothyroidism. Cost-Minimization-Analysis in the Follow-up Care of Differentiated Thyroid Carcinoma. Nuklearmedizin 2010, 49, 216–224. [Google Scholar] [CrossRef]
- Sohn, S.Y.; Jang, H.W.; Cho, Y.Y.; Kim, S.W.; Chung, J.H. Economic Evaluation of Recombinant Human Thyroid Stimulating Hormone Stimulation vs. Thyroid Hormone Withdrawal Prior to Radioiodine Ablation for Thyroid Cancer: The Korean Perspective. Endocrinol. Metab. 2015, 30, 531–542. [Google Scholar] [CrossRef]
- Fu, H.; Ma, C.; Tang, L.; Wu, F.; Liu, B.; Wang, H. Recombinant Human Thyrotropin versus Thyroid Hormone Withdrawal in Radioiodine Remnant Ablation for Differentiated Thyroid Cancer: A Meta-Analysis. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 121–128. [Google Scholar] [PubMed]
- Iakovou, I.; Goulis, D.G.; Tsinaslanidou, Z.; Giannoula, E.; Katsikaki, G.; Konstantinidis, I. Effect of Recombinant Human Thyroid-Stimulating Hormone or Levothyroxine Withdrawal on Salivary Gland Dysfunction after Radioactive Iodine Administration for Thyroid Remnant Ablation. Head Neck 2016, 38 (Suppl. S1), E227–E230. [Google Scholar] [CrossRef] [PubMed]
- Mariani, G.; Ferdeghini, M.; Augeri, C.; Villa, G.; Taddei, G.Z.; Scopinaro, G.; Boni, G.; Bodei, L.; Rabitti, C.; Molinari, E.; et al. Clinical Experience with Recombinant Human Thyrotrophin (RhTSH) in the Management of Patients with Differentiated Thyroid Cancer. Cancer Biother. Radiopharm. 2000, 15, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, R.; Yamane, S.; Seto, T. Long-Term Safety and Effectiveness of Thyrotropin Alfa in Japanese Patients: A Post-Marketing Surveillance Study. Adv. Ther. 2021, 38, 4949–4960. [Google Scholar] [CrossRef]
- Vargas, G.E.; Uy, H.; Bazan, C.; Guise, T.A.; Bruder, J.M. Hemiplegia after Thyrotropin Alfa in a Hypothyroid Patient with Thyroid Carcinoma Metastatic to the Brain. J. Clin. Endocrinol. Metab. 1999, 84, 3867–3871. [Google Scholar] [CrossRef]
- Braga, M.; Ringel, M.D.; Cooper, D.S. Sudden Enlargement of Local Recurrent Thyroid Tumor after Recombinant Human TSH Administration. J. Clin. Endocrinol. Metab. 2001, 86, 5148–5151. [Google Scholar] [CrossRef]
- Goffman, T.; Ioffe, V.; Tuttle, M.; Bowers, J.T.; Mason, M.E. Near-Lethal Respiratory Failure after Recombinant Human Thyroid-Stimulating Hormone Use in a Patient with Metastatic Thyroid Carcinoma. Thyroid 2003, 13, 827–830. [Google Scholar] [CrossRef]
- Vethakkan, S.R.; Roberts, V.; Ward, G.M. Sudden Onset of Haemoptysis and Hypoxia after Recombinant Human Thyroid-Stimulating Hormone Use in a Patient with Papillary Thyroid Carcinoma and Pulmonary Metastases. Intern. Med. J. 2009, 39, 854–855. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Coppes, M.H.; Bongaerts, A.H.H.; Glaudemans, A.W.J.M.; Links, T.P. Unexpected Symptoms after RhTSH Administration Due to Occult Thyroid Carcinoma Metastasis. Neth. J. Med. 2013, 71, 253–256. [Google Scholar]
- Dowling, E.; Kasperbauer, J.; Morris, J.; Bayan, S. Acute Airway Compromise after Recombinant Human TSH Administration: A Case Report and Review of the Literature. Laryngoscope 2020, 130, 2725–2727. [Google Scholar] [CrossRef]
- Pötzi, C.; Moameni, A.; Karanikas, G.; Preitfellner, J.; Becherer, A.; Pirich, C.; Dudczak, R. Comparison of Iodine Uptake in Tumour and Nontumour Tissue under Thyroid Hormone Deprivation and with Recombinant Human Thyrotropin in Thyroid Cancer Patients. Clin. Endocrinol. 2006, 65, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Hung, G.U.; Ho, M.; Kao, C.H. Faster Radioiodine Washout in the Treatment of Pulmonary Metastases of Papillary Thyroid Cancer Prepared with Recombinant Human Thyroid-Stimulating Hormone. Clin. Nucl. Med. 2009, 34, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Plyku, D.; Hobbs, R.F.; Huang, K.; Atkins, F.; Garcia, C.; Sgouros, G.; Van Nostrand, D. Recombinant Human Thyroid-Stimulating Hormone Versus Thyroid Hormone Withdrawal in 124 I PET/CT-Based Dosimetry for 131 I Therapy of Metastatic Differentiated Thyroid Cancer. J. Nucl. Med. 2017, 58, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.Y.; Ehya, H.; et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef] [PubMed]


| Author | Design | Country | Period | Sample | Age (years) | Sex (Male/Female) | DTC | Follow-Up | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | rhTSH | THW | rhTSH | TWH | rhTSH | TWH | ||||||
| Campopiano et al. (2020) [28] | RS | Italy | 2001–2017 | 77 | 43 | 34 | rhTSH: 48 ± 20 | rhTSH: 21/43 | PTC: 35; FTC: 8 | PTC: 29; DTC: 5 | 45 ± 46 mo | 48 ± 27 mo |
| THW: 41 ± 17 | THW:16/34 | |||||||||||
| Gomes-Lima et al. (2022) [22] | RS | USA | 1996–2017 | 55 | 27 | 28 | rhTSH: 59 (47.5–65.5) | rhTSH: 5/22 | PTC: 19; FTC: 6; HCC: 1; PD: 1 | PTC: 21; FTC: 4;HCC: 3; PD: 0 | 4.2 years (3.3–5.5) | 6.9 yr (4.2–11.6) |
| THW: 41 (30.9–63.5) | THW: 11/17 | |||||||||||
| Higuchi et al. (2021) [26] | RS | Brazil | 1997–2019 | 70 | 21 | 49 | rhTSH: 63 (24–83) | rhTSH: 9/12 | PTC: 13; FTC: 5; HCC: 1; PD: 2 | PTC: 31; FTC: 9; HCC: 5; PD: 4 | 61 mo (19–149) | 88 mo (13–241) |
| THW: 63 (31–79) | THW: 20/29 | |||||||||||
| Hugo et al. (2012) [23] | RS | USA | 1994–2004 | 586 | 265 | 321 | rhTSH: 46 ± 15 | rhTSH: 180/85 | PTC: 225; PD: 12; FTC: 10; HCC: 18 | PTC: 271; PD: 26; FTC: 13; HCC: 11 | 8.1 ± 3.3 years | 8.8 ± 3.6 yr |
| THW: 47 ± 15 | THW: 221/100 | |||||||||||
| Klubo-Gwiezdzinska et al. (2011) [24] | RS | USA | 1996–2009 | 56 | 15 | 41 | rhTSH: 62.4 ± 12.6 | rhTSH: 4/11 | PTC: 36; FTC: 8; HCC: 7; Other: 5 | 72 ± 36.2 mo | ||
| THW: 48.8 ± 18.2 | THW: 21/20 | |||||||||||
| Rosario et al. (2017) [27] | RS | Brazil | 2006–2014 | 178 | 91 | 87 | rhTSH: 47 (18–76) | rhTSH:23/68 | Only PTC | 64 mo (24–118) | 68 mo (18–118) | |
| THW: 48 (18–72) | THW: 21/66 | |||||||||||
| Simoes-Pereira et al. (2021) [20] | RS | Portugal | 2006–2018 | 94 | 67 | 27 | rhTSH *: 65.5 (22–85) | rhTSH: 27/41 | Only PTC | 68 mo * (8–332) | 120 mo * (9–332) | |
| THW *: 58.9 (20–77) | THW: 7/20 | |||||||||||
| Tala et al. (2011) [25] | RS | USA | 1993–2010 | 93 | 58 | 35 | rhTSH: 60 (20–89) | rhTSH: 28/30 | PTC: 29; FTC. 5; HCC: 4; PD: 16; Other: 4 | PTC: 20; FTC. 6; PD: 8; Other: 1 | 3.4 mo (1.3–10.3) | 6.9 mo (1.4–17.1) |
| THW: 56 (24–80) | THW: 15/20 | |||||||||||
| Tsai et al. (2023) [29] | RS | Taiwan | 2013–2018 | 647 | 324 | 323 | rhTSH: 49.66 ± 14.40 | rhTSH: 72/252 | PTC: 307; HCC: 2; FTC: 15 | PTC: 306; HCC: 5; FTC: 12 | NR | NR |
| THW: 49.29 ± 13.06 | THW: 88/235 | |||||||||||
| Wolfson et al. (2016) [30] | RS | Canada | 2007–2018 | 73 | 42 | 31 | rhTSH: 45.7 ± 16.2 | rhTSH: 17/25 | PTC: 41; PD: 1 | PTC: 29; PD: 1; Other: 1 | 6.8 ± 2.1 | 8.6 ± 2.4 |
| THW: 38.2 ± 12.4 | THW: 6/25 | |||||||||||
| Authors | Selection | Comparability | Outcome |
|---|---|---|---|
| Campopiano et al. (2020) [28] | **** | * | ** |
| Gomes-Lima et al. (2022) [22] | **** | * | ** |
| Higuchi et al. (2021) [26] | **** | * | ** |
| Hugo et al. (2012) [23] | **** | * | ** |
| Klubo-Gwiezdzinska et al. (2011) [24] | **** | * | ** |
| Rosario et al. (2017) [27] | **** | * | ** |
| Simoes-Pereira et al. (2021) [20] | **** | * | ** |
| Tala et al. (2011) [25] | **** | * | ** |
| Tsai et al. (2023) [29] | **** | * | ** |
| Wolfson et al. (2016) [30] | **** | * | ** |
| Author | Outcome |
|---|---|
| Campopiano et al. (2020) [28] | RECIST 1.1 criteria |
| Gomes-Lima et al. (2022) [22] | RECIST 1.1 criteria |
| Higuchi et al. (2021) [26] | Stable disease = no structural progression in the last year of follow-up; Disease progression = an increase or appearance of a new structural lesion in the last year of follow-up |
| Hugo et al. (2012) [23] | Best response to initial therapy (first two years of follow-up): excellent response (suppressed and stimulated Tg < 1 ng/mL; neck US with no evidence of disease and no other cross sectional or functional evidence of disease); acceptable response (suppressed Tg < 1 ng/mL with stimulated Tg 1–10 ng/mL or non-specific findings on neck US or other imaging); incomplete response (suppressed Tg > 1 ng/mL, stimulated Tg > 10 ng/mL, or structural evidence of persistent disease). Clinical status at the time of last follow-up: no evidence of disease (suppressed Tg < 1 ng/mL or no detectable anti-Tg antibody and no structural or functional evidence of disease); persistent disease (suppressed Tg values > 1 ng/mL; stimulated Tg values > 2 ng/mL; evidence of persistent disease in structural or function imaging or biopsy-proven disease); recurrent disease (suppressed Tg > 1 ng/mL or structural or functional evidence of disease identified following a period of no evidence of disease) |
| Klubo-Gwiezdzinska et al. (2011) [24] | RECIST 1.1 criteria |
| Rosario et al. (2017) [27] | (i) Rate of excellent response to therapy, i.e., nonstimulated Tg ≤ 0.2 ng/mL, with negative TgAb and negative neck US, one year after RAI (1–4); (ii) structural disease one year after RAI; (iii) structural or biochemical (nonstimulated Tg > 1 ng/mL, with increment) recurrence during follow-up, and (iv) percentage of patients without disease in the last assessment, i.e., nonstimulated Tg < 1 ng/mL and no evidence of structural disease |
| Simoes-Pereira et al. (2021) [20] | (i) Malignant tissue that does not concentrate RAI on a post-RAIT WBS; (ii) tumor tissue that loses the ability to concentrate RAI after previous evidence of RAI-avid disease; (iii) RAI uptake that is concentrated in some lesions, but not in others; (iv) metastatic disease that progresses despite significant concentration of RAI; and (v) 600 mCi of cumulative RAIT |
| Tala et al. (2011) [25] | Progression disease |
| Tsai et al. (2023) [29] | Excellent treatment response (non-stimulated Tg levels of <0.2 ng/mL, undetectable TgAb, and negative imaging on neck ultrasonography and DxWBS); biological incomplete response (abnormal Tg levels, rising anti-Tg antibody levels, and lack of localizable disease on imaging); structural incomplete response (persistent, newly loco-regional, or distant metastases revealed on thyroid ultrasonography or other imaging); indeterminate response (non-specific biochemical or structural findings, which could not be classified as either benign or malignant) |
| Wolfson et al. (2016) [30] | Response to initial treatment: excellent (both suppressed and stimulated Tg were <1 ng/mL and no evidence of disease on neck ultrasound, whole body iodine scan, or CT scan); acceptable response (suppressed Tg < 1 ng/mL, stimulated Tg 1–10 ng/mL, and/or equivocal findings on diagnostic imaging); incomplete response (suppressed Tg > 1 ng/mL, stimulated Tg > 10 ng/mL, and/or evidence of persistent disease on diagnostic imaging). Final outcome: no evidence of disease (suppressed Tg < 1 ng/mL, no detectable anti-Tg antibody, and no structural evidence of disease on clinical examination or radiological studies); persistent disease (suppressed Tg values > 1 ng/mL, stimulated Tg values > 2 ng/ mL, and/or evidence of persistent disease in structural or functional imaging); recurrent disease (suppressed Tg > 1 ng/mL and/or structural or functional evidence of disease identified following a period of no evidence of disease) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanella, L.; Garo, M.L.; Campenní, A.; Petranović Ovčariček, P.; Görges, R. Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 2510. https://doi.org/10.3390/cancers15092510
Giovanella L, Garo ML, Campenní A, Petranović Ovčariček P, Görges R. Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(9):2510. https://doi.org/10.3390/cancers15092510
Chicago/Turabian StyleGiovanella, Luca, Maria Luisa Garo, Alfredo Campenní, Petra Petranović Ovčariček, and Rainer Görges. 2023. "Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis" Cancers 15, no. 9: 2510. https://doi.org/10.3390/cancers15092510
APA StyleGiovanella, L., Garo, M. L., Campenní, A., Petranović Ovčariček, P., & Görges, R. (2023). Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers, 15(9), 2510. https://doi.org/10.3390/cancers15092510






