Comprehensive Brain Tumour Characterisation with VERDICT-MRI: Evaluation of Cellular and Vascular Measures Validated by Histology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisition
2.3. Processing of Diffusion and Perfusion MRI
2.4. Diffusion Models of Tissue
2.5. Selection of the VERDICT Mathematical Model for Brain Tumours
- To select the best extracellular compartment model without the confounding effect of a low-b dMRI signal that is mainly related to pseudo diffusion, we used the Corrected Akaike’s Information Criterion (AICc) to evaluate the fitting performance of the two- and three-compartment models on high-b data (b > 200 s/mm2, excluding the first 4 shells in Table 1);
- To assess the fitting performance on the full signal, we evaluated the AICc again on the best-performing models from (1) with the addition of the vascular compartment (Ball or AstroSticks), and with and without FWE;
- We evaluated anisotropic measures in the extracellular compartment of the same models as in (2), fitted to the full signal. ODI from NODDI was considered as the gold standard;
- To highlight issues of ambiguity between pseudo-diffusion and diffusion with high diffusivity, we investigated estimates of the Vascular Fraction (fvasc) from the same models as in (2), fitted to the full signal, in areas where NODDI provided fiso >0.5 and we did not expect any significant vascularity.
2.6. Histology
2.7. Statistical Analysis
3. Results
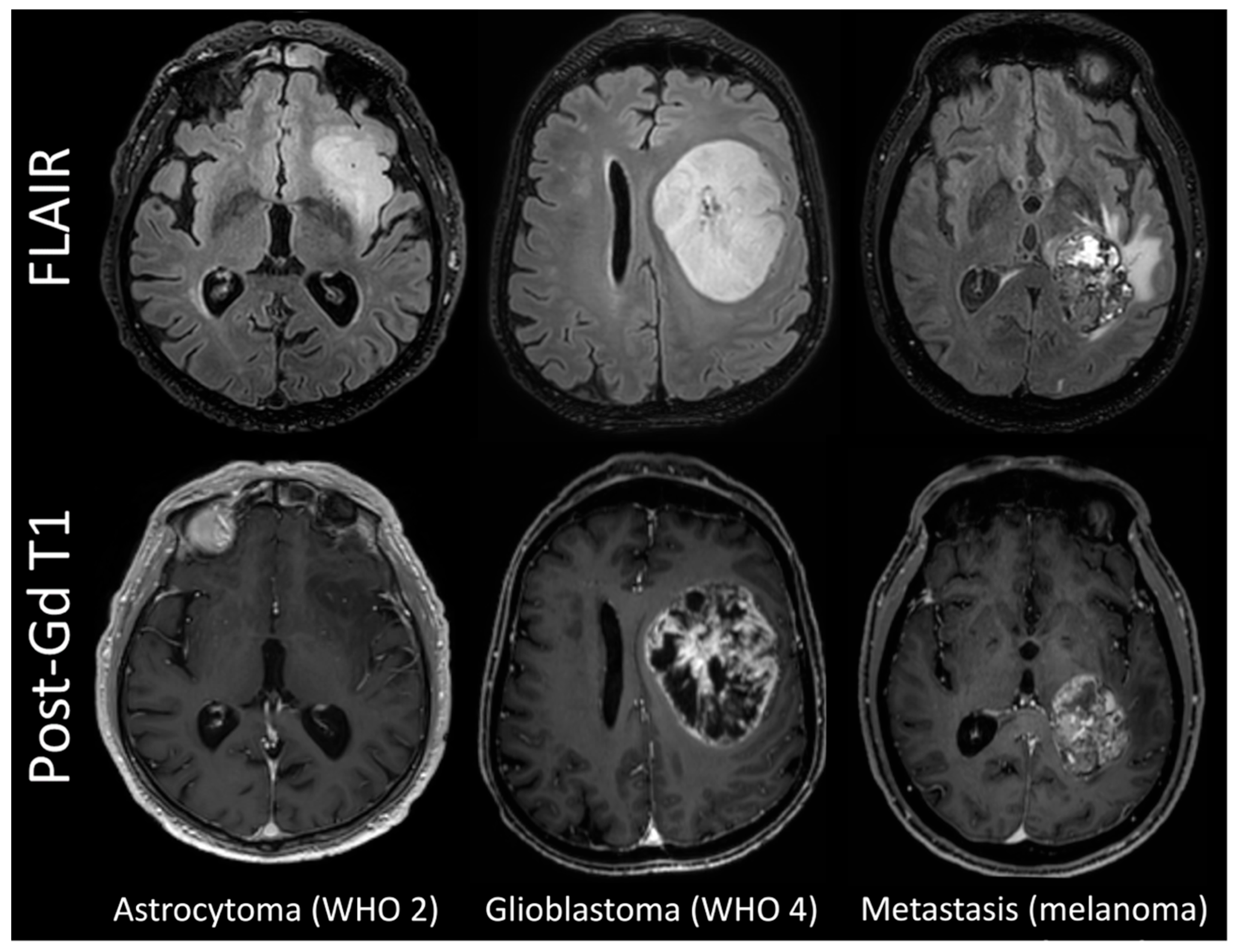
3.1. Patient Population
3.2. Selection of the VERDICT Mathematical Model for Brain Tumours
- In the comparison between two- and three-compartment models at high b, all of the top-performing (lower AICs) models included Tensor and/or Zeppelin to describe the extracellular and extravascular compartments, whereas models including Stick, Watson-distributed Sticks, or Cylinder to describe the extracellular and extravascular compartments performed worse on average. For this reason, only Tensor and Zeppelin were considered as candidates for the extracellular compartment in the following experiments. The considered models are ranked according to the average AICc across patients in Supplementary Table S3;
- In all cases, the AICc of the model with FWE was significantly lower (better fitting) or very similar than that without FWE. The difference was higher in peritumoural areas and when the pseudo-diffusivity was fixed in the vascular compartment. The average AICc values of the considered models, fitted to the full signal, are reported in Table 3;
- Comparing equivalent models in which the only difference was the form of the extracellular compartment (Zeppelin or Tensor), models with Zeppelin showed stronger correlations with NODDI. The correlation coefficients between the ROI-averaged FA of the extracellular compartment from each model and the ROI-averaged ODI from NODDI are listed in Table 4;
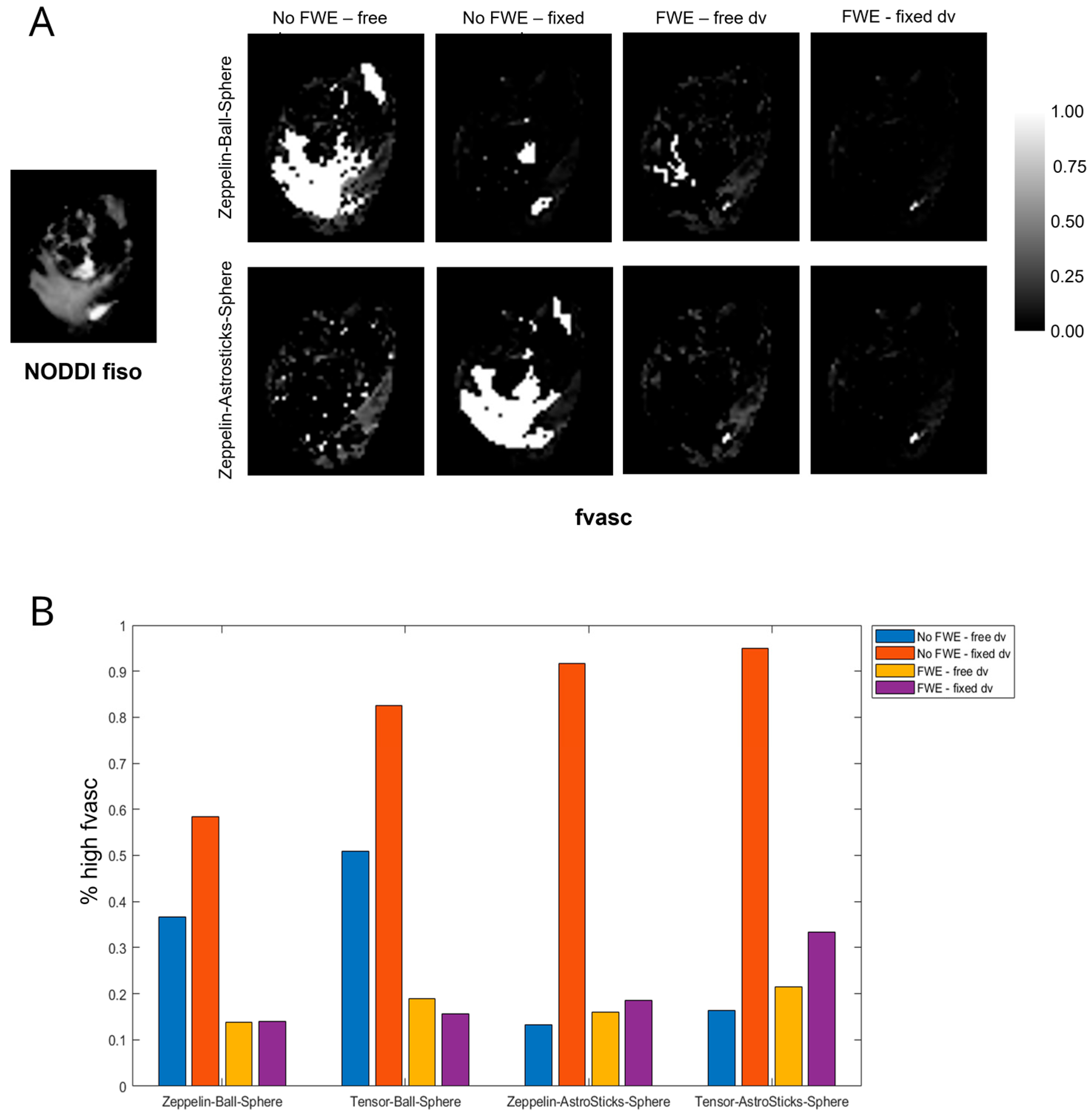
- Very high values of fvasc were often estimated in areas of high fiso in models without FWE and when using Ball (as opposed to AstroSticks) to model the vascular compartment. We assumed that such high values were biased and symptomatic of model degeneracy, as vascularity should be negligible in extracellular areas with high free water content; an example is shown in Figure 2A. To quantitatively assess this observation, we measured the percentage of voxels with fvasc >0.9 for each model out of those with fiso >0.5 in NODDI. The highest values were found for models with fixed diffusivity of the vascular compartment without FWE; FWE reduced the extent of this issue especially when dv was fixed and the AstroSticks model seemed to be more robust (Figure 2B).
3.3. Comparison between Histotypes
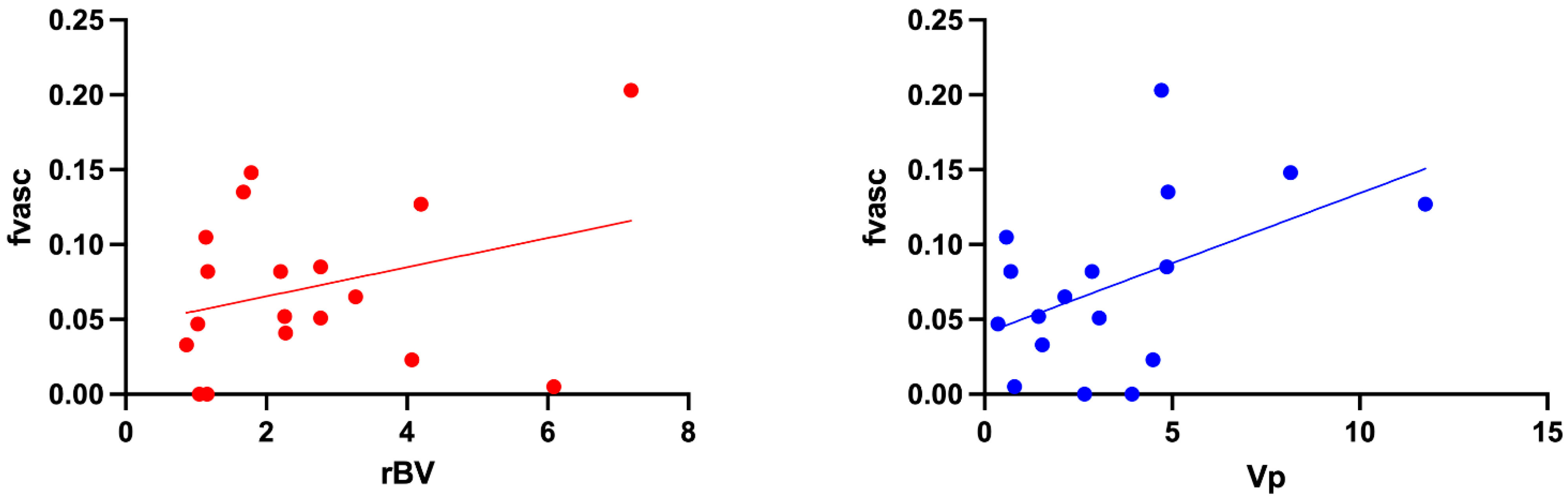
3.4. Comparison between VERDICT Fvasc and PWI Parameters
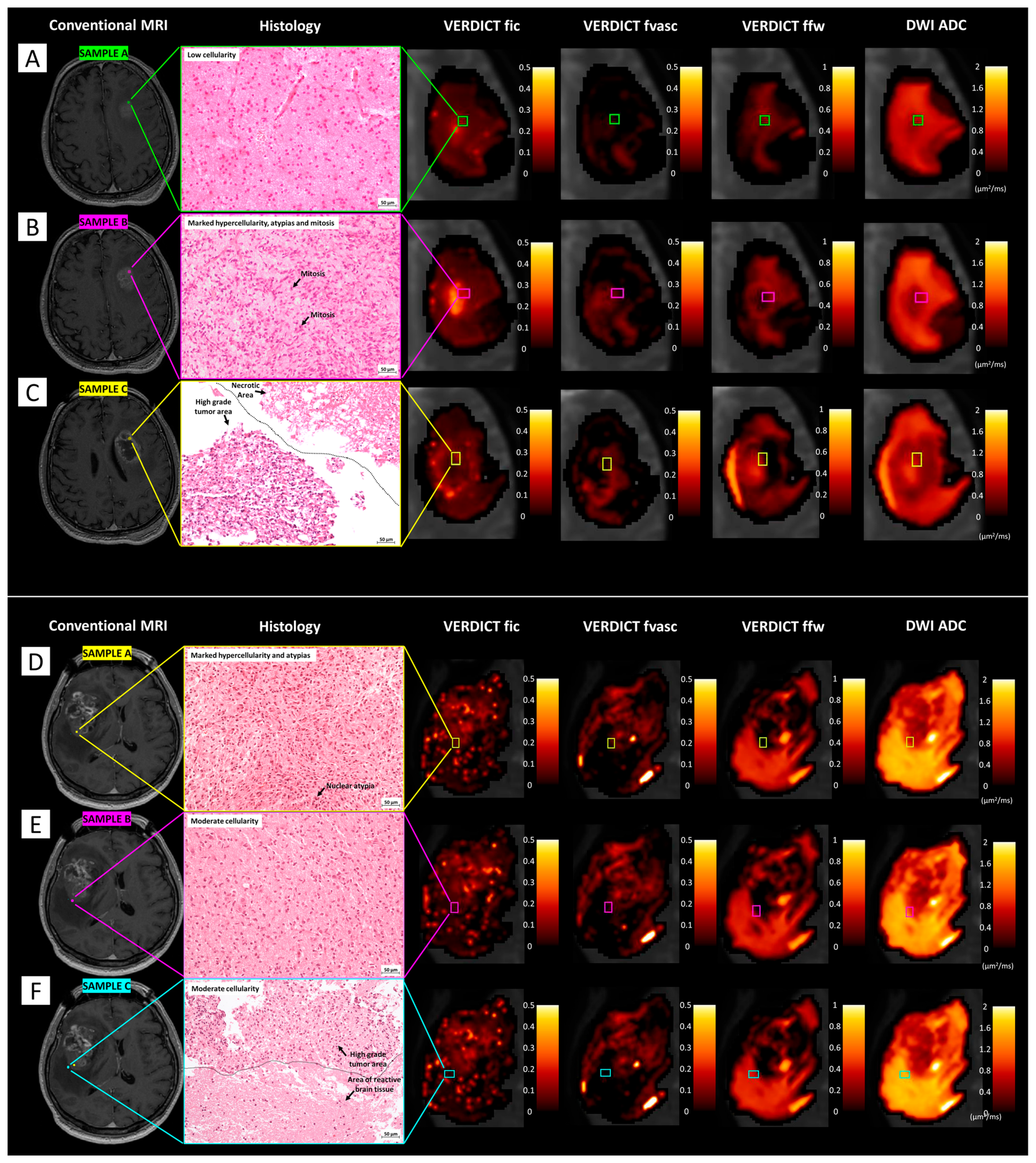
3.5. Comparison between Histology and VERDICT Maps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellingson, B.M.; Wen, P.Y.; van den Bent, M.J.; Cloughesy, T.F. Pros and cons of current brain tumor imaging. Neuro Oncol. 2014, 16 (Suppl. S7), vii2–vii11. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, F.; Castellano, A.; Falini, A. Advancements in Neuroimaging to Unravel Biological and Molecular Features of Brain Tumors. Cancers 2021, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Kochar, P. Brain Cancer: Implication to Disease, Therapeutic Strategies and Tumor Targeted Drug Delivery Approaches. Recent Pat. Anticancer Drug Discov. 2018, 13, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Bendszus, M.; Boxerman, J.; Barboriak, D.; Erickson, B.J.; Smits, M.; Nelson, S.J.; Gerstner, E.; Alexander, B.; Goldmacher, G.; et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015, 17, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.; Thulasi Seetha, S.; Refaee, T.A.G.; Lieverse, R.I.Y.; Granzier, R.W.Y.; Ibrahim, A.; Keek, S.A.; Sanduleanu, S.; Primakov, S.P.; Beuque, M.P.L.; et al. Radiomics: From qualitative to quantitative imaging. Br. J. Radiol. 2020, 93, 20190948. [Google Scholar] [CrossRef]
- Zhou, M.; Scott, J.; Chaudhury, B.; Hall, L.; Goldgof, D.; Yeom, K.W.; Iv, M.; Ou, Y.; Kalpathy-Cramer, J.; Napel, S.; et al. Radiomics in Brain Tumor: Image Assessment, Quantitative Feature Descriptors, and Machine-Learning Approaches. Am. J. Neuroradiol. 2018, 39, 208–216. [Google Scholar] [CrossRef]
- Thust, S.C.; Heiland, S.; Falini, A.; Jager, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef]
- Castellano, A.; Falini, A. Progress in neuro-imaging of brain tumors. Curr. Opin. Oncol. 2016, 28, 484–493. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Pietrzak, A.; Marszałek, A.; Kunikowska, J.; Piotrowski, T.; Medak, A.; Pietrasz, K.; Wojtowicz, J.; Cholewiński, W. Detection of clinically silent brain lesions in [18F]FDG PET/CT study in oncological patients: Analysis of over 10,000 studies. Sci. Rep. 2021, 11, 18293. [Google Scholar] [CrossRef]
- Treglia, G.; Muoio, B.; Trevisi, G.; Mattoli, M.V.; Albano, D.; Bertagna, F.; Giovanella, L. Diagnostic Performance and Prognostic Value of PET/CT with Different Tracers for Brain Tumors: A Systematic Review of Published Meta-Analyses. Int. J. Mol. Sci. 2019, 20, 4669. [Google Scholar] [CrossRef]
- Nilsson, M.; Englund, E.; Szczepankiewicz, F.; van Westen, D.; Sundgren, P.C. Imaging brain tumour microstructure. Neuroimage 2018, 182, 232–250. [Google Scholar] [CrossRef]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Shigematu, Y.; Hirai, T.; Okuda, T.; Liang, L.; Ge, Y.; Komohara, Y.; et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 1999, 9, 53–60. [Google Scholar] [CrossRef]
- Doskaliyev, A.; Yamasaki, F.; Ohtaki, M.; Kajiwara, Y.; Takeshima, Y.; Watanabe, Y.; Takayasu, T.; Amatya, V.J.; Akiyama, Y.; Sugiyama, K.; et al. Lymphomas and glioblastomas: Differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3T. Eur. J. Radiol. 2012, 81, 339–344. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Bao, J.; Xia, Y.; Zhang, J.; Zhang, L.; Huang, X.; Wang, J. The Correlation between Apparent Diffusion Coefficient and Tumor Cellularity in Patients: A Meta-Analysis. PLoS ONE 2013, 8, e79008. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; du Plessis, D.G.; Smith, T.S.; Brodbelt, A.R.; Joyce, K.A.; Walker, C. Cellularity and apparent diffusion coefficient in oligodendroglial tumours characterized by genotype. J. Neuro-Oncol. 2010, 96, 385–392. [Google Scholar] [CrossRef]
- Maier, S.E.; Sun, Y.; Mulkern, R.V. Diffusion imaging of brain tumors. NMR Biomed. 2010, 23, 849–864. [Google Scholar] [CrossRef]
- Raab, P.; Hattingen, E.; Franz, K.; Zanella, F.E.; Lanfermann, H. Cerebral Gliomas: Diffusional Kurtosis Imaging Analysis of Microstructural Differences. Radiology 2010, 254, 876–881. [Google Scholar] [CrossRef]
- Raja, R.; Sinha, N.; Saini, J.; Mahadevan, A.; Rao, K.V.L.N.; Swaminathan, A. Assessment of tissue heterogeneity using diffusion tensor and diffusion kurtosis imaging for grading gliomas. Neuroradiology 2016, 58, 1217–1231. [Google Scholar] [CrossRef]
- Szczepankiewicz, F.; van Westen, D.; Englund, E.; Westin, C.-F.; Ståhlberg, F.; Lätt, J.; Sundgren, P.C.; Nilsson, M. The link between diffusion MRI and tumor heterogeneity: Mapping cell eccentricity and density by diffusional variance decomposition (DIVIDE). NeuroImage 2016, 142, 522–532. [Google Scholar] [CrossRef]
- Nilsson, M.; Szczepankiewicz, F.; Brabec, J.; Taylor, M.; Westin, C.F.; Golby, A.; van Westen, D.; Sundgren, P.C. Tensor-valued diffusion MRI in under 3 minutes: An initial survey of microscopic anisotropy and tissue heterogeneity in intracranial tumors. Magn. Reson. Med. 2020, 83, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Panagiotaki, E.; Schneider, T.; Siow, B.; Hall, M.G.; Lythgoe, M.F.; Alexander, D.C. Compartment models of the diffusion MR signal in brain white matter: A taxonomy and comparison. Neuroimage 2012, 59, 2241–2254. [Google Scholar] [CrossRef] [PubMed]
- Jelescu, I.O.; Veraart, J.; Fieremans, E.; Novikov, D.S. Degeneracy in model parameter estimation for multi-compartmental diffusion in neuronal tissue. NMR Biomed. 2016, 29, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef]
- Qiuting, W.; Douglas, A.C.K.; Suchandrima, B.; Janine, M.L.; Susan, M.C.; Duan, X.; Christopher, P.H.; Sarah, J.N. Clinically feasible NODDI characterization of glioma using multiband EPI at 7 T. NeuroImage Clin. 2015, 9, 291–299. [Google Scholar] [CrossRef]
- Caverzasi, E.; Papinutto, N.; Castellano, A.; Zhu, A.H.; Scifo, P.; Riva, M.; Bello, L.; Falini, A.; Bharatha, A.; Henry, R.G. Neurite Orientation Dispersion and Density Imaging Color Maps to Characterize Brain Diffusion in Neurologic Disorders. J. Neuroimaging 2016, 26, 494–498. [Google Scholar] [CrossRef]
- Figini, M.; Riva, M.; Graham, M.; Castelli, G.M.; Fernandes, B.; Grimaldi, M.; Baselli, G.; Pessina, F.; Bello, L.; Zhang, H.; et al. Prediction of Isocitrate Dehydrogenase Genotype in Brain Gliomas with MRI: Single-Shell versus Multishell Diffusion Models. Radiology 2018, 289, 788–796. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Xie, J.; McKinley, E.T.; Zhao, P.; Gore, J.C.; Xu, J. In vivo imaging of cancer cell size and cellularity using temporal diffusion spectroscopy. Magn. Reson. Med. 2017, 78, 156–164. [Google Scholar] [CrossRef]
- Svolos, P.; Kousi, E.; Kapsalaki, E.; Theodorou, K.; Fezoulidis, I.; Kappas, C.; Tsougos, I. The role of diffusion and perfusion weighted imaging in the differential diagnosis of cerebral tumors: A review and future perspectives. Cancer Imaging 2014, 14, 20. [Google Scholar] [CrossRef]
- Villanueva-Meyer, J.E.; Mabray, M.C.; Cha, S. Current Clinical Brain Tumor Imaging. Neurosurgery 2017, 81, 397–415. [Google Scholar] [CrossRef]
- Le Bihan, D. What can we see with IVIM MRI? Neuroimage 2019, 187, 56–67. [Google Scholar] [CrossRef]
- Federau, C.; O’Brien, K.; Meuli, R.; Hagmann, P.; Maeder, P. Measuring brain perfusion with intravoxel incoherent motion (IVIM): Initial clinical experience. J. Magn. Reson. Imaging 2014, 39, 624–632. [Google Scholar] [CrossRef]
- Jabehdar Maralani, P.; Myrehaug, S.; Mehrabian, H.; Chan, A.K.M.; Wintermark, M.; Heyn, C.; Conklin, J.; Ellingson, B.M.; Rahimi, S.; Lau, A.Z.; et al. Intravoxel incoherent motion (IVIM) modeling of diffusion MRI during chemoradiation predicts therapeutic response in IDH wildtype glioblastoma. Radiother. Oncol. 2021, 156, 258–265. [Google Scholar] [CrossRef]
- Panagiotaki, E.; Walker-Samuel, S.; Siow, B.; Johnson, S.P.; Rajkumar, V.; Pedley, R.B.; Lythgoe, M.F.; Alexander, D.C. Noninvasive quantification of solid tumor microstructure using VERDICT MRI. Cancer Res. 2014, 74, 1902–1912. [Google Scholar] [CrossRef]
- Panagiotaki, E.; Chan, R.W.; Dikaios, N.; Ahmed, H.U.; O’Callaghan, J.; Freeman, A.; Atkinson, D.; Punwani, S.; Hawkes, D.J.; Alexander, D.C. Microstructural characterization of normal and malignant human prostate tissue with vascular, extracellular, and restricted diffusion for cytometry in tumours magnetic resonance imaging. Investig. Radiol. 2015, 50, 218–227. [Google Scholar] [CrossRef]
- Johnston, E.W.; Bonet-Carne, E.; Ferizi, U.; Yvernault, B.; Pye, H.; Patel, D.; Clemente, J.; Piga, W.; Heavey, S.; Sidhu, H.S.; et al. VERDICT MRI for Prostate Cancer: Intracellular Volume Fraction versus Apparent Diffusion Coefficient. Radiology 2019, 291, 391–397. [Google Scholar] [CrossRef]
- Bailey, C.; Collins, D.J.; Tunariu, N.; Orton, M.R.; Morgan, V.A.; Feiweier, T.; Hawkes, D.J.; Leach, M.O.; Alexander, D.C.; Panagiotaki, E. Microstructure Characterization of Bone Metastases from Prostate Cancer with Diffusion MRI: Preliminary Findings. Front. Oncol. 2018, 8, 26. [Google Scholar] [CrossRef]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-Aron, B.; Sijbers, J.; Fieremans, E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016, 142, 394–406. [Google Scholar] [CrossRef]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef]
- Tournier, J.D.; Smith, R.; Raffelt, D.; Tabbara, R.; Dhollander, T.; Pietsch, M.; Christiaens, D.; Jeurissen, B.; Yeh, C.H.; Connelly, A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019, 202, 116137. [Google Scholar] [CrossRef]
- Nilsson, M.; Szczepankiewicz, F.; Lampinen, B.; Ahlgren, A.; de Almeida Martins, J.P.; Lasic, S.; Westin, C.-F.; Topgaard, D. An open-source framework for analysis of multidimensional diffusion MRI data implemented in MATLAB. In Proceedings of the Joint Annual Meeting ISMRM-ESMRMB, Paris, France, 16–21 June 2018; p. 5355. [Google Scholar]
- Nilsson, M.; Szczepankiewicz, F.; van Westen, D.; Hansson, O. Extrapolation-Based References Improve Motion and Eddy-Current Correction of High B-Value DWI Data: Application in Parkinson’s Disease Dementia. PLoS ONE 2015, 10, e0141825. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Palombo, M.; Ianus, A.; Guerreri, M.; Nunes, D.; Alexander, D.C.; Shemesh, N.; Zhang, H. SANDI: A compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI. NeuroImage 2020, 215, 116835. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Federau, C. Intravoxel incoherent motion MRI as a means to measure in vivo perfusion: A review of the evidence. NMR Biomed. 2017, 30, e3780. [Google Scholar] [CrossRef] [PubMed]
- Dolgorsuren, E.A.; Harada, M.; Kanazawa, Y.; Abe, T.; Otomo, M.; Matsumoto, Y.; Mizobuchi, Y.; Nakajima, K. Correlation and Characteristics of Intravoxel Incoherent Motion and Arterial Spin Labeling Techniques Versus Multiple Parameters Obtained on Dynamic Susceptibility Contrast Perfusion MRI for Brain Tumors. J. Med. Investig. 2019, 66, 308–313. [Google Scholar] [CrossRef]
- Roberts, T.A.; Hyare, H.; Agliardi, G.; Hipwell, B.; d’Esposito, A.; Ianus, A.; Breen-Norris, J.O.; Ramasawmy, R.; Taylor, V.; Atkinson, D.; et al. Noninvasive diffusion magnetic resonance imaging of brain tumour cell size for the early detection of therapeutic response. Sci. Rep. 2020, 10, 9223. [Google Scholar] [CrossRef]
- Zaccagna, F.; Riemer, F.; Priest, A.N.; McLean, M.A.; Allinson, K.; Grist, J.T.; Dragos, C.; Matys, T.; Gillard, J.H.; Watts, C.; et al. Non-invasive assessment of glioma microstructure using VERDICT MRI: Correlation with histology. Eur. Radiol. 2019, 29, 5559–5566. [Google Scholar] [CrossRef]
- Alexander, D.C. A general framework for experiment design in diffusion MRI and its application in measuring direct tissue-microstructure features. Magn. Reson. Med. 2008, 60, 439–448. [Google Scholar] [CrossRef]
- Blumberg, S.B.; Lin, H.; Grussu, F.; Zhou, Y.; Figini, M.; Alexander, D.C. Progressive Subsampling for Oversampled Data—Application to Quantitative MRI. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2022, Singapore, 18–22 September 2022; pp. 421–431. [Google Scholar]
- Grussu, F.; Battiston, M.; Palombo, M.; Schneider, T.; Gandini Wheeler-Kingshott, C.A.M.; Alexander, D.C. Deep learning model fitting for diffusion-relaxometry: A comparative study. bioRxiv 2020. [Google Scholar] [CrossRef]
- de Almeida Martins, J.P.; Nilsson, M.; Lampinen, B.; Palombo, M.; While, P.T.; Westin, C.-F.; Szczepankiewicz, F. Neural networks for parameter estimation in microstructural MRI: Application to a diffusion-relaxation model of white matter. NeuroImage 2021, 244, 118601. [Google Scholar] [CrossRef]
- Molina-Romero, M.; Wiestler, B.; Gómez, P.A.; Menzel, M.I.; Menze, B.H. Deep Learning with Synthetic Diffusion MRI Data for Free-Water Elimination in Glioblastoma Cases. In Proceedings of the Medical Image Computing and Computer Assisted Intervention–MICCAI 2018: 21st International Conference, Granada, Spain, 16–20 September 2018; pp. 98–106. [Google Scholar]
- Zhang, J.; Lemberskiy, G.; Moy, L.; Fieremans, E.; Novikov, D.S.; Kim, S.G. Measurement of cellular-interstitial water exchange time in tumors based on diffusion-time-dependent diffusional kurtosis imaging. NMR Biomed. 2021, 34, e4496. [Google Scholar] [CrossRef]



| b (s/mm2) | 50 | 70 | 90 | 110 | 350 | 1000 | 1500 | 2500 | 3000 | 3500 | 711 | 3000 |
| TE (ms) | 45 | 53 | 43 | 43 | 54 | 78 | 118 | 88 | 103 | 123 | 78 | 78 |
| δ (ms) | 5 | 5 | 5 | 5 | 10 | 10 | 10 | 20 | 15 | 15 | 20 | 20 |
| Δ (ms) | 22 | 30 | 20 | 20 | 26 | 50 | 90 | 50 | 70 | 90 | 42 | 42 |
| Ndir | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 38 | 63 |
| Age | mean 52 years (range 19–77 years) |
| Sex | |
| Male | 16 |
| Female | 5 |
| Histopathology | |
| Glioma | 17 |
| Lower grade glioma (WHO 2-3) | 10 |
| IDH1/2 mutated a | 4 (1 WHO 2, 3 WHO 3) |
| IDH1/2 wild-type a | 6 (3 WHO 2, 3 WHO 3) |
| Glioblastoma (WHO 4) | 5 |
| IDH1/2 wild-type | 5 |
| Other glial tumours (ependymal) a | 2 (1 subependymoma WHO 1, 1 ependymoma WHO 3) |
| Metastasis | 2 (melanoma) |
| Other | 1 radiation necrosis, 1 focal cortical dysplasia |
| Model | Core AICc | Periphery AICc | ||
|---|---|---|---|---|
| No FWE | FWE | No FWE | FWE | |
| Zeppelin–Ball–Sphere | 2538 | 2541 | 2595 | 2582 |
| Zeppelin–Ball–Sphere with fixed dv | 2538 | 2545 | 2583 | 2582 |
| Tensor–Ball–Sphere | 2536 | 2546 | 2606 | 2606 |
| Tensor–Ball–Sphere with fixed dv | 2546 | 2543 | 2632 | 2571 |
| Zeppelin–AstroSticks–Sphere | 2542 | 2543 | 2583 | 2583 |
| Zeppelin–AstroSticks–Sphere with fixed dv | 2616 | 2553 | 3436 | 2598 |
| Tensor–AstroSticks–Sphere | 2541 | 2541 | 2572 | 2574 |
| Tensor–AstroSticks–Sphere with fixed dv | 2668 | 2641 | 5993 | 2726 |
| Zeppelin–Ball –Sphere | 2538 | 2541 | 2595 | 2582 |
| Model (Excluding Extracellular Compartment) | Core | Periphery | ||
|---|---|---|---|---|
| Zeppelin | Tensor | Zeppelin | Tensor | |
| Ball–Sphere | −0.63 | −0.49 | +0.13 | +0.09 |
| Ball–Sphere with fixed dv | −0.50 | −0.48 | −0.19 | −0.04 |
| Ball–Sphere with FWE | −0.65 | −0.44 | −0.35 | −0.11 |
| Ball–Sphere with FWE and fixed dv | −0.46 | −0.45 | −0.39 | −0.42 |
| AstroSticks–Sphere | −0.62 | −0.45 | −0.19 | −0.05 |
| AstroSticks–Sphere with fixed dv | −0.29 | −0.27 | +0.13 | +0.13 |
| AstroSticks–Sphere with FWE | −0.65 | −0.42 | −0.36 | −0.32 |
| AstroSticks–Sphere with FWE and fixed dv | −0.42 | −0.16 | −0.42 | −0.16 |
| Ball–Sphere | −0.63 | −0.49 | +0.13 | +0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figini, M.; Castellano, A.; Bailo, M.; Callea, M.; Cadioli, M.; Bouyagoub, S.; Palombo, M.; Pieri, V.; Mortini, P.; Falini, A.; et al. Comprehensive Brain Tumour Characterisation with VERDICT-MRI: Evaluation of Cellular and Vascular Measures Validated by Histology. Cancers 2023, 15, 2490. https://doi.org/10.3390/cancers15092490
Figini M, Castellano A, Bailo M, Callea M, Cadioli M, Bouyagoub S, Palombo M, Pieri V, Mortini P, Falini A, et al. Comprehensive Brain Tumour Characterisation with VERDICT-MRI: Evaluation of Cellular and Vascular Measures Validated by Histology. Cancers. 2023; 15(9):2490. https://doi.org/10.3390/cancers15092490
Chicago/Turabian StyleFigini, Matteo, Antonella Castellano, Michele Bailo, Marcella Callea, Marcello Cadioli, Samira Bouyagoub, Marco Palombo, Valentina Pieri, Pietro Mortini, Andrea Falini, and et al. 2023. "Comprehensive Brain Tumour Characterisation with VERDICT-MRI: Evaluation of Cellular and Vascular Measures Validated by Histology" Cancers 15, no. 9: 2490. https://doi.org/10.3390/cancers15092490
APA StyleFigini, M., Castellano, A., Bailo, M., Callea, M., Cadioli, M., Bouyagoub, S., Palombo, M., Pieri, V., Mortini, P., Falini, A., Alexander, D. C., Cercignani, M., & Panagiotaki, E. (2023). Comprehensive Brain Tumour Characterisation with VERDICT-MRI: Evaluation of Cellular and Vascular Measures Validated by Histology. Cancers, 15(9), 2490. https://doi.org/10.3390/cancers15092490






