Conservative Surgery in cT4 Breast Cancer: Single-Center Experience in the Neoadjuvant Setting
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Characteristics
2.2. Neoadjuvant Treatment
2.3. Surgery and Pathological Evaluation
- Skin involvement post NA. In case of extensive skin involvement, patients underwent RBS without reconstruction; in case of limited involvement or complete resolution, patients underwent conservative surgery by removing the affected skin area.
- Extension of muscle involvement post NA. Patients with extensive muscle involvement underwent RBS, while for those with limited involvement, conservative surgery was considered by removing the involved muscle area.
- Extension of neoplastic residue at radiological stadiation post NA. In the presence of <20% involvement of the mammary gland there was indication for Q, between 20% and 50% quadrantectomy with OPSII and >50% conservative mastectomy with immediate reconstruction [19].
- Site of neoplasm and expected aesthetic outcome. Lesions of the upper-outer quadrant (UOQ) or axillary tail (AT) or external quadrant had indication to quadrantectomy (Q), lesions of the inner quadrants (as upper-inner quadrant—UIQ or sub-areolar quadrant-SQ—had indication to OPSII and finally lesions of the lower-inner quadrant (LIQ) had indication to conservative mastectomy.
- Presence of any pathogenic mutations that were subjected to conservative mastectomy [20].
- Patient’s preferences.
2.4. Adjuvant Treatment
2.5. Follow-Up and Oncological Outcomes
2.6. Statistical Analysis
3. Results
3.1. Pathological Results
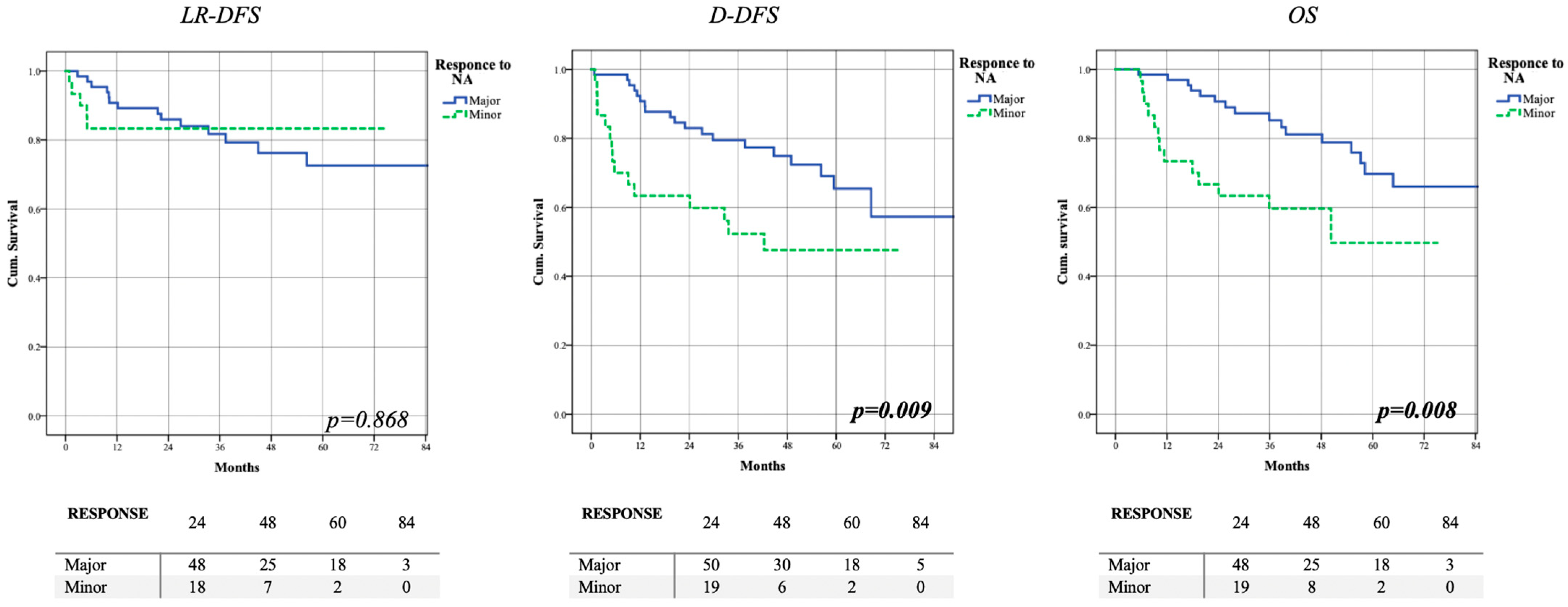
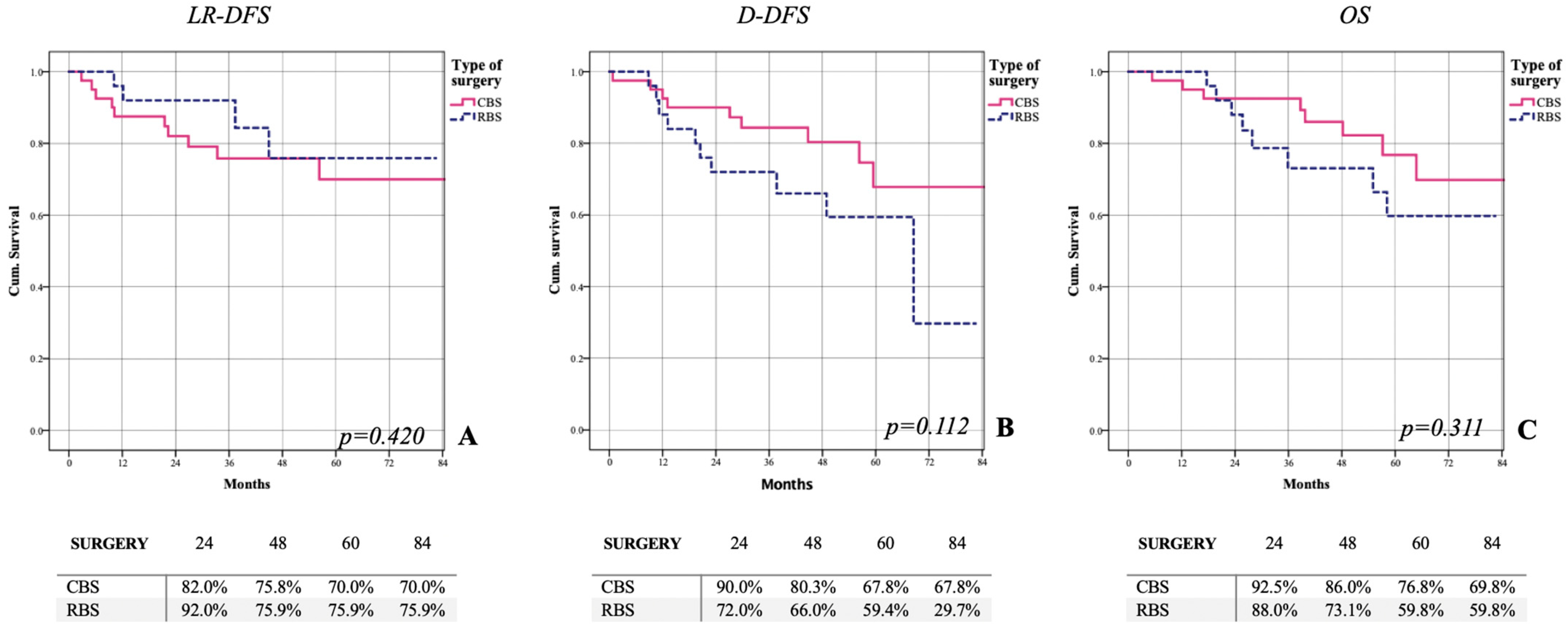
3.2. Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ionta, M.T.; Atzori, F.; Massidda, B. Inflammatory breast cancer in Italy: Epidemiological and clinical aspects. Cancer 2010, 116 (Suppl. 11), 2736–2740. [Google Scholar] [CrossRef]
- AIOM (Italian Association of Medical Oncology). Guidelines for Breast Cancer, Ver 11.11.2021; AIOM (Italian Association of Medical Oncology): Milano, Italy, 2021. [Google Scholar]
- NCCN (National Comprehensive Cancer Network). Breast Cancer Guidelines Ver. 04.2022; NCCN (National comprehensive cancer network): Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Kim, H.J.; Kim, H.J.; Lee, S.B.; Moon, H.G.; Noh, W.C.; Cho, Y.U.; Yoo, Y.; Ahn, S.H.; Korean Breast Cancer Society. A proposal for a new classification of T4 breast cancer as stage IIIC: A report from the Korean Breast Cancer Society. Breast Cancer Res. Treat. 2015, 153, 153–160. [Google Scholar] [CrossRef]
- Dawood, S.; Merajver, S.D.; Viens, P.; Vermeulen, P.B.; Swain, S.M.; Buchholz, T.A.; Dirix, L.Y.; Levine, P.H.; Lucci, A.; Krishnamurthy, S.; et al. International expert panel on inflammatory breast cancer: Consensus statement for standardized diagnosis and treatment. Ann. Oncol. 2011, 22, 515–523. [Google Scholar] [CrossRef]
- Liu, J.; Chen, K.; Jiang, W.; Mao, K.; Li, S.; Kim, M.J.; Liu, Q.; Jacobs, L.K. Chemotherapy response and survival of inflammatory breast cancer by hormone receptor- and HER2- defined molecular subtypes approximation: An analysis from the National Cancer Database. J. Cancer Res. Clin. Oncol. 2017, 143, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Postlewait, L.M.; Teshome, M.; DeSnyder, S.M.; Lim, B.; Kuerer, H.M.; Bedrosian, I.; Woodward, W.A.; Ueno, N.T.; Lucci, A. Factors associated with pathological node negativity in inflammatory breast cancer: Are there patients who may be candidates for a de-escalation of axillary surgery? Ann. Surg. Oncol. 2020, 27, 4603–4612. [Google Scholar] [CrossRef] [PubMed]
- Abraham, H.G.; Xia, Y.; Mukherjee, B.; Merajver, S.D. Incidence and survival of inflammatory breast cancer between 1973 and 2015 in the SEER database. Breast Cancer Res. Treat. 2021, 185, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F.; Schairer, C.; Chen, B.E.; Hance, K.W.; Levine, P.H. Epidemiology of inflammatory breast cancer (IBC). Breast Dis. 2005, 22, 9–23. [Google Scholar] [CrossRef]
- Corso, G.; Kahler-Ribeiro-Fontana, S.; Pagan, E.; Bagnardi, V.; Magnoni, F.; Munzone, E.; Bottiglieri, L.; Veronesi, P.; Galimberti, V. Ten-year outcome results of cT4 breast cancer after neoadjuvant treatment. J. Surg. Oncol. 2021, 124, 1242–1250. [Google Scholar] [CrossRef]
- Giordano, S.H. Update on locally advanced breast cancer. Oncologist 2003, 8, 521–530. [Google Scholar] [CrossRef]
- Chen, H.; Wu, K.; Wang, M.; Wang, F.; Zhang, M.; Zhang, P. A standard mastectomy should not be the only recommended breast surgical treatment for non-metastatic inflammatory breast cancer: A large population-based study in the Surveillance, Epidemiology, and End Results database 18. Breast 2017, 35, 48–54. [Google Scholar] [CrossRef]
- Aebi, S.; Karlsson, P.; Wapnir, I.L. Locally advanced breast cancer. Breast 2022, 62 (Suppl. S1), S58–S62. [Google Scholar] [CrossRef] [PubMed]
- van Uden, D.J.; van Laarhoven, H.W.; Westenberg, A.H.; de Wilt, J.H.; Blanken-Peeters, C.F. Inflammatory breast cancer: An overview. Crit. Rev. Oncol./Hematol. 2015, 93, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Di Leone, A.; Terribile, D.; Magno, S.; Sanchez, A.M.; Scardina, L.; Mason, E.J.; D’archi, S.; Maggiore, C.; Rossi, C.; Di Micco, A.; et al. Neoadjuvant Chemotherapy in Breast Cancer: An Advanced Personalized Multidisciplinary Prehabilitation Model (APMP-M) to Optimize Outcomes. J. Pers. Med. 2021, 11, 324. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Feig, B.A.; Hsiang, D.J.-B.; Butler, J.A.; Mehta, R.S.; Bahri, S.; Nalcioglu, O.; Su, M.-Y. Impact of MRI-evaluated neoadjuvant chemotherapy response on change of surgical recommendation in breast cancer. Ann. Surg. 2009, 249, 448–454. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; De La Haba-Rodríguez, J.R.; Im, S.-A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicenter, open-label, phase 2 randomized trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Di Leone, A.; Franco, A.; Terribile, D.A.; Magno, S.; Fabi, A.; Sanchez, A.M.; D’Archi, S.; Scardina, L.; Natale, M.; Mason, E.J.; et al. Level II Oncoplastic Surgery as an Alternative Option to Mastectomy with Immediate Breast Reconstruction in the Neoadjuvant Setting: A Multidisciplinary Single Center Experience. Cancers 2022, 14, 1275. [Google Scholar] [CrossRef]
- Terribile, D.A.; Mason, E.J.; Murando, F.; DI Leone, A.; Sanchez, A.M.; Scardina, L.; Magno, S.; Franco, A.; D’archi, S.; Natale, M.; et al. Surgical management of BRCA pathogenic variant carriers with breast cancer: A recent literature review and current state of the art. Minerva Surg. 2021, 76, 564–574. [Google Scholar] [CrossRef]
- Povoski, S.P.; Jimenez, R.E.; Wang, W.P.; Xu, R.X. Standardized and reproducible methodology for the comprehensive and systematic assessment of surgical resection margins during breast-conserving surgery for invasive breast cancer. BMC Cancer 2009, 9, 254. [Google Scholar] [CrossRef]
- Untch, M.; Fasching, A.P.; Konecny, E.G.; Hasmüller, S.; Lebeau, A.; Kreienberg, R.; Camara, O.; Müller, V.; Du Bois, A.; Kühn, T.; et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: Results from the TECHNO trial of the AGO and GBG study groups. J. Clin. Oncol. 2021, 29, 3351–3357. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Huo, X.; Li, J.; Zhao, F.; Ren, D.; Ahmad, R.; Yuan, X.; Du, F.; Zhao, J. The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Garufi, G.; Carbognin, L.; Schettini, F.; Seguí, E.; Di Leone, A.; Franco, A.; Paris, I.; Scambia, G.; Tortora, G.; Fabi, A. Updated Neoadjuvant Treatment Landscape for Early Triple Negative Breast Cancer: Immunotherapy, Potential Predictive Biomarkers, and Novel Agents. Cancers 2022, 14, 4064. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet Lond. Engl. 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Goss, P.E.; Ingle, J.N.; Martino, S.; Robert, N.J.; Muss, H.B.; Piccart, M.J.; Castiglione, M.; Tu, D.; Shepherd, L.E.; Pritchard, K.I.; et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 2003, 349, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Recht, A.; Comen, E.A.; Fine, R.E.; Fleming, G.F.; Hardenbergh, P.H.; Ho, A.Y.; Hudis, C.A.; Hwang, E.S.; Kirshner, J.J.; Morrow, M.; et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Pr. Radiat. Oncol. 2016, 6, e219–e234. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Baldassarre, F.; Eisen, A.; Dayes, I.; Engel, J.; Cil, T.; Kornecki, A.; George, R.; SenGupta, S.; Brackstone, M. A systematic review of axillary nodal irradiation for the management of the axilla in patients with early-stage breast cancer. Surg. Oncol. 2022, 42, 101754. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Scardina, L.; Franceschini, G.; Terribile, D.; Franco, A.; Salgarello, M.; Masetti, R. Treatment protocol to allow reconstructive breast surgery during COVID-19 pandemic. Br. J. Surg. 2020, 107, e573–e574. [Google Scholar]
- Robertson, F.M.; Bondy, M.; Yang, W.; Yamauchi, H.; Wiggins, S.; Kamrudin, S.; Krishnamurthy, S.; Le-Petross, H.; Bidaut, L.; Player, A.N.; et al. Inflammatory breast cancer: The disease, the biology, the treatment. CA Cancer J. Clin. 2010, 60, 351–375. [Google Scholar] [CrossRef]
- Adesoye, T.; Lucci, A. Current Surgical Management of Inflammatory Breast Cancer. Ann. Surg. Oncol. 2021, 28, 5461–5467. [Google Scholar] [CrossRef]
- Nakhlis, F. Inflammatory Breast Cancer: Is There a Role for Deescalation of Surgery? Ann. Surg. Oncol. 2022, 29, 6106–6113. [Google Scholar] [CrossRef] [PubMed]
- Scardina, L.; Di Leone, A.; Biondi, E.; Carnassale, B.; Sanchez, A.M.; D’archi, S.; Franco, A.; Moschella, F.; Magno, S.; Terribile, D.; et al. Prepectoral vs. Submuscular Immediate Breast Reconstruction in Patients Undergoing Mastectomy after Neoadjuvant Chemotherapy: Our Early Experience. J. Pers. Med. 2022, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Nakhlis, F.; Regan, M.M.; Warren, L.E.; Bellon, J.R.; Hirshfield-Bartek, J.; Duggan, M.M.; Dominici, L.S.; Golshan, M.; Jacene, H.A.; Yeh, E.D.; et al. The impact of residual disease after preoperative systemic therapy on clinical outcomes in patients with inflammatory breast cancer. Ann. Surg. Oncol. 2017, 24, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Vicini, E.; Corso, G.; Morigi, C.; Fontana, S.; Sacchini, V.; Veronesi, P. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast 2017, 34 (Suppl. S1), S82–S84. [Google Scholar] [CrossRef]
- Conti, M.; Morciano, F.; Bufi, E.; D’Angelo, A.; Panico, C.; Di Paola, V.; Gori, E.; Russo, G.; Cimino, G.; Palma, S.; et al. Surgical Planning after Neoadjuvant Treatment in Breast Cancer: A Multimodality Imaging-Based Approach Focused on MRI. Cancers 2023, 15, 1439. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, Z.; Liang, M.; Liu, Z.; Hou, J.; Chen, X.; Xu, D.; Fei, Y.; Tang, J. Diagnostic accuracy of de-escalated surgical procedure in axilla for node-positive breast cancer patients treated with neoadjuvant systemic therapy: A systematic review and meta-analysis. Cancer Med. 2022, 11, 4085–4103. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Cristofanilli, M.; Kau, S.W.; Broglio, K.; Fornage, B.; Singletary, S.E.; Sahin, A.; Buzdar, A.U.; et al. Disease-free and overall survival after pathologic complete disease remission of cytologically proven inflammatory breast carcinoma axillary lymph node metastases after primary systemic chemotherapy. Cancer 2006, 106, 1000–1006. [Google Scholar] [CrossRef]
- Weiss, A.; Menen, R.S.; Lin, H.Y.; Shen, Y.; Rosso, K.J.; Shaitelman, S.; Woodward, W.; Valero, V.; Ueno, N.T.; Bedrosian, I.; et al. Factors associated with improved outcomes for metastatic inflammatory breast cancer patients. Breast Cancer Res. Treat. 2018, 169, 615–623. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Khalil, H.; Assi, H.I.; Dargham, T.B. Treatment Strategies for Residual Disease following Neoadjuvant Chemotherapy in Patients with Early-Stage Breast Cancer. Curr. Oncol. 2022, 29, 5810–5822. [Google Scholar] [CrossRef] [PubMed]
- Adesoye, T.; Irwin, S.; Sun, S.X.; Lucci, A.; Teshome, M. Contemporary surgical management of inflammatory breast cancer: A narrative review. Chin. Clin. Oncol. 2021, 10, 57. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | ALL 96 Patients | CBS 51 (53.1%) | RBS 45 (46.9%) | p-Value |
|---|---|---|---|---|
| Epidemic and anatomical characteristics | ||||
| Age (years) | 53.7 ± 11.3 (52; 45.5–62.3) | 53 ± 11.6 (51; 45.6–60.6) | 54 ± 11.4 (53.9; 45.4–63.7) | p = 0.697 |
| Menopausal Status | 57 (59.4%) | 29 (56.9%) | 28 (62.2%) | p = 0.679 |
| BMI (Kg/m2) | 25.8 ± 5.1 (25; 22.1–28.6) | 26.1 ± 5 (25.4; 22.3–29.4) | 25.5 ± 5.2 (23.8; 22–27.9) | p = 0.531 |
| BRCA 1/2 pathological mutations | 6 (6.3%) | 5 (9.8%) | 1 (2.2%) | p = 0.209 |
| Tumor site | p = 0.159 | |||
| UOQ/AT | 59 (61.5%) | 36 (70.6%) | 23 (51.1%) | |
| UIQ | 3 (3.1%) | 1 (2.0%) | 2 (4.4%) | |
| LIQ | 9 (9.4%) | 5 (9.8%) | 4 (8.9%) | |
| LOQ | 10 (10.4%) | 5 (9.8%) | 5 (11.1%) | |
| SQ | 15 (15.6%) | 4 (7.8%) | 11 (24.4%) | |
| cT4 Types | p < 0.0001 | |||
| - 4a | 1 (1.0%) | 1 (2.0%) | 0 (0%) | |
| - 4b | 58 (60.4%) | 41 (80.4%) | 17 (37.8%) | |
| - 4c | 9 (9.4%) | 2 (3.9%) | 7 (15.6%) | |
| - 4d | 28 (29.2%) | 7 (13.7%) | 21 (46.7%) | |
| Initial stage | p = 0.018 | |||
| - III B | 82 (85.4%) | 48 (94.1%) | 34 (75.6%) | |
| - III C | 14 (14.6%) | 3 (5.9%) | 11 (24.4%) | |
| Biological characteristics | ||||
| Istotype | p = 0.419 | |||
| - DIC | 54 (56.3%) | 31 (60.8%) | 23 (51.1%) | |
| - LIC | 9 (9.4%) | 3 (5.9%) | 6 (13.3%) | |
| - IC NST | 33 (34.4%) | 17 (33.3%) | 16 (35.6%) | |
| Grading | p = 0.675 | |||
| - G2 | 37 (38.5%) | 21 (41.2%) | 16 (35.6%) | |
| - G3 | 59 (61.5%) | 30 (58.8%) | 29 (64.4%) | |
| Estrogen Receptors | p = 1.000 | |||
| - Negative | 32 (33.3%) | 17 (33.3%) | 15 (33.3%) | |
| - Positive | 64 (56.3%) | 34 (66.7%) | 30 (66.7%) | |
| Progesterone Receptors | p = 0.396 | |||
| - Negative | 35 (36.5%) | 21 (41.2%) | 14 (31.1%) | |
| - Positive | 61 (63.5%) | 30 (58.8%) | 31 (68.9%) | |
| Ki-67 | p = 0.468 | |||
| - <20 | 8 (8.3%) | 3 (5.9%) | 5 (11.1%) | |
| - ≥20 | 88 (91.7%) | 48 (94.1%) | 40 (88.9%) | |
| HER2 | p = 0.723 | |||
| - 0 | 23 (24.0%) | 13 (25.5%) | 10 (22.2%) | |
| - 1+ | 25 (26.0%) | 15 (29.4%) | 10 (22.2%) | |
| - 2+ SISH negative | 19 (19.8%) | 7 (13.7%) | 12 (26.7% | |
| - 2+ SISH positive | 8 (8.3%) | 5 (9.8%) | 3 (6.7%) | |
| - 3+ | 21 (21.9%) | 11 (21.6%) | 10 (22.2%) | |
| Tumor subtype | p = 0.900 | |||
| - Luminal A | 7 (7.3%) | 4 (7.8%) | 3 (6.7%) | |
| - Luminal B | 41 (42.7%) | 20 (39.2%) | 21 (46.7%) | |
| - Her2 Positive | 29 (30.2%) | 16 (31.4%) | 13 (28.9%) | |
| - Triple Negative | 19 (19.8%) | 11 (21.6%) | 8 (17.8%) | |
| Characteristics | ALL 96 Patients | CBS 51 (53.1%) | RBS 45 (46.9%) | p-Value |
|---|---|---|---|---|
| Baseline | ||||
| Median tumor size (mm) | 57.4 ± 26.7 (51.5; 39.3–73.8) | 52.3 ± 26.4 (46; 33–70) | 63.1 ± 26.1 (60; 44.5–82.5) | p = 0.046 |
| Multifocality | 39 (40.6%) | 23 (45.1%) | 16 (35.6%) | p = 0.411 |
| Clinical Node Stadiation | p = 0.191 | |||
| - N0 | 16 (16.7%) | 10 (19.6%) | 6 (13.3%) | |
| - N1 | 39 (40.6%) | 23 (45.1%) | 16 (35.6%) | |
| - N2 | 27 (28.1%) | 15 (29.4%) | 12 (26.7%) | |
| - N3 | 14 (14.6%) | 3 (5.9%) | 11 (24.4%) | |
| Post-NA radiological characteristics | ||||
| Residual tumor | 30.2 ± 28.2 | 21.9 ± 20.4 | 39.6 ± 32.8 | p = 0.002 |
| dimension (mm) | (25; 6.3–43.8) | (22; 0–35) | (36; 9–62) | |
| ycT | p < 0.00001 | |||
| - rCR | 20 (20.8%) | 13 (25.5%) | 7 (15.5%) | |
| - 1 | 19 (19.8%) | 12 (23.5%) | 7 (15.5%) | |
| - 2 | 32 (33.3%) | 23 (45.1%) | 9 (20.1%) | |
| - 3 | 11 (11.5%) | 3 (5.9%) | 8 (17.8%) | |
| - 4 | 14 (14.6%) | 0 (0%) | 14 (31.1%) | |
| RECIST CRITERIA | p = 0.097 | |||
| - CR | 20 (20.8%) | 13 (25.5%) | 7 (15.6%) | |
| - PR | 41 (42.7%) | 24 (47.0%) | 17 (37.8%) | |
| - SD | 28 (29.2%) | 14 (27.5%) | 14 (31.1%) | |
| - PD | 7 (7.3%) | 0 (0%) | 7 (15.5%) | |
| Reduction of maximum size (%) | p = 0.036 | |||
| - 100 | 20 (20.8%) | 13 (25.5%) | 7 (15.6%) | |
| - 99.9–25.1 | 46 (47.9%) | 28 (54.9%) | 18 (40.0%) | |
| - <25 or progression | 30 (31.3%) | 10 (19.6%) | 20 (44.4%) | |
| Multifocality | 26 (27.1%) | 13 (25.5%) | 13 (28.9%) | p = 0.819 |
| ycN | p = 0.007 | |||
| - ycN0 | 50 (52.1%) | 33 (64.7%) | 17 (37.8%) | |
| - ycN+ | 46 (47.9%) | 18 (35.3%) | 28 (62.2%) | |
| Conservative Breast Surgery (CBS) | |
| Quadrantectomy | 25 (49.0%) |
| Oncoplastic surgery level II | 8 (15.8%) |
| Conservative Mastectomy - Nipple Sparing - Skin Sparing | 18 (35.2%) - 9 (17.6%) - 9 (17.6%) |
| Radical Breast Surgery (RBS) | |
| Modified radical mastectomy | 45 (100%) |
| Axillary Surgery | |
| Only Sentinel Lymph Node Biopsy Axillary Dissection | 22 (22.9%) 73 (76.0%) |
| Characteristics | All | CBS | RBS | p-Value |
|---|---|---|---|---|
| 96 Patients | 51 (53.1%) | 45 (46.9%) | ||
| BREAST | ||||
| pCR | 25 (26.0%) | 16 (31.4%) | 9 (20.0%) | p = 0.248 |
| ypT | p < 0.0001 | |||
| - 0 | 25 (26.0%) | 16 (31.4%) | 9 (20.0%) | |
| - mic | 6 (6.3%) | 4 (7.8%) | 2 (4.4%) | |
| - 1a | 7 (7.3%) | 6 (11.8%) | 1 (2.2%) | |
| - 1b | 7 (7.3%) | 6 (11.8%) | 1 (2.2%) | |
| - 1c | 14 (14.6%) | 9 (17.6%) | 5 (11.1%) | |
| - 2 | 16 (16.7%) | 8 (15.7%) | 8 (17.8%) | |
| - 3 | 7 (7.3%) | 2 (3.9%) | 5 (11.1%) | |
| - 4 | 14 (14.6%) | 0 (0%) | 14 (31.1%) | |
| Multifocality | 36 (37.5%) | 18 (35.3%) | 18 (40.0%) | p = 0.677 |
| Pathological stage | p < 0.0001 | |||
| - 0 | 19 (19.8%) | 12 (23.5%) | 7 (15.6%) | |
| - I | 11 (11.5%) | 9 (17.6%) | 2 (4.4%) | |
| - IIA | 21 (21.9%) | 17 (33.4%) | 4 (8.9%) | |
| - IIB | 10 (10.4%) | 5 (9.8%) | 5 (11.1%) | |
| - IIIA | 13 (13.5%) | 5 (9.8%) | 8 (17.8%) | |
| - IIIB | 8 (8.3%) | 0 (0%) | 8 (17.8%) | |
| - IIIC | 14 (14.6%) | 3 (5.9%) | 11 (24.4%) | |
| Skin involvement | 13 (13.5%) | 0 (0%) | 13 (28.9%) | p < 0.0001 |
| Muscle involvement | 5 (5.2%) | 0 (0%) | 5 (11.1%) | p = 0.020 |
| AXILLA | ||||
| Sentinel node biopsy | 22 (22.9%) | 16 (31.4%) | 6 (13.3%) | p = 0.051 |
| Axillary dissection | 74 (77.1%) | 35 (68.6%) | 39 (86.7%) | p = 0.051 |
| Number of lymph nodes excised | 12.3 ± 7 | 11.4 ± 6.1 | 13.4 ± 7.8 | p = 0.146 |
| (11; 7–16) | (10; 7–16) | (11; 8–15) | ||
| Number of metastatic lymph nodes | 3.9 ± 6.1 | 2 ± 4 | 6.1 ± 7.4 | p = 0.001 |
| (1; 0–5) | (0.3; 0–2) | (4; 0.4–9) | ||
| ypN | p = 0.004 | |||
| - 0 | 31 (32.3%) | 22 (43.1%) | 9 (20.0%) | |
| - 1 | 33 (34.4%) | 20 (39.2%) | 13 (28.9%) | |
| - 2 | 18 (18.8%) | 6 (11.8%) | 12 (26.7%) | |
| - 3 | 14 (14.6%) | 3 (5.9%) | 11 (24.4%) | |
| Positive lymph nodes/total lymph nodes excised ratio | 0.28 ± 0.3 | 0.15 ± 0.3 | 0.41 ± 0.4 | p < 0.0001 |
| (0.11; 0–0.6) | (0.03; 0–0.2) | (0.33; 0.04–0.76) | ||
| Characteristics | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | p Value | 95% CI | OR | p Value | 95% CI | |
| Menopausal status | 1.249 | 0.594 | 0.551–2.833 | |||
| BRCA pathological mutations | 4.783 | 0.161 | 0.537–42.582 | |||
| Istotype | 0.871 | 0.531 | 0.565–1.342 | |||
| Grading | 0.788 | 0.573 | 0.345–1.802 | |||
| cT4 | ||||||
| - a | 21.098 | 1.000 | 0 | |||
| - b | 6.530 | <0.0001 | 2.699–16.895 | 6.941 | 0.021 | 1.245–35.543 |
| - c | 0.222 | 0.07 | 0.044–1.128 | |||
| - d | 0.168 | 0.001 | 0.068–0.489 | 0.934 | 0.941 | 0.155–5.620 |
| Tumor Subtype | ||||||
| - Luminal | 0.778 | 0.54 | 0.348–1.737 | |||
| - Triple negative | 1.272 | 0.642 | 0.461–3.508 | |||
| - HER2 positive | 1.125 | 0.791 | 0.469–2.699 | |||
| Median tumor size (mm) | ||||||
| - <50 | 2.491 | 0.033 | 1.079–5.753 | 2.692 | 0.095 | 0.841–8.620 |
| - 50.1–80 | 0.906 | 0.819 | 0.390–2.107 | |||
| - >80.1 | 0.318 | 0.026 | 0.116–0.874 | 0.936 | 0.926 | 0.228–3.837 |
| cN | 0.665 | 0.073 | 0.413–1.041 | |||
| Reduction of max size (%) | ||||||
| - 100 | 0.538 | 0.236 | 0.194–1.498 | |||
| - 99.9–25.1 | 1.826 | 0.146 | 0.810–4.115 | |||
| - <25 or progression | 0.305 | 0.01 | 0.123–0.756 | 0.291 | 0.042 | 0.090–0.955 |
| ycN1 | 0.331 | 0.009 | 0.144–0.761 | 0.395 | 0.078 | 0.140–1.111 |
| All 96 Patients | CBS 51 (53.1%) | RBS 45 (46.9%) | p-Value | |
|---|---|---|---|---|
| Locoregional Recurrence | ||||
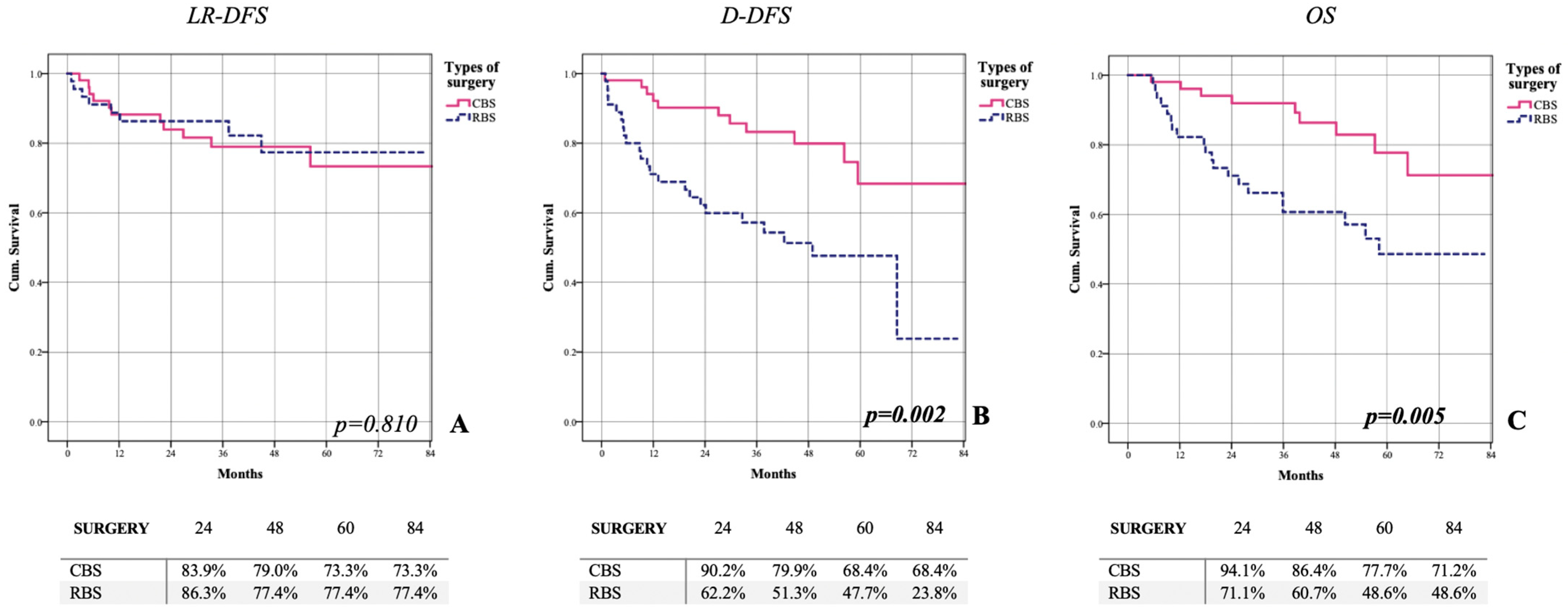
| N. of patients | 19 (19.8%) | 11 (21.6%) | 8 (17.8%) | p = 0.798 |
| Locoregional disease-free survival | 74.9% | 73.3% | 77.4% | LR = 0.801 |
| Distant Recurrence | ||||
| N. of patients | 34 (35.4%) | 11 (21.6%) | 23 (51.5%) | p = 0.003 |
| Distant disease-free survival | 52.3% | 64.8% | 44.7% | LR = 0.002 |
| Death | ||||
| N. of patients | 29 (30.2%) | 9 (17.6%) | 20 (44.4%) | p = 0.007 |
| Overall survival | 60.2% | 71.2% | 48.6% | LR = 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, A.; Di Leone, A.; Fabi, A.; Belli, P.; Carbognin, L.; Gambaro, E.; Marazzi, F.; Mason, E.J.; Mulè, A.; Orlandi, A.; et al. Conservative Surgery in cT4 Breast Cancer: Single-Center Experience in the Neoadjuvant Setting. Cancers 2023, 15, 2450. https://doi.org/10.3390/cancers15092450
Franco A, Di Leone A, Fabi A, Belli P, Carbognin L, Gambaro E, Marazzi F, Mason EJ, Mulè A, Orlandi A, et al. Conservative Surgery in cT4 Breast Cancer: Single-Center Experience in the Neoadjuvant Setting. Cancers. 2023; 15(9):2450. https://doi.org/10.3390/cancers15092450
Chicago/Turabian StyleFranco, Antonio, Alba Di Leone, Alessandra Fabi, Paolo Belli, Luisa Carbognin, Elisabetta Gambaro, Fabio Marazzi, Elena Jane Mason, Antonino Mulè, Armando Orlandi, and et al. 2023. "Conservative Surgery in cT4 Breast Cancer: Single-Center Experience in the Neoadjuvant Setting" Cancers 15, no. 9: 2450. https://doi.org/10.3390/cancers15092450
APA StyleFranco, A., Di Leone, A., Fabi, A., Belli, P., Carbognin, L., Gambaro, E., Marazzi, F., Mason, E. J., Mulè, A., Orlandi, A., Palazzo, A., Paris, I., Rossi, A., Scardina, L., Terribile, D. A., Tiberi, G., Giannarelli, D., Scambia, G., Masetti, R., & Franceschini, G. (2023). Conservative Surgery in cT4 Breast Cancer: Single-Center Experience in the Neoadjuvant Setting. Cancers, 15(9), 2450. https://doi.org/10.3390/cancers15092450











