Feasibility, Reliability, and Safety of Remote Five Times Sit to Stand Test in Patients with Gastrointestinal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participant Recruitment
2.3. Demographic Measures
2.4. Five Times Sit to Stand Test
2.5. Primary Outcome Measures
- (i)
- Feasibility: The feasibility of the 5STS test was as the proportion of eligible patients that incurred issues with at home assessment, including inadequate space, chair, or internet connectivity. The remote assessment was considered feasible if the minority (i.e., <20%) of included participants presented with the abovementioned issues.
- (ii)
- Reliability: This was measured by comparing the 5STS test scores (i.e., time) between the remote (videoconferencing measurement) and direct assessment (face-to-face measurement), within the same participant. This was performed to explore whether remote physical assessments produce similar scores (i.e., agreement) as the face-to-face assessments.
- (iii)
- Safety: Safety was defined by the number of adverse events which occurred during the 5STS tests. A serious adverse event was defined as an event which required medical intervention and results in death, a life-threatening situation, hospitalisation, incapacity, and/or disability. A minor adverse event was defined as an event that requires medical review and resolves without intervention, resulting in no hospitalisation, incapacity, or disability [22].
2.6. Sample Size
2.7. Analyses
3. Results
3.1. Characteristics of the Included Sample
3.2. Feasibility
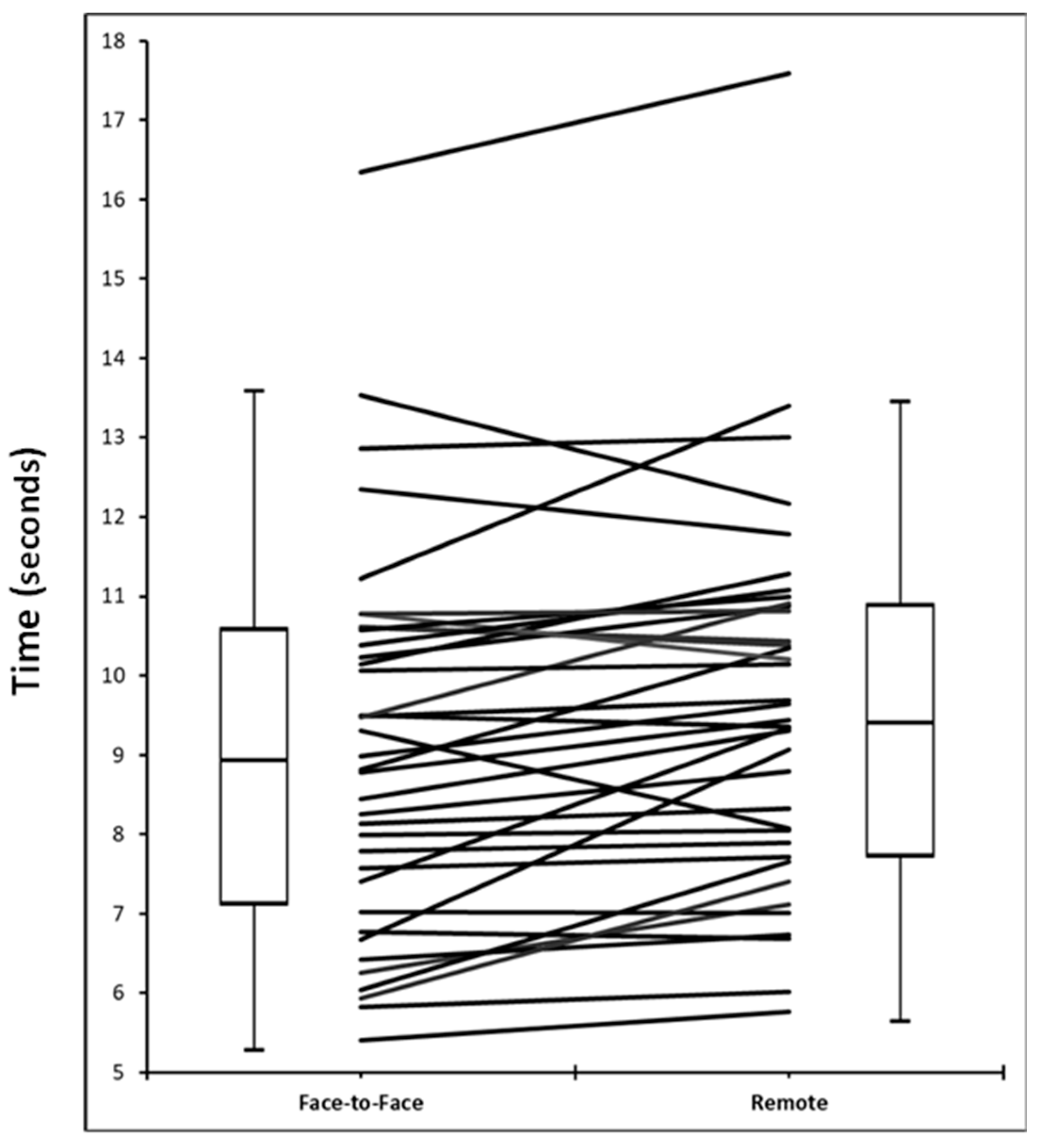
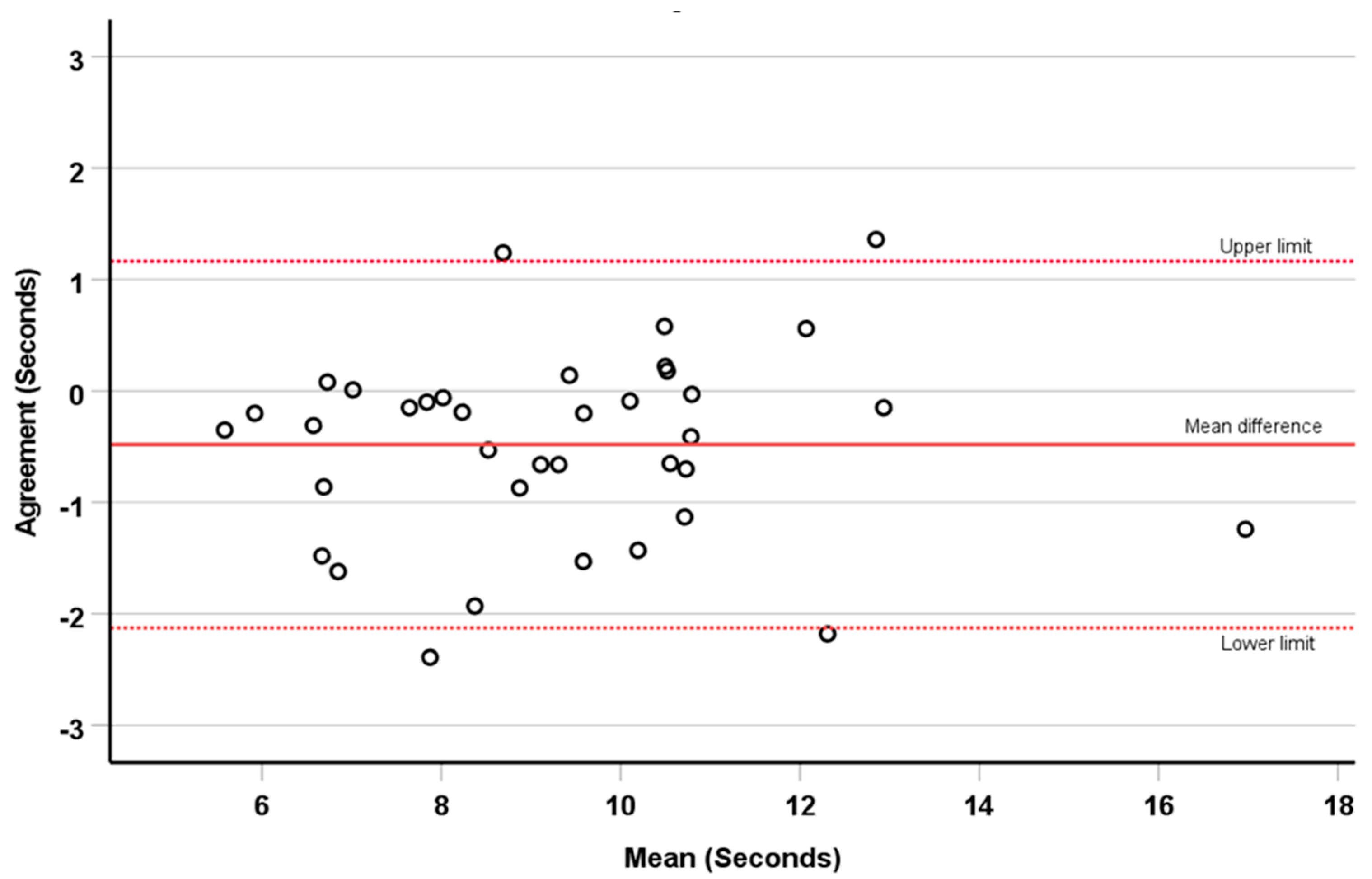
3.3. Reliability
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffens, D.; Beckenkamp, P.R.; Hancock, M.; Solomon, M.; Young, J. Preoperative exercise halves the postoperative complication rate in patients with lung cancer: A systematic review of the effect of exercise on complications, length of stay and quality of life in patients with cancer. Br. J. Sport. Med. 2018, 52, 344. [Google Scholar] [CrossRef] [PubMed]
- Waterland, J.L.; Chahal, R.; Ismail, H.; Sinton, C.; Riedel, B.; Francis, J.J.; Denehy, L. Implementing a telehealth prehabilitation education session for patients preparing for major cancer surgery. BMC Health Serv. Res. 2021, 21, 443. [Google Scholar] [CrossRef] [PubMed]
- McBride, K.E.; Brown, K.G.M.; Fisher, O.M.; Steffens, D.; Yeo, D.A.; Koh, C.E. Impact of the COVID-19 pandemic on surgical services: Early experiences at a nominated COVID-19 centre. ANZ J. Surg. 2020, 90, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Steffens, D.; Young, J.; Riedel, B.; Morton, R.; Denehy, L.; Heriot, A.; Koh, C.; Li, Q.; Bauman, A.; Sandroussi, C.; et al. PrehabIlitation with preoperatIve exercise and education for patients undergoing major abdominal cancer surgery: Protocol for a multicentre randomised controlled TRIAL (PRIORITY TRIAL). BMC Cancer 2022, 22, 443. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Kalfa, N.; Beckers, G.M.A.; Kaefer, M.; Nieuwhof-Leppink, A.J.; Fossum, M.; Herbst, K.W.; Bagli, D. The impact of COVID-19 on research. J. Pediatr. Urol. 2020, 16, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Monaghesh, E.; Hajizadeh, A. The role of telehealth during COVID-19 outbreak: A systematic review based on current evidence. BMC Public Health 2020, 20, 1193. [Google Scholar] [CrossRef]
- Dijkstra, H.P.; Ergen, E.; Holtzhausen, L.; Beasley, I.; Alonso, J.M.; Geertsema, L.; Geertsema, C.; Nelis, S.; Ngai, A.S.H.; Stankovic, I.; et al. Remote assessment in sport and exercise medicine (SEM): A narrative review and teleSEM solutions for and beyond the COVID-19 pandemic. Br. J. Sport. Med. 2020, 54, 1162. [Google Scholar] [CrossRef]
- Lord, S.R.; Murray, S.M.; Chapman, K.; Munro, B.; Tiedemann, A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M539–M543. [Google Scholar] [CrossRef]
- Makker, P.G.S.; Koh, C.E.; Solomon, M.J.; Ratcliffe, J.; Steffens, D. Functional outcomes following pelvic exenteration: Results from a prospective cohort study. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2021, 23, 2647–2658. [Google Scholar] [CrossRef]
- Quinn, T.J.; McArthur, K.; Ellis, G.; Stott, D.J. Functional assessment in older people. BMJ Br. Med. J. 2011, 343, d4681. [Google Scholar] [CrossRef]
- Goldberg, A.; Chavis, M.; Watkins, J.; Wilson, T. The five-times-sit-to-stand test: Validity, reliability and detectable change in older females. Aging Clin. Exp. Res. 2012, 24, 339–344. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Nakakubo, S.; Hotta, R.; Suzuki, T. Predictive Cutoff Values of the Five-Times Sit-to-Stand Test and the Timed “Up & Go” Test for Disability Incidence in Older People Dwelling in the Community. Phys. Ther. 2017, 97, 417–424. [Google Scholar] [PubMed]
- Peyrusqué, E.; Granet, J.; Pageaux, B.; Buckinx, F.; Aubertin-Leheudre, M. Assessing Physical Performance in Older Adults during Isolation or Lockdown Periods: Web-Based Video Conferencing as a Solution. J. Nutr. Health Aging 2022, 26, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.F.; Harris, R.; Dufour, A.B.; Morey, M.C.; Bean, J. Reliability of Virtual Physical Performance Assessments in Veterans During the COVID-19 Pandemic. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100146. [Google Scholar] [CrossRef]
- Lawford, B.J.; Dobson, F.; Bennell, K.L.; Merolli, M.; Graham, B.; Haber, T.; Teo, P.L.; Mackenzie, D.; McManus, F.; Lamb, K.E.; et al. Clinician-administered performance-based tests via telehealth in people with chronic lower limb musculoskeletal disorders: Test–retest reliability and agreement with in-person assessment. J. Telemed. Telecare 2022, 1357633X221137387. [Google Scholar] [CrossRef]
- Blair, C.K.; Harding, E.; Herman, C.; Boyce, T.; Demark-Wahnefried, W.; Davis, S.; Kinney, A.Y.; Pankratz, V.S. Remote Assessment of Functional Mobility and Strength in Older Cancer Survivors: Protocol for a Validity and Reliability Study. JMIR Res. Protoc. 2020, 9, e20834. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ (Clin. Res. Ed.) 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Ownby, K.K. Use of the Distress Thermometer in Clinical Practice. J. Adv. Pract. Oncol. 2019, 10, 175–179. [Google Scholar] [PubMed]
- Learmonth, Y.C.; Dlugonski, D.; Pilutti, L.A.; Sandroff, B.M.; Klaren, R.; Motl, R.W. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J. Neurol. Sci. 2013, 331, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Safety Monitoring and Reporting in Clinical Trials Involving Therapeutic Goods; goods Smarictit; National Health and Medical Research Council: Canberra, Australia, 2016. [Google Scholar]
- Patridge, E.F.; Bardyn, T.P. Research Electronic Data Capture (REDCap). J. Med. Libr. Assoc. 2018, 106, 142–144. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [PubMed]
- Suttanon, P.; Hill, K.D.; Dodd, K.J.; Said, C.M. Retest reliability of balance and mobility measurements in people with mild to moderate Alzheimer’s disease. Int. Psychogeriatr. 2011, 23, 1152–1159. [Google Scholar] [CrossRef]
- Melo, T.A.; Duarte, A.C.M.; Bezerra, T.S.; França, F.; Soares, N.S.; Brito, D. The Five Times Sit-to-Stand Test: Safety and reliability with older intensive care unit patients at discharge. Rev. Bras. De Ter. Intensiv. 2019, 31, 27–33. [Google Scholar] [CrossRef]
- Bowman, A.; Denehy, L.; Benjemaa, A.; Crowe, J.; Bruns, E.; Hall, T.; Traill, A.; Edbrooke, L. Feasibility and safety of the 30-second sit-to-stand test delivered via telehealth: An observational study. PM R J. Inj. Funct. Rehabil. 2022, 15, 31–40. [Google Scholar] [CrossRef]
- Gill, S.; Hely, R.; Page, R.S.; Hely, A.; Harrison, B.; Landers, S. Thirty second chair stand test: Test-retest reliability, agreement and minimum detectable change in people with early-stage knee osteoarthritis. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2022, 27, e1957. [Google Scholar] [CrossRef]
- Özkeskin, M.; Özden, F.; Ar, E.; Yüceyar, N. The reliability and validity of the 30-second chair stand test and modified four square step test in persons with multiple sclerosis. Physiother. Theory Pract. 2022, 1–7. [Google Scholar] [CrossRef]
- Hoenemeyer, T.W.; Cole, W.W.; Oster, R.A.; Pekmezi, D.W.; Pye, A.; Demark-Wahnefried, W. Test/Retest Reliability and Validity of Remote vs. In-Person Anthropometric and Physical Performance Assessments in Cancer Survivors and Supportive Partners. Cancers 2022, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Pelicioni, P.H.S.; Waters, D.L.; Still, A.; Hale, L. A pilot investigation of reliability and validity of balance and gait assessments using telehealth with healthy older adults. Exp. Gerontol. 2022, 162, 111747. [Google Scholar] [CrossRef] [PubMed]
- Makker, P.G.S.; Koh, C.E.; Ansari, N.; Gonzaga, N.; Bartyn, J.; Solomon, M.; Steffens, D. Functional Outcomes Following Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Prospective Cohort Study. Ann. Surg. Oncol. 2022, 30, 447–458. [Google Scholar] [CrossRef] [PubMed]
| Baseline Variables | Frequency (Percentage) or Median (Interquartile Range) |
|---|---|
| Age, years | 54.0 (46.0 to 61.5) |
| Gender, female | 24 (64.9%) |
| Body mass index, kg/m2 | 24.1 (22.7 to 28.4) |
| Country of birth | |
| Australia | 25 (67.6%) |
| Overseas | 12 (32.4%) |
| Language spoken at home | |
| English | 31 (83.8%) |
| Other | 6 (16.2%) |
| Caring responsibilities | 13 (35.1%) |
| Level of education | |
| Primary school—Year 12 | 13 (35.1%) |
| Technical certificate or diploma | 7 (18.9%) |
| University degree | 17 (46.0%) |
| Employment status | |
| Full-time/Part-time | 23 (62.2%) |
| Retired/sick leave | 12 (32.4%) |
| Unemployed | 2 (5.4%) |
| Type of cancer | |
| Anal | 2 (5.4%) |
| Appendix | 3 (8.1%) |
| Colorectal | 23 (62.2%) |
| Gastrointestinal Stromal Tumour | 1 (2.7%) |
| Pseudomyxoma Peritonei | 6 (16.2%) |
| Retroperitoneal Liposarcoma | 1 (2.7%) |
| Small Bowel Adenocarcinoma | 1 (2.7%) |
| Familiarity with technology | |
| Smartphone or computer | |
| Very familiar | 26 (70.3%) |
| Familiar | 9 (24.3%) |
| Not at all familiar | 2 (5.4%) |
| iPad or tablet device | |
| Very familiar | 27 (73.0%) |
| Familiar | 8 (21.6%) |
| Not at all familiar | 2 (5.4%) |
| Numerical pain rating score a | 1.0 (0.0 to 3.0) |
| Distress thermometer b | 1.0 (0.0 to 2.5) |
| Fatigue Severity Scale c | 26.0 (14.0 to 38.5) |
| Meeting WHO physical activity recommendations d | |
| Yes | 15 (40.5%) |
| No | 22 (59.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffens, D.; Pocovi, N.C.; Bartyn, J.; Delbaere, K.; Hancock, M.J.; Koh, C.; Denehy, L.; van Schooten, K.S.; Solomon, M.; on behalf of the Priority Trial Collaboration. Feasibility, Reliability, and Safety of Remote Five Times Sit to Stand Test in Patients with Gastrointestinal Cancer. Cancers 2023, 15, 2434. https://doi.org/10.3390/cancers15092434
Steffens D, Pocovi NC, Bartyn J, Delbaere K, Hancock MJ, Koh C, Denehy L, van Schooten KS, Solomon M, on behalf of the Priority Trial Collaboration. Feasibility, Reliability, and Safety of Remote Five Times Sit to Stand Test in Patients with Gastrointestinal Cancer. Cancers. 2023; 15(9):2434. https://doi.org/10.3390/cancers15092434
Chicago/Turabian StyleSteffens, Daniel, Natasha C. Pocovi, Jenna Bartyn, Kim Delbaere, Mark J. Hancock, Cherry Koh, Linda Denehy, Kimberley S. van Schooten, Michael Solomon, and on behalf of the Priority Trial Collaboration. 2023. "Feasibility, Reliability, and Safety of Remote Five Times Sit to Stand Test in Patients with Gastrointestinal Cancer" Cancers 15, no. 9: 2434. https://doi.org/10.3390/cancers15092434
APA StyleSteffens, D., Pocovi, N. C., Bartyn, J., Delbaere, K., Hancock, M. J., Koh, C., Denehy, L., van Schooten, K. S., Solomon, M., & on behalf of the Priority Trial Collaboration. (2023). Feasibility, Reliability, and Safety of Remote Five Times Sit to Stand Test in Patients with Gastrointestinal Cancer. Cancers, 15(9), 2434. https://doi.org/10.3390/cancers15092434











