Long Axial Field-of-View PET/CT Could Answer Unmet Needs in Gynecological Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. LAFOV PET/CT Scanners
3. Diagnostic Unmet Needs in Gynecological Malignancies
3.1. Assessment of Disease Extent and Quantitative Global Disease Assessment
3.2. Differential Diagnosis between Physiological, Inflammatory, Benign, and Malignant Findings
3.3. Detection of Peritoneal Carcinomatosis and Sub-Centimetric Metastases
3.4. Effective Assessment of Post-Therapy Changes
3.5. Motion Artifacts
3.6. Radiation Exposure
3.7. Pain
3.8. Detection of Cancer-Associated Vascular Complications
3.9. Bone Metabolism and Osteoporosis Assessment
4. Discussion
5. Conclusions
- -
- larger FOV (up to total-body coverage);
- -
- higher scanner sensitivity and consequently improved image quality, demonstrating sites of uptake previously unseen on conventional PET that may result in earlier detection of tumor lesions;
- -
- possibility of lower radiotracer activity administration, with decreased overall radiation exposure of patients, or shorter image protocol acquisition, with increased tolerability of imaging;
- -
- dynamic scanning, with the attainment of non-invasive kinetic modeling and better quantification;
- -
- delayed scanning, which may improve the visualization of tumors by increasing the ratio between tumor uptake and background.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute, Bethesda. SEER Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/ (accessed on 31 January 2023).
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.W. Combined Positron Emission Tomography–Computed Tomography: The Historical Perspective. Semin. Ultrasound CT MRI 2008, 29, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.E.; Fine, G.C.; Covington, M.F.; Koppula, B.R.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology-IV. Gynecologic and Genitourinary Malignancies. Cancers 2022, 14, 3000. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, A.; Chow, D.Z.; Lee, S.I. FDG-PET for Assessment of Endometrial and Vulvar Cancer. Semin. Nucl. Med. 2019, 49, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Kakhki, V.R.D.; Shahriari, S.; Treglia, G.; Hasanzadeh, M.; Zakavi, S.R.; Yousefi, Z.; Kadkhodayan, S.; Sadeghi, R. Diagnostic Performance of Fluorine 18 Fluorodeoxyglucose Positron Emission Tomography Imaging for Detection of Primary Lesion and Staging of Endometrial Cancer Patients: Systematic Review and Meta-Analysis of the Literature. Int. J. Gynecol. Cancer 2013, 23, 1536–1543. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.-Y.; Lee, J.J.; Kim, M.H.; Kim, D.-Y.; Suh, D.-S.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Comparison of MRI and 18F-FDG PET/CT in the Preoperative Evaluation of Uterine Carcinosarcoma. Gynecol. Oncol. 2016, 140, 409–414. [Google Scholar] [CrossRef]
- Shweel, M.A.; Abdel-Gawad, E.A.; Abdel-Gawad, E.A.; Abdelghany, H.S.; Abdel-Rahman, A.M.; Ibrahim, E.M. Uterine Cervical Malignancy: Diagnostic Accuracy of MRI with Histopathologic Correlation. J. Clin. Imaging Sci. 2012, 2, 42. [Google Scholar] [CrossRef]
- Paredes, P.; Paño, B.; Díaz, B.; Vidal-Sicart, S. Endometrial Cancer. In Nuclear Medicine Manual on Gynaecological Cancers and Other Female Malignancies, 1st ed.; Collarino, A., Vidal-Sicart, S., Olmos, R.A.V., Eds.; Springer: Cham, Switzerland, 2022; Volume 1, pp. 71–88. [Google Scholar]
- Gee, M.S.; Atri, M.; Bandos, A.I.; Mannel, R.S.; Gold, M.A.; Lee, S.I. Identification of Distant Metastatic Disease in Uterine Cervical and Endometrial Cancers with FDG PET/CT: Analysis from the ACRIN 6671/GOG 0233 Multicenter Trial. Radiology 2018, 287, 176–184. [Google Scholar] [CrossRef]
- Rao, Y.J.; Grigsby, P.W. The Role of PET Imaging in Gynecologic Radiation Oncology. PET Clin. 2018, 13, 225–237. [Google Scholar] [CrossRef]
- Triumbari, E.K.A.; de Koster, E.J.; Rufini, V.; Fragomeni, S.M.; Garganese, G.; Collarino, A. 18F-FDG PET and 18F-FDG PET/CT in Vulvar Cancer: A Systematic Review and Meta-Analysis. Clin. Nucl. Med. 2021, 46, 125–132. [Google Scholar] [CrossRef]
- Rufini, V.; Garganese, G.; Ieria, F.P.; Pasciuto, T.; Fragomeni, S.M.; Gui, B.; Florit, A.; Inzani, F.; Zannoni, G.F.; Scambia, G.; et al. Diagnostic Performance of Preoperative [18F]FDG-PET/CT for Lymph Node Staging in Vulvar Cancer: A Large Single-Centre Study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3303–3314. [Google Scholar] [CrossRef]
- Prat, J. FIGO Committee on Gynecologic Oncology Staging Classification for Cancer of the Ovary, Fallopian Tube, and Peritoneum: Abridged Republication of Guidelines From the International Federation of Gynecology and Obstetrics (FIGO). Obstet. Gynecol. 2015, 126, 171–174. [Google Scholar] [CrossRef]
- Kemppainen, J.; Hynninen, J.; Virtanen, J.; Seppänen, M. PET/CT for Evaluation of Ovarian Cancer. Semin. Nucl. Med. 2019, 49, 484–492. [Google Scholar] [CrossRef]
- Dejanovic, D.; Hansen, N.L.; Loft, A. PET/CT Variants and Pitfalls in Gynecological Cancers. Semin. Nucl. Med. 2021, 51, 593–610. [Google Scholar] [CrossRef]
- Katal, S.; Eibschutz, L.S.; Saboury, B.; Gholamrezanezhad, A.; Alavi, A. Advantages and Applications of Total-Body PET Scanning. Diagnostics 2022, 12, 426. [Google Scholar] [CrossRef]
- Alberts, I.; Prenosil, G.; Sachpekidis, C.; Weitzel, T.; Shi, K.; Rominger, A.; Afshar-Oromieh, A. Digital versus Analogue PET in [68 Ga] Ga-PSMA-11 PET/CT for Recurrent Prostate Cancer: A Matched-Pair Comparison. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 614–623. [Google Scholar] [CrossRef]
- Alberts, I.; Sachpekidis, C.; Prenosil, G.; Viscione, M.; Bohn, K.P.; Mingels, C.; Shi, K.; Ashar-Oromieh, A.; Rominger, A. Digital PET/CT Allows for Shorter Acquisition Protocols or Reduced Radiopharmaceutical Dose in [18F]-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 485–492. [Google Scholar] [CrossRef]
- Badawi, R.D.; Shi, H.; Hu, P.; Chen, S.; Xu, T.; Price, P.M.; Ding, Y.; Spencer, B.A.; Nardo, L.; Liu, W.; et al. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 299–303. [Google Scholar] [CrossRef]
- Cherry, S.R.; Badawi, R.D.; Karp, J.S.; Moses, W.W.; Price, P.; Jones, T. Total-Body Imaging: Transforming the Role of Positron Emission Tomography. Sci. Transl. Med. 2017, 9, eaaf6169. [Google Scholar] [CrossRef]
- Yu, H.; Gu, Y.; Fan, W.; Gao, Y.; Wang, M.; Zhu, X.; Wu, Z.; Liu, J.; Li, B.; Wu, H.; et al. Expert Consensus on Oncological [18F]FDG Total-Body PET/CT Imaging (Version 1). Eur. Radiol. 2022, 33, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Lv, X.; Liu, W.; Judenhofer, M.S.; Zwingenberger, A.; Wisner, E.; Berg, E.; McKenney, S.; Leung, E.; Spencer, B.A.; et al. Mini EXPLORER II: A Prototype High-Sensitivity PET/CT Scanner for Companion Animal Whole Body and Human Brain Scanning. Phys. Med. Biol. 2019, 64, 075004. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.A.; Berg, E.; Schmall, J.P.; Omidvari, N.; Leung, E.K.; Abdelhafez, Y.G.; Tang, S.; Deng, Z.; Dong, Y.; Lv, Y.; et al. Performance Evaluation of the UEXPLORER Total-Body PET/CT Scanner Based on NEMA NU 2-2018 with Additional Tests to Characterize PET Scanners with a Long Axial Field of View. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.S.; Viswanath, V.; Geagan, M.J.; Muehllehner, G.; Pantel, A.R.; Parma, M.J.; Perkins, A.E.; Schmall, J.P.; Werner, M.E.; Daube-Witherspoon, M.E. PennPET Explorer: Design and Preliminary Performance of a Whole-Body Imager. J. Nucl. Med. 2020, 61, 136–143. [Google Scholar] [CrossRef]
- Pantel, A.R.; Viswanath, V.; Daube-Witherspoon, M.E.; Dubroff, J.G.; Muehllehner, G.; Parma, M.J.; Pryma, D.A.; Schubert, E.K.; Mankoff, D.A.; Karp, J.S. PennPET Explorer: Human Imaging on a Whole-Body Imager. J. Nucl. Med. 2020, 61, 144–151. [Google Scholar] [CrossRef]
- Available online: https://www.med.upenn.edu/pennpetexplorer/scanner-progress.html (accessed on 31 January 2023).
- Rodriguez, J.A.; Selvaraj, S.; Bravo, P.E. Potential Cardiovascular Applications of Total-Body PET Imaging. PET Clin. 2021, 16, 129–136. [Google Scholar] [CrossRef]
- Chaudhari, A.J.; Raynor, W.Y.; Gholamrezanezhad, A.; Werner, T.J.; Rajapakse, C.S.; Alavi, A. Total-Body PET Imaging of Musculoskeletal Disorders. PET Clin. 2021, 16, 99–117. [Google Scholar] [CrossRef]
- Saboury, B.; Morris, M.A.; Nikpanah, M.; Werner, T.J.; Jones, E.C.; Alavi, A. Reinventing Molecular Imaging with Total-Body PET, Part II: Clinical Applications. PET Clin. 2020, 15, 463–475. [Google Scholar] [CrossRef]
- Alberts, I.; Hünermund, J.-N.; Prenosil, G.; Mingels, C.; Bohn, K.P.; Viscione, M.; Sari, H.; Vollnberg, B.; Shi, K.; Afshar-Oromieh, A.; et al. Clinical Performance of Long Axial Field of View PET/CT: A Head-to-Head Intra-Individual Comparison of the Biograph Vision Quadra with the Biograph Vision PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2395–2404. [Google Scholar] [CrossRef]
- Prenosil, G.A.; Sari, H.; Fürstner, M.; Afshar-Oromieh, A.; Shi, K.; Rominger, A.; Hentschel, M. Performance Characteristics of the Biograph Vision Quadra PET/CT System with a Long Axial Field of View Using the NEMA NU 2-2018 Standard. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 476–484. [Google Scholar] [CrossRef]
- Nardo, L.; Pantel, A.R. Oncologic Applications of Long Axial Field-of-View PET/Computed Tomography. PET Clin. 2021, 16, 65–73. [Google Scholar] [CrossRef]
- Nardo, L.; Abdelhafez, Y.G.; Spencer, B.A.; Badawi, R.D. Clinical Implementation of Total-Body PET/CT at University of California, Davis. PET Clin. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Chondronikola, M.; Sarkar, S. Total-Body PET Imaging. PET Clin. 2021, 16, 75–87. [Google Scholar] [CrossRef]
- Henrich, T.J.; Jones, T.; Beckford-Vera, D.; Price, P.M.; VanBrocklin, H.F. Total-Body PET Imaging in Infectious Diseases. PET Clin. 2021, 16, 89–97. [Google Scholar] [CrossRef]
- Saboury, B.; Morris, M.A.; Farhadi, F.; Nikpanah, M.; Werner, T.J.; Jones, E.C.; Alavi, A. Reinventing Molecular Imaging with Total-Body PET, Part I: Technical Revolution in Evolution. PET Clin. 2020, 15, 427–438. [Google Scholar] [CrossRef]
- NCCN Guidelines. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 31 January 2023).
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO Staging for Carcinoma of the Cervix Uteri. Int. J. Gynecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- NCCN Guidelines. Uterine Neoplasms, Version 1. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 21 January 2023).
- NCCN Guidelines. Cervical Cancer, Version 1. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 21 January 2023).
- NCCN Guidelines. Vulvar Cancer, Version 1. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 21 January 2023).
- NCCN Guidelines. Ovarian Cancer, Version 1. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 21 January 2023).
- Alavi, A.; Saboury, B.; Nardo, L.; Zhang, V.; Wang, M.; Li, H.; Raynor, W.Y.; Werner, T.J.; Høilund-Carlsen, P.F.; Revheim, M.-E. Potential and Most Relevant Applications of Total Body PET/CT Imaging. Clin. Nucl. Med. 2022, 47, 43–55. [Google Scholar] [CrossRef]
- van Sluis, J.; Borra, R.; Tsoumpas, C.; van Snick, J.H.; Roya, M.; ten Hove, D.; Brouwers, A.H.; Lammertsma, A.A.; Noordzij, W.; Dierckx, R.A.J.O.; et al. Extending the Clinical Capabilities of Short- and Long-Lived Positron-Emitting Radionuclides through High Sensitivity PET/CT. Cancer Imaging 2022, 22, 69. [Google Scholar] [CrossRef]
- De Gaetano, A.M.; Calcagni, M.L.; Rufini, V.; Valentini, A.L.; Gui, B.; Giordano, A.; Bonomo, L. Imaging of Gynecologic Malignancies with FDG PET–CT: Case Examples, Physiolocic Activity, and Pitfalls. Abdom. Imaging 2009, 34, 696–711. [Google Scholar] [CrossRef]
- Hernandez Pampaloni, M.; Facchetti, L.; Nardo, L. Pitfalls in [18F]FDG PET Imaging in Gynecological Malignancies. Q. J. Nucl. Med. Mol. Imaging 2016, 60, 124–138. [Google Scholar]
- Basu, S.; Kung, J.; Houseni, M.; Zhuang, H.; Tidmarsh, G.F.; Alavi, A. Temporal Profile of Fluorodeoxyglucose Uptake in Malignant Lesions and Normal Organs over Extended Time Periods in Patients with Lung Carcinoma: Implications for Its Utilization in Assessing Malignant Lesions. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 9–19. [Google Scholar]
- Zhuang, H.; Pourdehnad, M.; Lambright, E.S.; Yamamoto, A.J.; Lanuti, M.; Li, P.; Mozley, P.D.; Rossman, M.D.; Albelda, S.M.; Alavi, A. Dual Time Point 18F-FDG PET Imaging for Differentiating Malignant from Inflammatory Processes. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2001, 42, 1412–1417. [Google Scholar]
- Schillaci, O. Use of Dual-Point Fluorodeoxyglucose Imaging to Enhance Sensitivity and Specificity. Semin. Nucl. Med. 2012, 42, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Torigian, D.A.; Zhuang, H.; Alavi, A. When Should We Recommend Use of Dual Time-Point and Delayed Time-Point Imaging Techniques in FDG PET? Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Alberts, I.; Prenosil, G.; Mingels, C.; Bohn, K.P.; Viscione, M.; Sari, H.; Rominger, A.; Afshar-Oromieh, A. Feasibility of Late Acquisition [68 Ga] Ga-PSMA-11 PET/CT Using a Long Axial Field-of-View PET/CT Scanner for the Diagnosis of Recurrent Prostate Cancer-First Clinical Experiences. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4456–4462. [Google Scholar] [CrossRef]
- Alberts, I.; Schepers, R.; Zeimpekis, K.; Sari, H.; Rominger, A.; Afshar-Oromieh, A. Combined [68 Ga] Ga-PSMA-11 and Low-Dose 2-[18F]FDG PET/CT Using a Long-Axial Field of View Scanner for Patients Referred for [177Lu]-PSMA-Radioligand Therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 951–956. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Tamburini, K.; Mori, Y.; Cardinale, J.; Haberkorn, U.; Giesel, F.L. Advancement and Future Perspective of FAPI PET/CT In Gynecological Malignancies. Semin. Nucl. Med. 2022, 52, 628–634. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Makino, A.; Mori, T.; Tsuyoshi, H.; Kiyono, Y.; Yoshida, Y.; Okazawa, H. PET Imaging of Estrogen Receptors for Gynecological Tumors. Clin. Nucl. Med. 2022, 47, e481–e488. [Google Scholar] [CrossRef]
- Sari, H.; Mingels, C.; Alberts, I.; Hu, J.; Buesser, D.; Shah, V.; Schepers, R.; Caluori, P.; Panin, V.; Conti, M.; et al. First Results on Kinetic Modelling and Parametric Imaging of Dynamic 18F-FDG Datasets from a Long Axial FOV PET Scanner in Oncological Patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1997–2009. [Google Scholar] [CrossRef]
- Narayanan, P.; Sahdev, A. The Role of 18 F-FDG PET CT in Common Gynaecological Malignancies. Br. J. Radiol. 2017, 90, 20170283. [Google Scholar] [CrossRef]
- Wang, G.; Nardo, L.; Parikh, M.; Abdelhafez, Y.G.; Li, E.; Spencer, B.A.; Qi, J.; Jones, T.; Cherry, S.R.; Badawi, R.D. Total-Body PET Multiparametric Imaging of Cancer Using a Voxelwise Strategy of Compartmental Modeling. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1274–1281. [Google Scholar] [CrossRef]
- Viswanath, V.; Sari, H.; Pantel, A.R.; Conti, M.; Daube-Witherspoon, M.E.; Mingels, C.; Alberts, I.; Eriksson, L.; Shi, K.; Rominger, A.; et al. Abbreviated Scan Protocols to Capture 18F-FDG Kinetics for Long Axial FOV PET Scanners. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3215–3225. [Google Scholar] [CrossRef]
- Campos, N.M.F.; Almeida, V.; Curvo Semedo, L. Peritoneal Disease: Key Imaging Findings That Help in the Differential Diagnosis. Br. J. Radiol. 2022, 95, 20210346. [Google Scholar] [CrossRef]
- van’t Sant, I.; Engbersen, M.P.; Bhairosing, P.A.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; van Driel, W.J.; Aalbers, A.G.J.; Kok, N.F.M.; Lahaye, M.J. Diagnostic Performance of Imaging for the Detection of Peritoneal Metastases: A Meta-Analysis. Eur. Radiol. 2020, 30, 3101–3112. [Google Scholar] [CrossRef]
- De Gaetano, A.M.; Calcagni, M.L.; Rufini, V.; Valenza, V.; Giordano, A.; Bonomo, L. Imaging of Peritoneal Carcinomatosis with FDG PET-CT: Diagnostic Patterns, Case Examples and Pitfalls. Abdom. Imaging 2009, 34, 391–402. [Google Scholar] [CrossRef]
- van Sluis, J.; van Snick, J.H.; Brouwers, A.H.; Noordzij, W.; Dierckx, R.A.J.O.; Borra, R.J.H.; Slart, R.H.J.A.; Lammertsma, A.A.; Glaudemans, A.W.J.M.; Boellaard, R.; et al. EARL Compliance and Imaging Optimisation on the Biograph Vision Quadra PET/CT Using Phantom and Clinical Data. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4652–4660. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Pan, L.; Kopp-Schneider, A.; Weru, V.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Application of the Long Axial Field-of-View PET/CT with Low-Dose [18F]FDG in Melanoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 1158–1167. [Google Scholar] [CrossRef]
- Feudo, V.; Collarino, A.; Arciuolo, D.; Lorusso, M.; Ferrandina, G.; Rufini, V. Cervical Cancer. In Nuclear Medicine Manual on Gynaecological Cancers and Other Female Malignancies, 1st ed.; Collarino, A., Vidal-Sicart, S., Olmos, R.A.V., Eds.; Springer: Cham, Switzerland, 2022; Volume 1, pp. 53–70. [Google Scholar]
- Slart, R.H.J.A.; Tsoumpas, C.; Glaudemans, A.W.J.M.; Noordzij, W.; Willemsen, A.T.M.; Borra, R.J.H.; Dierckx, R.A.J.O.; Lammertsma, A.A. Long Axial Field of View PET Scanners: A Road Map to Implementation and New Possibilities. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4236–4245. [Google Scholar] [CrossRef]
- Lowry, K.P.; Lee, J.M.; Kong, C.Y.; McMahon, P.M.; Gilmore, M.E.; Cott Chubiz, J.E.; Pisano, E.D.; Gatsonis, C.; Ryan, P.D.; Ozanne, E.M.; et al. Annual Screening Strategies in BRCA1 and BRCA2 Gene Mutation Carriers: A Comparative Effectiveness Analysis. Cancer 2012, 118, 2021–2030. [Google Scholar] [CrossRef]
- Hepner, A.; Negrini, D.; Hase, E.A.; Exman, P.; Testa, L.; Trinconi, A.F.; Filassi, J.R.; Francisco, R.P.V.; Zugaib, M.; O’Connor, T.L.; et al. Cancer During Pregnancy: The Oncologist Overview. World J. Oncol. 2019, 10, 28–34. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Z.; Yu, H.; Wang, J.; Yang, X.; Shi, H. Ultra-Low-Dose CT Reconstructed with the Artificial Intelligence Iterative Reconstruction Algorithm (AIIR) in 18F-FDG Total-Body PET/CT Examination: A Preliminary Study. EJNMMI Phys. 2023, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Sari, H.; Teimoorisichani, M.; Mingels, C.; Alberts, I.; Panin, V.; Bharkhada, D.; Xue, S.; Prenosil, G.; Shi, K.; Conti, M.; et al. Quantitative Evaluation of a Deep Learning-Based Framework to Generate Whole-Body Attenuation Maps Using LSO Background Radiation in Long Axial FOV PET Scanners. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4490–4502. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xue, S.; Hu, J.; Sari, H.; Mingels, C.; Zeimpekis, K.; Prenosil, G.; Wang, Y.; Zhang, Y.; Viscione, M.; et al. Using Domain Knowledge for Robust and Generalizable Deep Learning-Based CT-Free PET Attenuation and Scatter Correction. Nat. Commun. 2022, 13, 5882. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Karim, F.W.; Kida, M.; Wentz, W.B.; Carter, J.R.; Sorensen, K.; Macfee, M.; Zika, J.; Makley, J.T. Bone Metastasis from Gynecologic Carcinomas: A Clinicopathologic Study. Gynecol. Oncol. 1990, 39, 108–114. [Google Scholar] [CrossRef]
- Rose, P.G.; Steven Piver, M.; Tsukada, Y.; Lau, T. Patterns of Metastasis in Uterine Sarcoma. An Autopsy Study. Cancer 1989, 63, 935–938. [Google Scholar] [CrossRef]
- Cormio, G.; Rossi, C.; Cazzolla, A.; Resta, L.; Loverro, G.; Greco, P.; Selvaggi, L. Distant Metastases in Ovarian Carcinoma. Int. J. Gynecol. Cancer 2003, 13, 125–129. [Google Scholar] [CrossRef]
- Thanapprapasr, D.; Nartthanarung, A.; Likittanasombut, P.; Na Ayudhya, N.I.; Charakorn, C.; Udomsubpayakul, U.; Subhadarbandhu, T.; Wilailak, S. Bone Metastasis in Cervical Cancer Patients Over a 10-Year Period. Int. J. Gynecol. Cancer 2010, 20, 373–378. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Hu, P.-C.; Wu, R.-Z.; Gu, Y.-S.; Chen, S.-G.; Yu, H.-J.; Wang, X.-Q.; Song, J.; Shi, H.-C. The Image Quality, Lesion Detectability, and Acquisition Time of 18F-FDG Total-Body PET/CT in Oncological Patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2507–2515. [Google Scholar] [CrossRef]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of Cancer-Associated Venous Thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef]
- Odajima, S.; Seki, T.; Kato, S.; Tomita, K.; Shoburu, Y.; Suzuki, E.; Takenaka, M.; Saito, M.; Takano, H.; Yamada, K.; et al. Efficacy of Edoxaban for the Treatment of Gynecological Cancer-Associated Venous Thromboembolism: Analysis of Japanese Real-World Data. J. Gynecol. Oncol. 2022, 33, e62. [Google Scholar] [CrossRef]
- O’Gorman, C.A.; Minnock, S.; Mulhall, J.; Gleeson, N. Attention to Bone Health in Follow-up of Gynaecological Cancers in Tertiary Care. Womens Health 2022, 18, 17455065211070747. [Google Scholar] [CrossRef]
- Calabro’, A.; Abdelhafez, Y.G.; Triumbari, E.K.A.; Spencer, B.A.; Chen, M.S.; Albano, D.; Cassim, C.R.; Bertagna, F.; Dondi, F.; Cherry, S.R.; et al. 18F-FDG Gallbladder Uptake: Observation from a Total-Body PET/CT Scanner. BMC Med. Imaging 2023, 23, 9. [Google Scholar] [CrossRef]
- Jiang, W.; Chalich, Y.; Deen, M.J. Sensors for Positron Emission Tomography Applications. Sensors 2019, 19, 5019. [Google Scholar] [CrossRef]
- Eakin, C.M.; Lai, T.; Cohen, J.G. Alarming Trends and Disparities in High-Risk Endometrial Cancer. Curr. Opin. Obstet. Gynecol. 2022, 35, 15–20. [Google Scholar] [CrossRef] [PubMed]
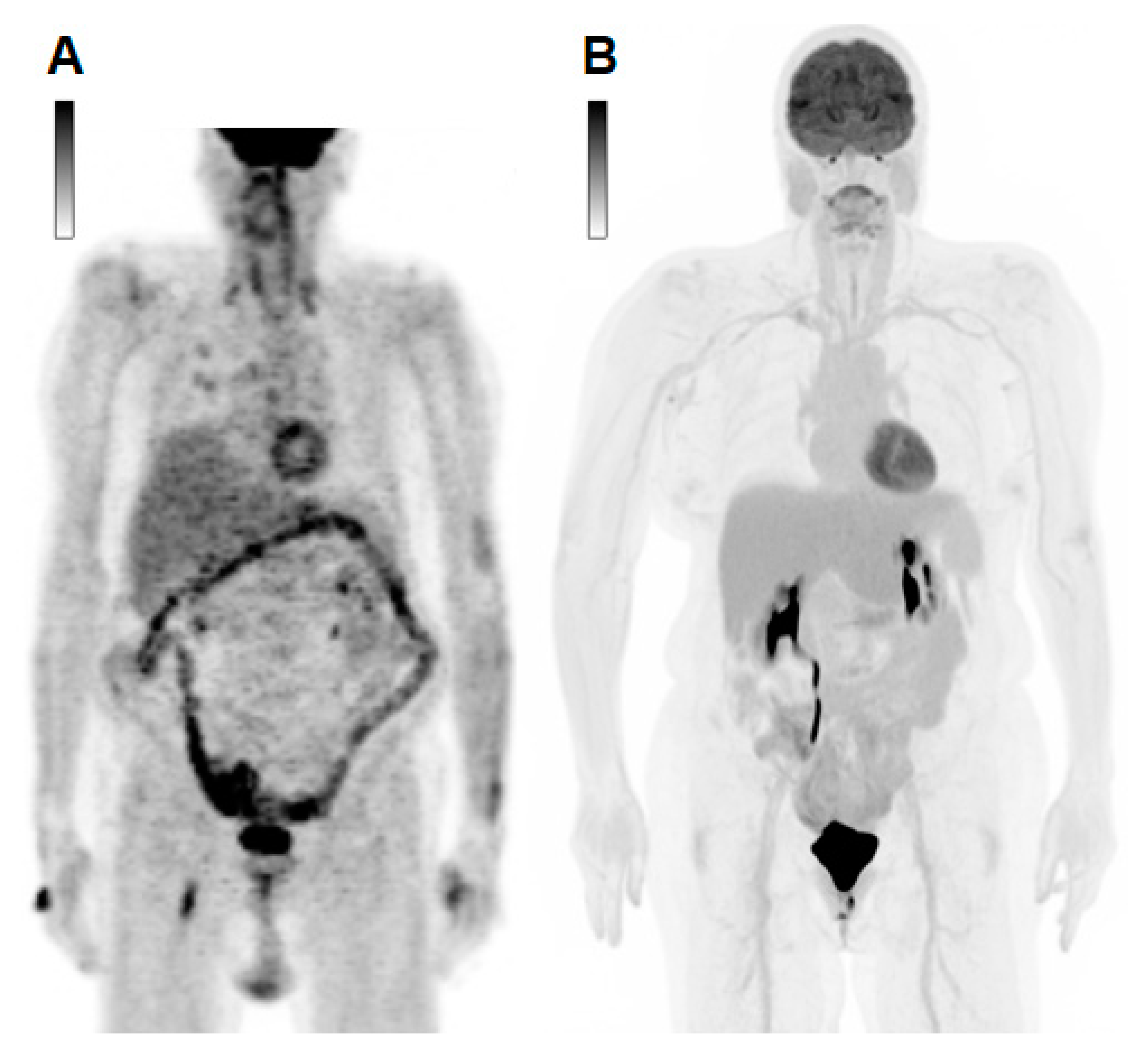

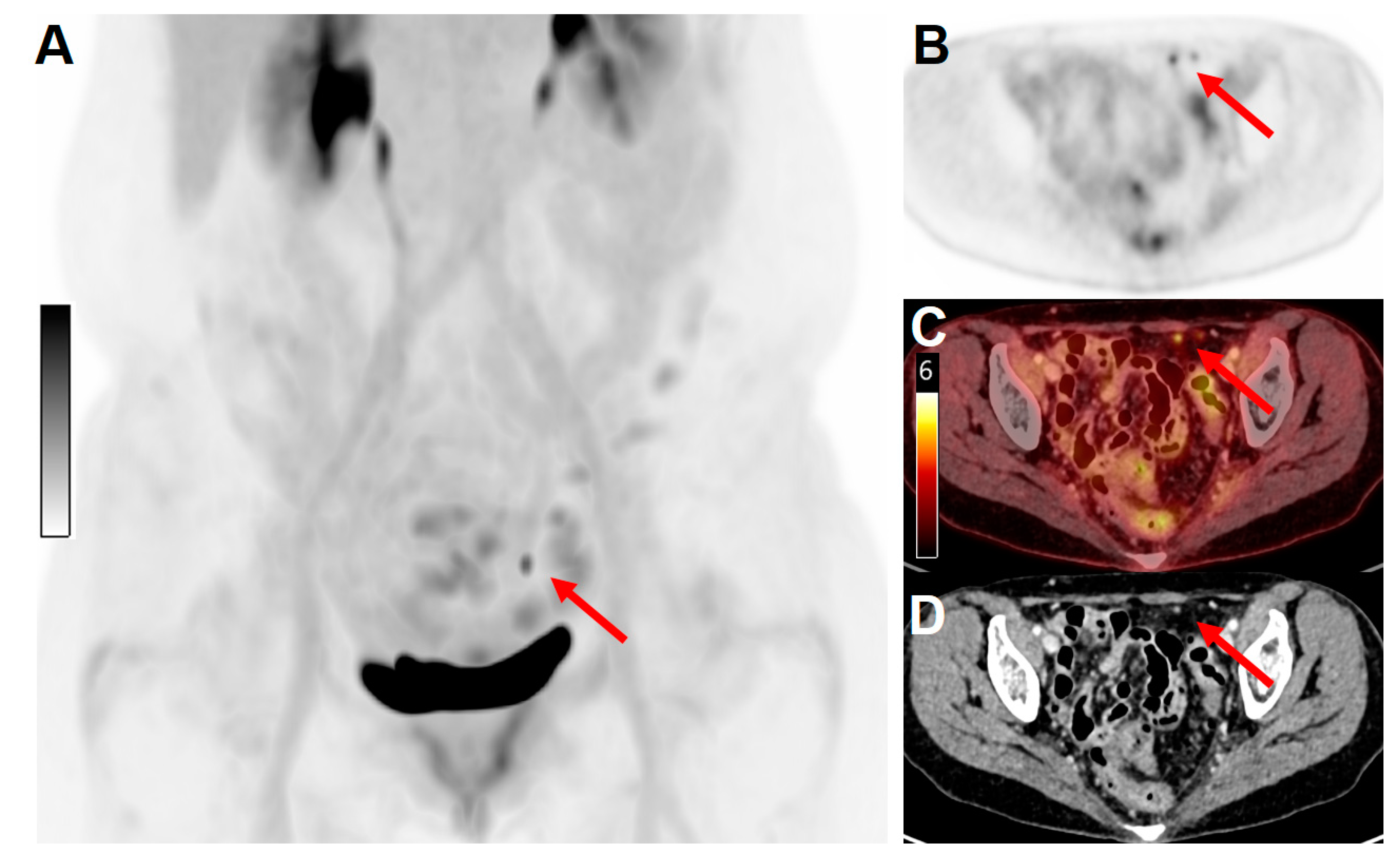
| Cancer Type | Incidence Rate (/100.000 Women/year) | Death Rate (/100.000 Women/year) | Diagnoses at Local Disease Stage (%) | Diagnoses at Loco-Regional Spread Stage (%) | Diagnoses at Metastatic Stage (%) | Mean 5-Year Relative Survival Rate (%) |
|---|---|---|---|---|---|---|
| Uterine | 27.8 | 5.1 | 67.0 | 20.0 | 9.0 | 81.3 |
| Cervical | 7.8 | 2.2 | 44.0 | 36.0 | 16.0 | 66.7 |
| Vaginal | 2.0 | Na | 66.0 | 54.0 | 24.0 | 49.0 |
| Vulvar | 2.6 | 0.6 | 60.0 | 28.0 | 6.0 | 70.3 |
| Ovarian | 10.6 | 6.3 | 17.0 | 21.0 | 57.0 | 49.7 |
| Scanner | uEXPLORER | PennPET Explorer | Biograph Vision Quadra |
|---|---|---|---|
| Company | UC Davis, United Imaging Healthcare | University of Pennsylvania | Siemens Healthineers |
| Bore diameter (cm) | 76 | 76.4 | 78 |
Scintillator
| LYSO 2.76 × 2.76 × 18.1 | LYSO 3.86 × 3.86 × 19 | LSO 3.2 × 3.2 × 20 |
| Photo-sensors | Analogue SiPMs | Digital SiPMs | Digital SiPMs |
| Axial field-of-view (cm) | 194 | 142 | 106 |
| Time of flight resolution (ps) | 505 | 250 | 228 |
| Sensitivity (kcps/MBq) | 174 | 140 | 176 |
| Spatial resolution (mm) | |||
| 2.9 | 4.0 | 3.4 |
| 3.0 | 4.0 | 3.4 |
| CT slice | 160 | 128 | 128 |
| Cancer Type | Indications |
|---|---|
| Uterine | (a) Endometrial carcinoma: initial workup, when metastatic disease is suspected in selected patients; suspected recurrence; for postoperative radiotherapy target delineation in patients at high risk of recurrence, especially if no nodes were sampled; for radiotherapy treatment planning in medically inoperable patients planned for definitive radiation by brachytherapy alone. (b) Uterine sarcoma: initial workup, to clarify ambiguous findings; follow-up/post-treatment surveillance, if metastatic disease is suspected. |
| Cervical | Initial workup of patients with revised 2018 FIGO stage >IB1, to evaluate nodal and distant disease; radiation therapy planning; response to treatment assessment in women with FIGO stage IB3 to IVA disease; recurrence; surveillance. |
| Vaginal | Initial workup, when metastatic disease is suspected in selected patients; follow-up/post-treatment surveillance; suspected recurrence. |
| Vulvar | Initial workup of patients with ≥T2 tumors or if metastatic disease is suspected; follow-up/surveillance if recurrence/metastasis is suspected; follow-up/surveillance, to assess treatment response if primary treatment was with definitive intent; follow-up/surveillance, in cases of high-risk disease every 4–12 months. |
| Ovarian | Initial workup in newly diagnosed ovarian cancer after a recent surgical procedure, if clinically indicated; stages II–IV post-primary treatment; follow-up/post-treatment surveillance in patients with rising Cancer Antigen-125 levels and equivocal findings at anatomic imaging studies for detection of recurrent disease; therapy monitoring. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triumbari, E.K.A.; Rufini, V.; Mingels, C.; Rominger, A.; Alavi, A.; Fanfani, F.; Badawi, R.D.; Nardo, L. Long Axial Field-of-View PET/CT Could Answer Unmet Needs in Gynecological Cancers. Cancers 2023, 15, 2407. https://doi.org/10.3390/cancers15092407
Triumbari EKA, Rufini V, Mingels C, Rominger A, Alavi A, Fanfani F, Badawi RD, Nardo L. Long Axial Field-of-View PET/CT Could Answer Unmet Needs in Gynecological Cancers. Cancers. 2023; 15(9):2407. https://doi.org/10.3390/cancers15092407
Chicago/Turabian StyleTriumbari, Elizabeth Katherine Anna, Vittoria Rufini, Clemens Mingels, Axel Rominger, Abass Alavi, Francesco Fanfani, Ramsey D. Badawi, and Lorenzo Nardo. 2023. "Long Axial Field-of-View PET/CT Could Answer Unmet Needs in Gynecological Cancers" Cancers 15, no. 9: 2407. https://doi.org/10.3390/cancers15092407
APA StyleTriumbari, E. K. A., Rufini, V., Mingels, C., Rominger, A., Alavi, A., Fanfani, F., Badawi, R. D., & Nardo, L. (2023). Long Axial Field-of-View PET/CT Could Answer Unmet Needs in Gynecological Cancers. Cancers, 15(9), 2407. https://doi.org/10.3390/cancers15092407









